Lymphatic Defects in Zebrafish sox18 Mutants Are Exacerbated by Perturbed VEGFC Signaling, While Masked by Elevated sox7 Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Lines and Maintenance
2.2. Genotyping
2.3. MO Microinjections
2.4. In Situ Hybridizations
2.5. Phenotypic Analyses
2.6. Statistical Analyses
3. Results
3.1. The sa12315 Mutation Is a Loss-of-Function Allele of sox18
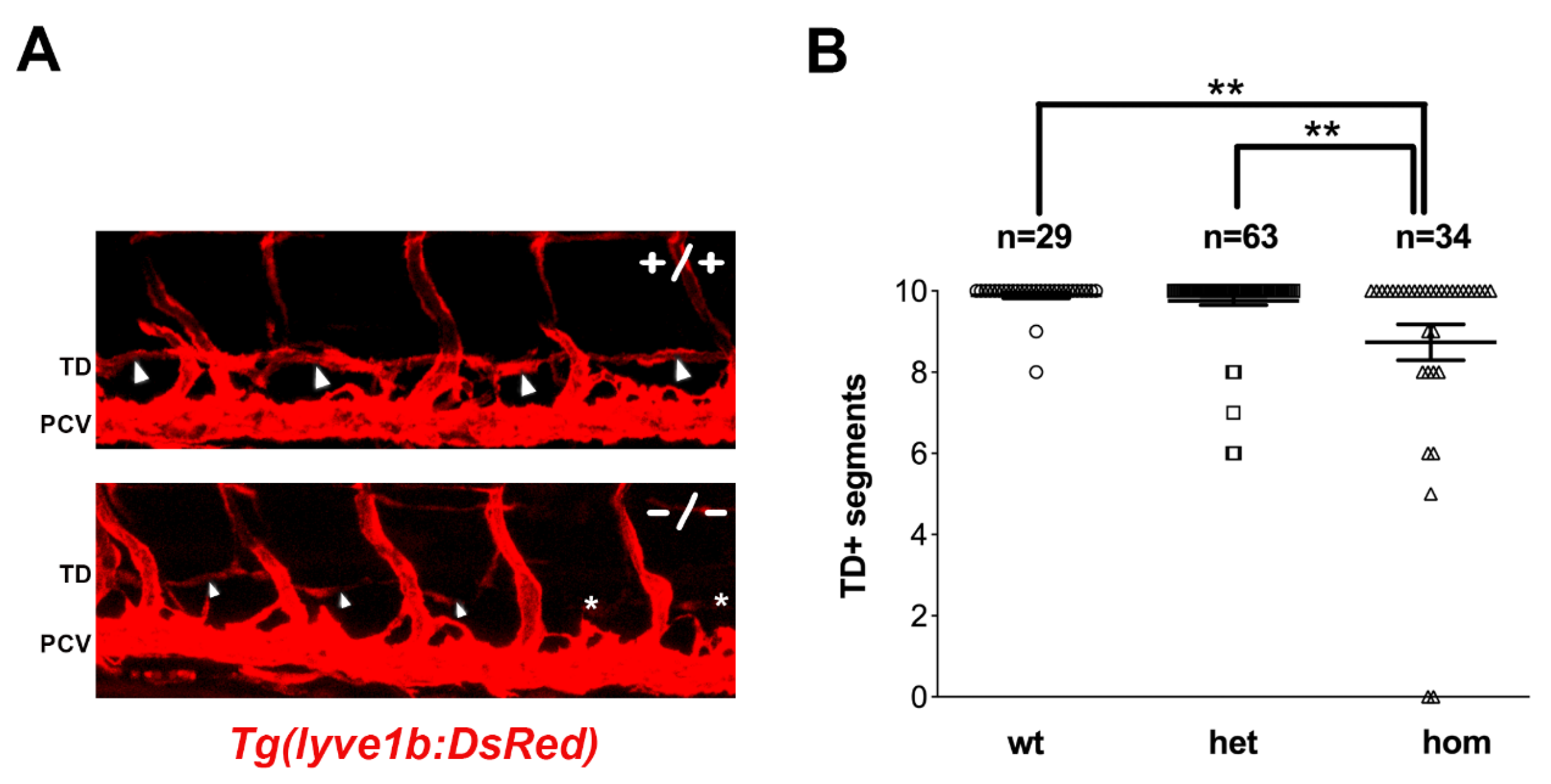
3.2. sa12315 Mutants Show Mild Lymphatic Defects, Which Are Exacerbated by Perturbed VEGFC Signaling
3.3. sox18sa12315 Mutants Have Milder Lymphatic Phenotypes Than sox18 Morphants
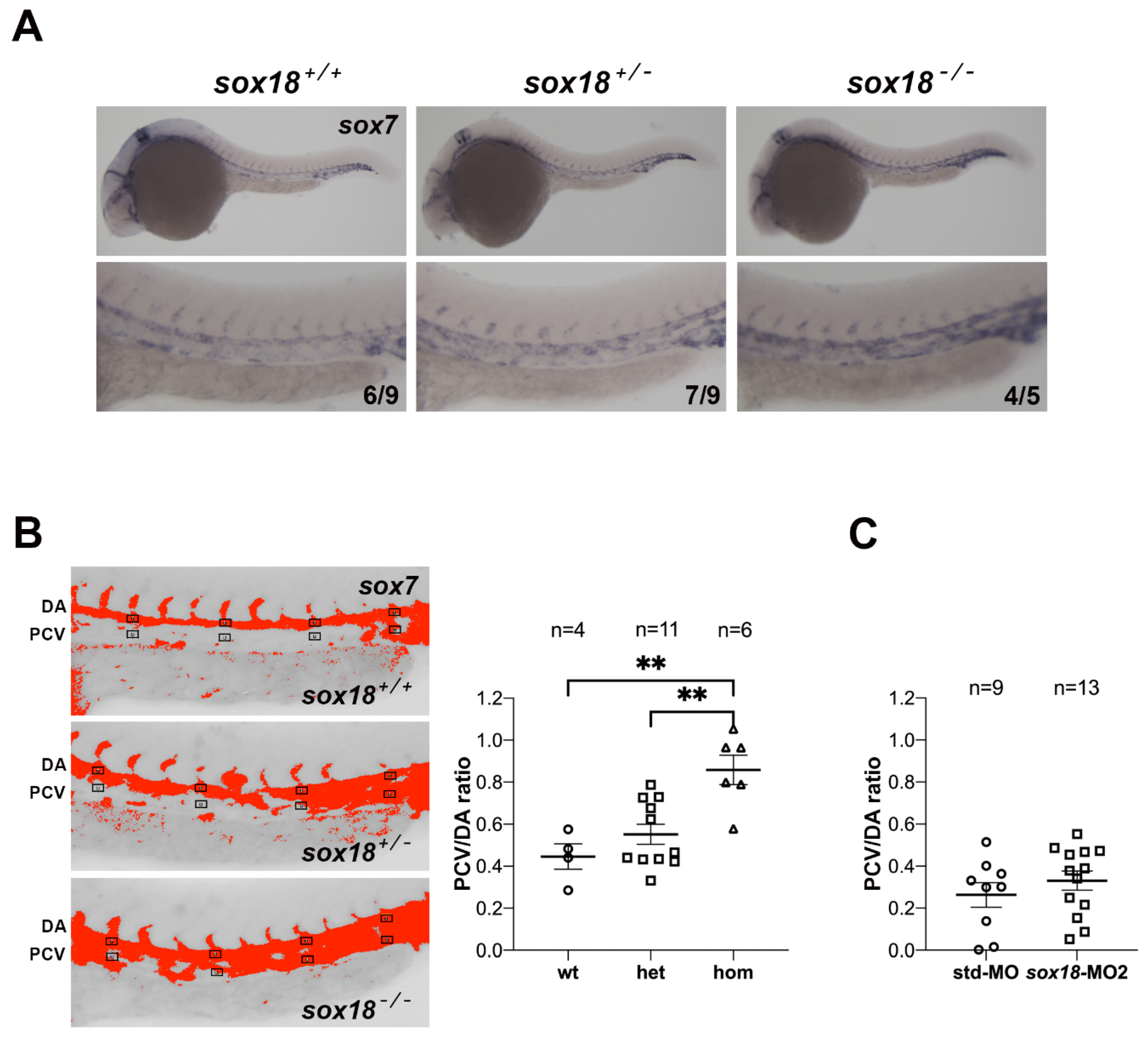
3.4. Ectopic Expression of sox7 in the PCV of sox18sa12315 Mutants, but Not sox18 Morphants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX Family of Developmental Transcription Factors Based on Sequence and Structural Indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef]
- François, M.; Koopman, P.; Beltrame, M. SoxF genes: Key players in the development of the cardio-vascular system. Int. J. Biochem. Cell Biol. 2010, 42, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Irrthum, A.; Devriendt, K.; Chitayat, D.; Matthijs, G.; Glade, C.; Steijlen, P.M.; Fryns, J.-P.; Van Steensel, M.A.M.; Vikkula, M. Mutations in the Transcription Factor Gene SOX18 Underlie Recessive and Dominant Forms of Hypotrichosis-Lymphedema Telangiectasia. Am. J. Hum. Genet. 2003, 72, 1470–1478. [Google Scholar] [CrossRef]
- Moalem, S.; Brouillard, P.; Kuypers, D.; Legius, E.; Harvey, E.; Taylor, G.; François, M.; Vikkula, M.; Chitayat, D. Hypotrichosis-lymphedema-telangiectasia-renal defect associated with a truncating mutation in the SOX18 gene. Clin. Genet. 2015, 87, 378–382. [Google Scholar] [CrossRef]
- Wünnemann, F.; Kokta, V.; Leclerc, S.; Thibeault, M.; McCuaig, C.; Hatami, A.; Stheneur, C.; Grenier, J.-C.; Awadalla, P.; Mitchell, G.A.; et al. Aortic Dilatation Associated with a De Novo Mutationin the SOX18 Gene: Expanding the Clinical Spectrum of Hypotrichosis-Lymphedema-Telangiectasia Syndrome. Can. J. Cardiol. 2016, 32, 135.e1–135.e7. [Google Scholar] [CrossRef]
