Could an Anterior Cruciate Ligament Be Tissue-Engineered from Silk?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
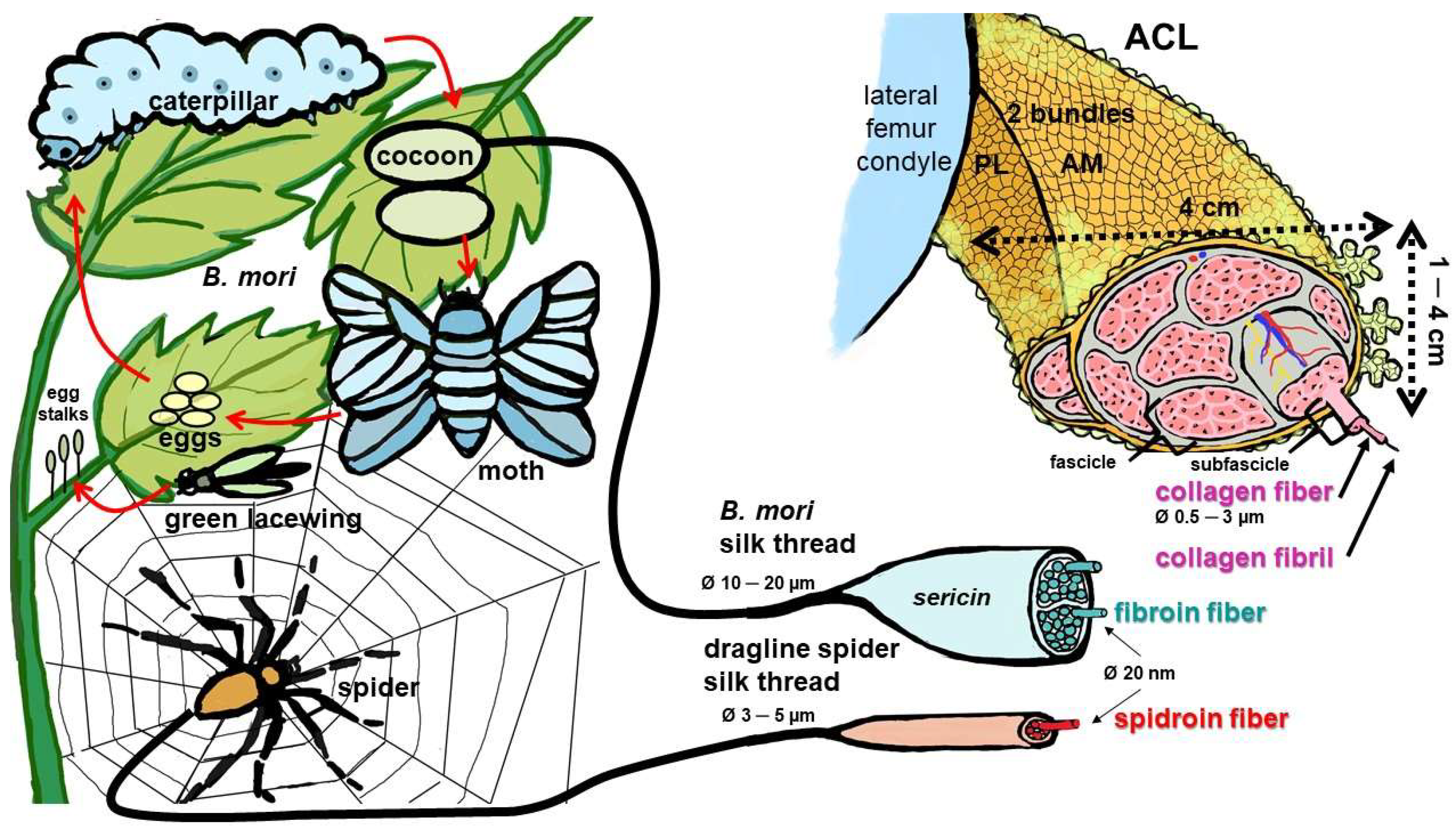
3.1. Mulberry and Non-Mulberry Silk Proteins, Sources of Silk
3.2. Structure of Silk
3.3. Biomechanical Properties of Silk
| Characteristics | B. mori, Silkworm Silk | Nephila Spider Dragline Silk | ACL | References |
|---|---|---|---|---|
| Fiber diameter | 10–20 µm | 2–5 µm | Human ACL: 1 cm × 1–4 cm Fascicles/subfascicles: 50–300 μm Collagen type 1 fiber: 0.5–3 µm Fibril: 10–100 nm | [29,69,70,71,72] |
| Ultimate tensile strength (UTS) | 500–740 MPa | 1.150–1.750 MPa | 600–2300 N Maximum stress: 21–41 MPa, depending on age, sex and health status | [15,71,73,74,75] |
| Strain/elongation at breakage | 4–20% | 19–40% | 15–30%, depending on age, sex and health status | [15,76] |
| Young’s modulus | 5–17 GPa, depending on with/without sericin, lower with sericin | 2–16 GPa | 99–129 MPa, depending on age, sex and health status | [15,60,73,77,78,79,80] |
3.4. Silk Processing for Tissue Engineering
3.4.1. Native Silk
3.4.2. Regenerated Silk Morphologies
3.5. Functionalization Strategies Applied to Silk
3.6. Cell Response to Silk and Its Components
3.7. Response of Cells to Silk Relevant for ACL Graft Integration
3.7.1. Fibroblasts
3.7.2. Ligamentocytes and Ligament-Derived Stem Cells
3.7.3. Tenocytes and Tendon-Derived Stem Cells
3.7.4. Synovial Fibroblasts
3.7.5. Adipose-Tissue-Derived Stem Cells
3.7.6. MSCs
3.7.7. Endothelial Cells
3.7.8. Osteoblasts
3.7.9. Chondrocytes
3.7.10. Macrophages
3.8. Signaling Pathways Stimulated with Silk Components in Cell Types Relevant for ACL Reconstruction
| Components | Cell Type | Effect | Reference |
|---|---|---|---|
| Fibroin | MSC | Integrin PIK3 pathway, immunomodulation | [118,127] |
| Silk (fibroin) | Ligamentocytes | Lesser effect on ligamentocytes compared with BM-MSCs | [132,133] |
| Fibroin | Fibroblasts | Support of growth | [131] |
| Fibroin | SaOs-2 cells (osteosarcoma) MC3T3-E1 (pre-osteoblasts) | Adhesion, proliferation, osteogenesis | [105,122] |
| Raw silk with SDF-1 | Ligament stem/progenitor cells | Cell recruitment | [112] |
| Fibroin (aligned/random fibers) | Periodontal ligament stem cells (wisdom teeth-derived) | Proliferation | [9] |
| Fibroin | Endothelial cells | Support of growth | [131] |
| Fibroin | Stromal vascular fraction | Support of tissue formation in vivo at 6 months | [128] |
| Sericin | MSC | Regulates glucose metabolism, oxidative stress, angiogenesis, cell adhesion, adaptation to hypoxia and immunomodulation in MSCs, glycolysis and angiogenesis Does not influence gene markers of adipogenic, osteogenic and chondrogenic lineage differentiation, as well as stemness maintenance | [127] |