- Atiş, G.; Sam Sari, A.; Soylu, E.; Akgün Dogan, O. A Case with Hypotrichosis-LymphedemaTelangiectasia Syndrome with Hair Shaft Fragility. Skin Appendage Disord. 2022, 8, 511–514. [Google Scholar] [CrossRef]
- Coulie, R.; Niyazov, D.M.; Gambello, M.J.; Fastré, E.; Brouillard, P.; Vikkula, M. Hypotrichosis-lymphedema-telangiectasia syndrome: Report of ileal atresia associated with a SOX18 de novo pathogenic variant and review of the phenotypic spectrum. Am. J. Med. Genet. 2021, 185A, 2153–2159. [Google Scholar] [CrossRef]
- Pennisi, D.; Gardner, J.; Chambers, D.; Hosking, B.; Peters, J.; Muscat, G.; Abbott, C.; Koopman, P. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat. Genet. 2000, 24, 434–437. [Google Scholar] [CrossRef]
- Downes, M.; Koopman, P. SOX18 and the Transcriptional Regulation of Blood Vessel Development. Trends Cardiovasc. Med. 2001, 11, 318–324. [Google Scholar] [CrossRef]
- Downes, M.; François, M.; Ferguson, C.; Parton, R.G.; Koopman, P. Vascular defects in a mouse model of hypotrichosis-lymphedema-telangiectasia syndrome indicate a role for SOX18 in blood vessel maturation. Hum. Mol. Genet. 2009, 18, 2839–2850. [Google Scholar] [CrossRef]
- François, M.; Caprini, A.; Hosking, B.; Orsenigo, F.; Wilhelm, D.; Browne, C.; Paavonen, K.; Karnezis, T.; Shayan, R.; Downes, M.; et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 2008, 456, 643–647. [Google Scholar] [CrossRef]
- Pennisi, D.; Bowles, J.; Nagy, A.; Muscat, G.; Koopman, P. Mice Null for Sox18 Are Viable and Display a Mild Coat Defect. Mol. Cell. Biol. 2000, 20, 9331–9336. [Google Scholar] [CrossRef]
- Wigle, J.T.; Oliver, G. Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef]
- Srinivasan, R.S.; Geng, X.; Yang, Y.; Wang, Y.; Mukatira, S.; Studer, M.; Porto, M.P.R.; Lagutin, O.; Oliver, G. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010, 24, 696–707. [Google Scholar] [CrossRef]
- Hosking, B.; François, M.; Wilhelm, D.; Orsenigo, F.; Caprini, A.; Svingen, T.; Tutt, D.; Davidson, T.; Browne, C.; Dejana, E.; et al. Sox7 and Sox17 are strain specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development 2009, 136, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Secker, G.A.; Harvey, N.L. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev. Dyn. 2015, 2443, 323–331. [Google Scholar] [CrossRef]