| Sericin | ASC | May stimulate the secretion of beneficial adhesion molecules from ASCs and activates the gene transcription associated with differentiation and migration of ASC, regulating regeneration of inflamed tissues | [137] |
| Sericin/fibroin | Macrophages | Sericin: improved differentiation of macrophages towards the M2 phenotype, ratio of fibroin/sericin determines macrophage phenotype, even the topology of both components elicited different macrophage responses | [137,152,153] |
3.9. Silk in View for ACL Tissue Engineering
3.10. ACL Enthesis and Osseointegrating Silk Scaffolds/Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbanek, O.; Moczulska-Heljak, M.; Wrobel, M.; Mioduszewski, A.; Kolbuk, D. Advanced Graft Development Approaches for ACL Reconstruction or Regeneration. Biomedicines 2023, 11, 507. [Google Scholar] [CrossRef]
- Rathbone, S.; Maffulli, N.; Cartmell, S.H. Most british surgeons would consider using a tissue-engineered anterior cruciate ligament: A questionnaire study. Stem Cells Int. 2012, 2012, 303724. [Google Scholar] [CrossRef]
- Riley, T.C.; Mafi, R.; Mafi, P.; Khan, W.S. Knee Ligament Injury and the Clinical Application of Tissue Engineering Techniques: A Systematic Review. Curr. Stem Cell Res. Ther. 2018, 13, 226–234. [Google Scholar] [CrossRef]
- Cooper, J.A., Jr.; Sahota, J.S.; Gorum, W.J., 2nd; Carter, J.; Doty, S.B.; Laurencin, C.T. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc. Natl. Acad. Sci. USA 2007, 104, 3049–3054. [Google Scholar] [CrossRef]
- Archer, D.E.; Mafi, R.; Mafi, P.; Khan, W.S. Preclinical Studies on Biomaterial Scaffold use in Knee Ligament Regeneration: A Systematic Review. Curr. Stem Cell Res. Ther. 2018, 13, 691–701. [Google Scholar] [CrossRef]
- Teuschl, A.H.; Tangl, S.; Heimel, P.; Schwarze, U.Y.; Monforte, X.; Redl, H.; Nau, T. Osteointegration of a Novel Silk Fiber-Based ACL Scaffold by Formation of a Ligament-Bone Interface. Am. J. Sports Med. 2019, 47, 620–627. [Google Scholar] [CrossRef]
- Bakirci, E.; Guenat, O.T.; Ahmad, S.S.; Gantenbein, B. Tissue engineering approaches for the repair and regeneration of the anterior cruciate ligament: Towards 3D bioprinted ACL-on-chip. Eur. Cell Mater. 2022, 44, 21–42. [Google Scholar] [CrossRef]
- Kokozidou, M.; Goegele, C.; Pirrung, F.; Hammer, N.; Werner, C.; Kohl, B.; Hahn, J.; Breier, A.; Schroepfer, M.; Meyer, M.; et al. In vivo ligamentogenesis in embroidered poly(lactic-co-epsilon-caprolactone)/polylactic acid scaffolds functionalized by fluorination and hexamethylene diisocyanate cross-linked collagen foams. Histochem. Cell Biol. 2023, 159, 275–292. [Google Scholar] [CrossRef]
- Chen, J.; Mo, Q.; Sheng, R.; Zhu, A.; Ling, C.; Luo, Y.; Zhang, A.; Chen, Z.; Yao, Q.; Cai, Z.; et al. The application of human periodontal ligament stem cells and biomimetic silk scaffold for in situ tendon regeneration. Stem Cell Res. Ther. 2021, 12, 596. [Google Scholar] [CrossRef]