- Martin-Almedina, S.; Mortimer, P.S.; Ostergaard, P. Development and physiological functions of the lymphatic system: Insights from human genetic studies of primary lymphedema. Physiol. Rev. 2021, 101, 1809–1871. [Google Scholar] [CrossRef]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef]
- Isogai, S.; Lawson, N.D.; Torrealday, S.; Horiguchi, M.; Weinstein, B.M. Angiogenic network formation in the developing vertebrate trunk. Development 2003, 130, 5281–5290. [Google Scholar] [CrossRef]
- Hogan, B.M.; Bos, F.L.; Bussmann, J.; Witte, M.; Chi, N.C.; Duckers, H.J.; Schulte-Merker, S. ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 2009, 41, 396–398. [Google Scholar] [CrossRef]
- Bussmann, J.; Bos, F.L.; Urasaki, A.; Kawakami, K.; Duckers, H.J.; Schulte-Merker, S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 2010, 137, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.M.; Schulte-Merker, S. How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev. Cell 2017, 42, 576–583. [Google Scholar] [CrossRef]
- Küchler, A.M.; Gjini, E.; Peterson-Maduro, J.; Cancilla, B.; Wolburg, H.; Schulte-Merker, S. Development of the Zebrafish Lymphatic System Requires Vegfc Signaling. Curr. Biol. 2006, 16, 1244–1248. [Google Scholar] [CrossRef]
- Hogan, B.M.; Herpers, R.; Witte, M.; Heloterä, H.; Alitalo, K.; Duckers, H.J.; Schulte-Merker, S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 2009, 136, 4001–4009. [Google Scholar] [CrossRef]
- Villefranc, J.A.; Nicoli, S.; Bentley, K.; Jeltsch, M.; Zarkada, G.; Moore, J.C.; Gerhardt, H.; Alitalo, K.; Lawson, N.D. A truncation allele in vascular endothelial growth factor c reveals distinct modes of signaling during lymphatic and vascular development. Development 2013, 140, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, L.; Karpanen, T.; Schulte, D.; Harris, N.C.; Koltowska, K.; Roukens, G.; Bower, N.I.; van Impel, A.; Stacker, S.A.; Achen, M.G.; et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 2014, 141, 1239–1249. [Google Scholar] [CrossRef]
- Aranguren, X.L.; Beerens, M.; Vandevelde, W.; Dewerchin, M.; Carmeliet, P.; Luttun, A. Transcription factor COUP-TFII is indispensable for venous and lymphatic development in zebrafish and Xenopus laevis. Biochem. Biophys. Res. Commun. 2011, 410, 121–126. [Google Scholar] [CrossRef]
- Cermenati, S.; Moleri, S.; Neyt, C.; Bresciani, E.; Carra, S.; Grassini, D.R.; Omini, A.; Goi, M.; Cotelli, F.; François, M.; et al. Sox18 genetically interacts with VegfC to regulate lymphangiogenesis in zebrafish. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1238–1247. [Google Scholar] [CrossRef]
- Cermenati, S.; Moleri, S.; Cimbro, S.; Corti, P.; Del Giacco, L.; Amodeo, R.; Dejana, E.; Koopman, P.; Cotelli, F.; Beltrame, M. Sox18 and Sox7 play redundant roles in vascular development. Blood 2008, 111, 2657–2666. [Google Scholar] [CrossRef]
- Herpers, R.; van de Kamp, E.; Duckers, H.J.; Schulte-Merker, S. Redundant Roles for Sox7 and Sox18 in Arteriovenous Specification in Zebrafish. Circ. Res. 2008, 102, 12–15. [Google Scholar] [CrossRef]
- Pendeville, H.; Winandy, M.; Manfroid, I.; Nivelles, O.; Motte, P.; Pasque, V.; Peers, B.; Struman, I.; Martial, J.A.; Voz, M.L. Zebrafish Sox7 and Sox18 function together to control arterial–venous identity. Dev. Biol. 2008, 317, 405–416. [Google Scholar] [CrossRef]
- Tao, S.; Witte, M.; Bryson-Richardson, R.J.; Currie, P.D.; Hogan, B.M.; Schulte-Merker, S. Zebrafish prox1b mutants develop a lymphatic vasculature, and prox1b does not specifically mark lymphatic endothelial cells. PLoS ONE. 2011, 6, e28934. [Google Scholar] [CrossRef][Green Version]
- van Impel, A.; Zhao, Z.; Hermkens, D.M.A.; Roukens, M.G.; Fischer, J.C.; Peterson-Maduro, J.; Duckers, H.; Ober, E.A.; Ingham, P.W.; Schulte-Merker, S. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 2014, 141, 1228–1238. [Google Scholar] [CrossRef]
- Koltowska, K.; Lagendijk, A.K.; Pichol-Thievend, C.; Fischer, J.C.; François, M.; Ober, E.A.; Yap, A.S.; Hogan, B.M. Vegfc Regulates Bipotential Precursor Division and Prox1 Expression to Promote Lymphatic Identity in Zebrafish. Cell Rep. 2015, 13, 1828–1841. [Google Scholar] [CrossRef]