- Vaishya, R.; Agarwal, A.K.; Ingole, S.; Vijay, V. Current Trends in Anterior Cruciate Ligament Reconstruction: A Review. Cureus 2015, 7, e378. [Google Scholar] [CrossRef]
- Aigner, T.B.; DeSimone, E.; Scheibel, T. Biomedical Applications of Recombinant Silk-Based Materials. Adv. Mater. 2018, 30, e1704636. [Google Scholar] [CrossRef]
- Yazawa, K.; Hidaka, K.; Negishi, J. Cell Adhesion Behaviors on Spider Silk Fibers, Films, and Nanofibers. Langmuir 2022, 38, 7766–7774. [Google Scholar] [CrossRef]
- Horan, R.L.; Toponarski, I.; Boepple, H.E.; Weitzel, P.P.; Richmond, J.C.; Altman, G.H. Design and characterization of a scaffold for anterior cruciate ligament engineering. J. Knee Surg. 2009, 22, 82–92. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Wong, E.J.; Toh, S.L.; Goh, J.C. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials 2008, 29, 3324–3337. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Toh, S.L.; Goh, J.C. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials 2009, 30, 4967–4977. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, J.; Mo, X.; Chen, S. Effect of silk fibroin/poly (L-lactic acid-co-e-caprolactone) nanofibrous scaffold on tendon-bone healing of rabbits. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2017, 31, 957–962. [Google Scholar]
- Zhi, Y.; Jiang, J.; Zhang, P.; Chen, S. Silk enhances the ligamentization of the polyethylene terephthalate artificial ligament in a canine anterior cruciate ligament reconstruction model. Artif. Organs 2019, 43, E94–E108. [Google Scholar] [CrossRef]
- Shiroud Heidari, B.; Muinos Lopez, E.; Harrington, E.; Ruan, R.; Chen, P.; Davachi, S.M.; Allardyce, B.; Rajkhowa, R.; Dilley, R.; Granero-Molto, F.; et al. Novel hybrid biocomposites for tendon grafts: The addition of silk to polydioxanone and poly(lactide-co-caprolactone) enhances material properties, in vitro and in vivo biocompatibility. Bioact. Mater. 2023, 25, 291–306. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Chen, J.; Chen, S. Silk fibroin coating through EDC/NHS crosslink is an effective method to promote graft remodeling of a polyethylene terephthalate artificial ligament. J. Biomater. Appl. 2019, 33, 1407–1414. [Google Scholar] [CrossRef]
- Jiang, J.; Ai, C.; Zhan, Z.; Zhang, P.; Wan, F.; Chen, J.; Hao, W.; Wang, Y.; Yao, J.; Shao, Z.; et al. Enhanced Fibroblast Cellular Ligamentization Process to Polyethylene Terepthalate Artificial Ligament by Silk Fibroin Coating. Artif. Organs 2016, 40, 385–393. [Google Scholar] [CrossRef]
- Jiang, J.; Wan, F.; Yang, J.; Hao, W.; Wang, Y.; Yao, J.; Shao, Z.; Zhang, P.; Chen, J.; Zhou, L.; et al. Enhancement of osseointegration of polyethylene terephthalate artificial ligament by coating of silk fibroin and depositing of hydroxyapatite. Int. J. Nanomed. 2014, 9, 4569–4580. [Google Scholar] [CrossRef]