- Kettleborough, R.N.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book; University of Oregon Press: Eugene, OR, USA, 1993. [Google Scholar]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef]
- Okuda, K.S.; Astin, J.W.; Misa, J.P.; Flores, M.V.; Crosier, K.E.; Crosier, P.S. lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 2012, 139, 2381–2391. [Google Scholar] [CrossRef]
- Jung, H.M.; Castranova, D.; Swift, M.R.; Pham, V.N.; Venero Galanternik, M.; Isogai, S.; Butler, M.G.; Mulligan, T.S.; Weinstein, B.M. Development of the larval lymphatic system. Development 2017, 144, 2070–2081. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
- Larson, J.D.; Wadman, S.A.; Chen, E.; Kerley, L.; Clark, K.J.; Eide, M.; Lippert, S.; Nasevicius, A.; Ekker, S.C.; Hackett, P.B.; et al. Expression of VE-cadherin in zebrafish embryos: A new tool to evaluate vascular development. Dev. Dyn. 2004, 231, 204–213. [Google Scholar] [CrossRef]
- Wilkinson, M.F. Genetic paradox explained by nonsense. Nature 2019, 568, 179–180. [Google Scholar] [CrossRef]
- El-Brolosy, M.A.; Kontarakis, Z.; Rossi, A.; Kuenne, C.; Günther, S.; Fukuda, N.; Kikhi, K.; Boezio, G.L.M.; Takacs, C.M.; Lai, S.L.; et al. Genetic compensation triggered by mutant mRNA degradation. Nature 2019, 568, 193–197. [Google Scholar] [CrossRef]
- Hermkens, D.M.A.; van Impel, A.; Urasaki, A.; Bussmann, J.; Duckers, H.J.; Schulte-Merker, S. Sox7 controls arterial specification in conjunction with hey2 and efnb2 function. Development 2015, 142, 1695–1704. [Google Scholar] [CrossRef]
- Arnold, H.; Panara, V.; Hubmann, M.; Filipek-Gorniok, B.; Skoczylas, R.; Ranefall, P.; Gloger, M.; Allalou, A.; Hogan, B.; Schulte-Merker, S.; et al. mafba and mafbb differentially regulate lymphatic endothelial cell migration in topographically distinct manners. Cell Rep. 2022, 39, 1–12. [Google Scholar] [CrossRef]
- Sinner, D.; Rankin, S.; Lee, M.; Zorn, A.M. Sox17 and β-catenin cooperate to regulate the transcription of endodermal genes. Development 2004, 131, 3069–3080. [Google Scholar] [CrossRef]