- Fare, S.; Torricelli, P.; Giavaresi, G.; Bertoldi, S.; Alessandrino, A.; Villa, T.; Fini, M.; Tanzi, M.C.; Freddi, G. In vitro study on silk fibroin textile structure for anterior cruciate ligament regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3601–3608. [Google Scholar] [CrossRef]
- Hohlrieder, M.; Teuschl, A.H.; Cicha, K.; van Griensven, M.; Redl, H.; Stampfl, J. Bioreactor and scaffold design for the mechanical stimulation of anterior cruciate ligament grafts. Biomed. Mater. Eng. 2013, 23, 225–237. [Google Scholar] [CrossRef]
- Bi, F.; Chen, Y.; Liu, J.; Wang, Y.; Xu, D.; Tian, K. Anterior cruciate ligament reconstruction in a rabbit model using a silk-collagen scaffold modified by hydroxyapatite at both ends: A histological and biomechanical study. J. Orthop. Surg. Res. 2021, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.C.; Weitzel, P.P. Bioresorbable scaffolds for anterior cruciate ligament reconstruction: Do we need an off-the-shelf ACL substitute? Sports Med. Arthrosc. Rev. 2010, 18, 40–42. [Google Scholar] [CrossRef]
- Neuenfeldt, M.; Scheibel, T. Sequence Identification, Recombinant Production, and Analysis of the Self-Assembly of Egg Stalk Silk Proteins from Lacewing Chrysoperla carnea. Biomolecules 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Blamires, S.J.; Rawal, A.; Edwards, A.D.; Yarger, J.L.; Oberst, S.; Allardyce, B.J.; Rajkhowa, R. Methods for Silk Property Analyses across Structural Hierarchies and Scales. Molecules 2023, 28, 2120. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.J.; Suzuki, S.; Harkin, D.G.; Chirila, T.V. Incorporation of Exogenous RGD Peptide and Inter-Species Blending as Strategies for Enhancing Human Corneal Limbal Epithelial Cell Growth on Bombyx mori Silk Fibroin Membranes. J. Funct. Biomater. 2013, 4, 74–88. [Google Scholar] [CrossRef]
- Yang, Y.; Greco, G.; Maniglio, D.; Mazzolai, B.; Migliaresi, C.; Pugno, N.; Motta, A. Spider (Linothele megatheloides) and silkworm (Bombyx mori) silks: Comparative physical and biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110197. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Jiang, Q.; Yang, Y. Investigation of the properties and potential medical applications of natural silk fibers produced by Eupackardia calleta. J. Biomater. Sci. Polym. Ed. 2013, 24, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Lee, J.; Park, T.Y.; Yang, Y.J.; Cha, H.J. Diverse silk and silk-like proteins derived from terrestrial and marine organisms and their applications. Acta Biomater. 2021, 136, 56–71. [Google Scholar] [CrossRef]
- Xu, J.; Dong, Q.; Yu, Y.; Niu, B.; Ji, D.; Li, M.; Huang, Y.; Chen, X.; Tan, A. Mass spider silk production through targeted gene replacement in Bombyx mori. Proc. Natl. Acad. Sci. USA 2018, 115, 8757–8762. [Google Scholar] [CrossRef]
- Wen, H.; Lan, X.; Zhang, Y.; Zhao, T.; Wang, Y.; Kajiura, Z.; Nakagaki, M. Transgenic silkworms (Bombyx mori) produce recombinant spider dragline silk in cocoons. Mol. Biol. Rep. 2010, 37, 1815–1821. [Google Scholar] [CrossRef]
- Ramezaniaghdam, M.; Nahdi, N.D.; Reski, R. Recombinant Spider Silk: Promises and Bottlenecks. Front. Bioeng. Biotechnol. 2022, 10, 835637. [Google Scholar] [CrossRef]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, J.; Pei, L.; Luo, Y.; Li, D.; Huang, Z. Recent Advances in Environmentally Friendly and Green Degumming Processes of Silk for Textile and Non-Textile Applications. Polymers 2022, 14, 659. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, J.; Rising, A. Silk Spinning in Silkworms and Spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; You, R.; Wang, L.; Li, M. The relationship between secondary structure and biodegradation behavior of silk fibroin scaffolds. Adv. Mater. Sci. Eng. 2012, 2012, 185905. [Google Scholar] [CrossRef]
- Asakura, T.; Williamson, M.P. A review on the structure of Bombyx mori silk fibroin fiber studied using solid-state NMR: An antipolar lamella with an 8-residue repeat. Int. J. Biol. Macromol. 2023, 245, 125537. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Petrini, P.; Bozzini, S.; Dal Pra, I.; Armato, U. Silk fibroin/poly(carbonate)-urethane as a substrate for cell growth: In vitro interactions with human cells. Biomaterials 2003, 24, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.K.; Hasturk, O.; Falcucci, T.; Kaplan, D.L. Silk chemistry and biomedical material designs. Nat. Rev. Chem. 2023, 7, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Nishino, K.; Nayar, S.K. The appearance of human skin: A survey. Found. Trends® Comput. Graph. Vision. 2007, 3, 1–95. [Google Scholar] [CrossRef]
- Takagi, J. Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins. Biochem. Soc. Trans. 2004, 32, 403–406. [Google Scholar] [CrossRef]
- Guerette, P.A.; Ginzinger, D.G.; Weber, B.H.; Gosline, J.M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science 1996, 272, 112–115. [Google Scholar] [CrossRef]
- Schaefer, S.; Aavani, F.; Koepf, M.; Drinic, A.; Stuermer, E.K.; Fuest, S.; Grust, A.L.C.; Gosau, M.; Smeets, R. Silk proteins in reconstructive surgery: Do they possess an inherent antibacterial activity? A systematic review. Wound Repair. Regen. 2023, 31, 99–110. [Google Scholar] [CrossRef]
- Sparkes, J.; Holland, C. The rheological properties of native sericin. Acta Biomater. 2018, 69, 234–242. [Google Scholar] [CrossRef]
- Ode Boni, B.O.; Bakadia, B.M.; Osi, A.R.; Shi, Z.; Chen, H.; Gauthier, M.; Yang, G. Immune Response to Silk Sericin-Fibroin Composites: Potential Immunogenic Elements and Alternatives for Immunomodulation. Macromol. Biosci. 2022, 22, e2100292. [Google Scholar] [CrossRef]