- Das, R.N.; Tevet, Y.; Safriel, S.; Han, Y.; Moshe, N.; Lambiase, G.; Bassi, I.; Nicenboim, J.; Brückner, M.; Hirsch, D.; et al. Generation of specialized blood vessels via lymphatic transdifferentiation. Nature 2022, 606, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Nicenboim, J.; Malkinson, G.; Lupo, T.; Asaf, L.; Sela, Y.; Mayseless, O.; Gibbs-Bar, L.; Senderovich, N.; Hashimshony, T.; Shin, M.; et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 2015, 522, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.S.; Escobedo, N.; Yang, Y.; Interiano, A.; Dillard, M.E.; Finkelstein, D.; Mukatira, S.; Gil, H.J.; Nurmi, H.; Alitalo, K.; et al. The Prox1–Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 2014, 28, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Chiang, I.K.N.; Graus, M.S.; Kirschnick, N.; Davidson, T.; Luu, W.; Harwood, R.; Jiang, K.; Li, B.; Wong, Y.Y.; Moustaqil, M.; et al. The blood vasculature instructs lymphatic patterning in a SOX7-dependent manner. EMBO J. 2023, 42, e109032. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moleri, S.; Mercurio, S.; Pezzotta, A.; D’Angelo, D.; Brix, A.; Plebani, A.; Lini, G.; Di Fuorti, M.; Beltrame, M. Lymphatic Defects in Zebrafish sox18 Mutants Are Exacerbated by Perturbed VEGFC Signaling, While Masked by Elevated sox7 Expression. Cells 2023, 12, 2309. https://doi.org/10.3390/cells12182309
Moleri S, Mercurio S, Pezzotta A, D’Angelo D, Brix A, Plebani A, Lini G, Di Fuorti M, Beltrame M. Lymphatic Defects in Zebrafish sox18 Mutants Are Exacerbated by Perturbed VEGFC Signaling, While Masked by Elevated sox7 Expression. Cells. 2023; 12(18):2309. https://doi.org/10.3390/cells12182309
Chicago/Turabian StyleMoleri, Silvia, Sara Mercurio, Alex Pezzotta, Donatella D’Angelo, Alessia Brix, Alice Plebani, Giulia Lini, Marialaura Di Fuorti, and Monica Beltrame. 2023. "Lymphatic Defects in Zebrafish sox18 Mutants Are Exacerbated by Perturbed VEGFC Signaling, While Masked by Elevated sox7 Expression" Cells 12, no. 18: 2309. https://doi.org/10.3390/cells12182309
APA StyleMoleri, S., Mercurio, S., Pezzotta, A., D’Angelo, D., Brix, A., Plebani, A., Lini, G., Di Fuorti, M., & Beltrame, M. (2023). Lymphatic Defects in Zebrafish sox18 Mutants Are Exacerbated by Perturbed VEGFC Signaling, While Masked by Elevated sox7 Expression. Cells, 12(18), 2309. https://doi.org/10.3390/cells12182309