- Teuschl, A.H.; van Griensven, M.; Redl, H. Sericin removal from raw Bombyx mori silk scaffolds of high hierarchical order. Tissue Eng. Part. C Methods 2014, 20, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Song, J.; Tian, C.; Jing, X.; Zhao, P.; Xia, Q. A novel method for silkworm cocoons self-degumming and its effect on silk fibers. J. Adv. Res. 2022, in press. [CrossRef] [PubMed]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef]
- Pereira, R.F.; Silva, M.M.; de Zea Bermudez, V. Bombyx mori silk fibers: An outstanding family of materials. Macromol. Mater. Eng. 2015, 300, 1171–1198. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Du, N.; Liu, X.Y.; Narayanan, J.; Li, L.; Lim, M.L.; Li, D. Design of superior spider silk: From nanostructure to mechanical properties. Biophys. J. 2006, 91, 4528–4535. [Google Scholar] [CrossRef]
- Xiao, S.; Stacklies, W.; Cetinkaya, M.; Markert, B.; Gräter, F. Mechanical response of silk crystalline units from force-distribution analysis. Biophys. J. 2009, 96, 3997–4005. [Google Scholar] [CrossRef]
- Rising, A.; Nimmervoll, H.; Grip, S.; Fernandez-Arias, A.; Storckenfeldt, E.; Knight, D.P.; Vollrath, F.; Engstrom, W. Spider silk proteins--mechanical property and gene sequence. Zoolog Sci. 2005, 22, 273–281. [Google Scholar] [CrossRef]
- Marhabaie, M.; Leeper, T.C.; Blackledge, T.A. Protein composition correlates with the mechanical properties of spider (Argiope trifasciata) dragline silk. Biomacromolecules 2014, 15, 20–29. [Google Scholar] [CrossRef]
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573. [Google Scholar] [CrossRef]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yoon, T.; Park, W.B.; Na, S. Predicting mechanical properties of silk from its amino acid sequences via machine learning. J. Mech. Behav. Biomed. Mater. 2023, 140, 105739. [Google Scholar] [CrossRef] [PubMed]
- Fazio, V.; Pugno, N.M.; Giustolisi, O.; Puglisi, G. Hierarchical physically based machine learning in material science: The case study of spider silk. arXiv 2023, arXiv:2307.12945. [Google Scholar]
- Zhang, Y.; Yang, H.; Shao, H.; Hu, X. Antheraea pernyi silk fiber: A potential resource for artificially biospinning spider dragline silk. J. Biomed. Biotechnol. 2010, 2010, 683962. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Xu, J.; Li, Y.; Zheng, T.; Zhang, T.; Han, K.; Chen, S.; Jiang, J.; Wu, S.; et al. Constructing high-strength nano-micro fibrous woven scaffolds with native-like anisotropic structure and immunoregulatory function for tendon repair and regeneration. Biofabrication 2023, 15, 025002. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xie, X.; Li, D.; Wang, L.; Jiang, J.; Mo, X.; Zhao, J. A novel knitted scaffold made of microfiber/nanofiber core-sheath yarns for tendon tissue engineering. Biomater. Sci. 2020, 8, 4413–4425. [Google Scholar] [CrossRef]
- Zhao, H.-P.; Feng, X.-Q.; Shi, H.-J. Variability in mechanical properties of Bombyx mori silk. Mater. Sci. Eng. C 2007, 27, 675–683. [Google Scholar] [CrossRef]
- Khademolqorani, S.; Tavanai, H.; Chronakis, I.S.; Boisen, A.; Ajalloueian, F. The determinant role of fabrication technique in final characteristics of scaffolds for tissue engineering applications: A focus on silk fibroin-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111867. [Google Scholar] [CrossRef]
- Rizzo, G.; Petrelli, V.; Sibillano, T.; De Caro, L.; Giangregorio, M.M.; Lo Presti, M.; Omenetto, F.G.; Giannini, C.; Mastrorilli, P.; Farinola, G.M. Raman, WAXS, and Solid-State NMR Characterizations of Regenerated Silk Fibroin Using Lanthanide Ions as Chaotropic Agents. ACS Omega 2023, 8, 24165–24175. [Google Scholar] [CrossRef]
- Vollrath, F.; Madsen, B.; Shao, Z. The effect of spinning conditions on the mechanics of a spider’s dragline silk. Proc. Biol. Sci. 2001, 268, 2339–2346. [Google Scholar] [CrossRef]
- Marieswaran, M.; Jain, I.; Garg, B.; Sharma, V.; Kalyanasundaram, D. A Review on Biomechanics of Anterior Cruciate Ligament and Materials for Reconstruction. Appl. Bionics Biomech. 2018, 2018, 4657824. [Google Scholar] [CrossRef] [PubMed]
- Ottani, V.; Raspanti, M.; Ruggeri, A. Collagen structure and functional implications. Micron 2001, 32, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kasperczyk, W.J.; Rosocha, S.; Bosch, U.; Oestern, H.J.; Tscherne, H. Age, activity and strength of knee ligaments. Unfallchirurg 1991, 94, 372–375. [Google Scholar] [PubMed]
- Babu, K. Spider silks and their applications. In Processing, Properties and Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 235–253. [Google Scholar]
- Handl, M.; Drzik, M.; Cerulli, G.; Povysil, C.; Chlpik, J.; Varga, F.; Amler, E.; Trc, T. Reconstruction of the anterior cruciate ligament: Dynamic strain evaluation of the graft. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Vehoff, T.; Glisovic, A.; Schollmeyer, H.; Zippelius, A.; Salditt, T. Mechanical properties of spider dragline silk: Humidity, hysteresis, and relaxation. Biophys. J. 2007, 93, 4425–4432. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F. Surprising strength of silkworm silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Mansouri, H.; Slauterbeck, J.; Hashemi, J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J. Biomech. 2006, 39, 2943–2950. [Google Scholar] [CrossRef]
- Woo, S.L.; Hollis, J.M.; Adams, D.J.; Lyon, R.M.; Takai, S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am. J. Sports Med. 1991, 19, 217–225. [Google Scholar] [CrossRef]
- Jones, R.S.; Nawana, N.S.; Pearcy, M.J.; Learmonth, D.J.; Bickerstaff, D.R.; Costi, J.J.; Paterson, R.S. Mechanical properties of the human anterior cruciate ligament. Clin. Biomech. 1995, 10, 339–344. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Chen, G.; He, J.; Han, Y.; Wang, X.; Kaplan, D.L. Silk-based biomaterials in biomedical textiles and fiber-based implants. Adv. Healthc. Mater. 2015, 4, 1134–1151. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Freeman, J.W.; Woods, M.D.; Laurencin, C.T. Tissue engineering of the anterior cruciate ligament using a braid-twist scaffold design. J. Biomech. 2007, 40, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sahoo, S.; He, P.; Ng, K.S.; Toh, S.L.; Goh, J.C. A hybrid silk/RADA-based fibrous scaffold with triple hierarchy for ligament regeneration. Tissue Eng. Part. A 2012, 18, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Zhu, T.; Huang, J.; Le, H.; Hu, Y.; Zheng, Z.; Tang, C.; Chen, Y.; Ran, J.; Chen, X.; et al. Knitted Silk-Collagen Scaffold Incorporated with Ligament Stem/Progenitor Cells Sheet for Anterior Cruciate Ligament Reconstruction and Osteoarthritis Prevention. ACS Biomater. Sci. Eng. 2019, 5, 5412–5421. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.K.; Kim, J.H.; Eo, S.R. Co-effect of silk and amniotic membrane for tendon repair. J. Biomater. Sci. Polym. Ed. 2016, 27, 1232–1247. [Google Scholar] [CrossRef]
- Hahn, J.; Schulze-Tanzil, G.; Schroepfer, M.; Meyer, M.; Goegele, C.; Hoyer, M.; Spickenheuer, A.; Heinrich, G.; Breier, A. Viscoelastic Behavior of Embroidered Scaffolds for ACL Tissue Engineering Made of PLA and P(LA-CL) After In Vitro Degradation. Int. J. Mol. Sci. 2019, 20, 4655. [Google Scholar] [CrossRef]
- Woltje, M.; Kunzelmann, L.; Belgucan, B.; Croft, A.S.; Voumard, B.; Bracher, S.; Zysset, P.; Gantenbein, B.; Cherif, C.; Aibibu, D. Textile Design of an Intervertebral Disc Replacement Device from Silk Yarn. Biomimetics 2023, 8, 152. [Google Scholar] [CrossRef]
- Lepore, E.; Marchioro, A.; Isaia, M.; Buehler, M.J.; Pugno, N.M. Evidence of the most stretchable egg sac silk stalk, of the European spider of the year Meta menardi. PLoS ONE 2012, 7, e30500. [Google Scholar] [CrossRef]
- Allmeling, C.; Radtke, C.; Vogt, P.M. Technical and biomedical uses of nature’s strongest fiber: Spider silk. In Spider Ecophysiology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 475–490. [Google Scholar]
- Radtke, C.; Allmeling, C.; Waldmann, K.-H.; Reimers, K.; Thies, K.; Schenk, H.C.; Hillmer, A.; Guggenheim, M.; Brandes, G.; Vogt, P.M. Spider silk constructs enhance axonal regeneration and remyelination in long nerve defects in sheep. PLoS ONE 2011, 6, e16990. [Google Scholar] [CrossRef]
- Hennecke, K.; Redeker, J.; Kuhbier, J.W.; Strauss, S.; Allmeling, C.; Kasper, C.; Reimers, K.; Vogt, P.M. Bundles of spider silk, braided into sutures, resist basic cyclic tests: Potential use for flexor tendon repair. PLoS ONE 2013, 8, e61100. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, e1800465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, M.; Hu, W.; Wang, X.; Hu, J. Spidroin-Based Biomaterials in Tissue Engineering: General Approaches and Potential Stem Cell Therapies. Stem Cells Int. 2021, 2021, 7141550. [Google Scholar] [CrossRef] [PubMed]
- Belbeoch, C.; Lejeune, J.; Vroman, P.; Salaun, F. Silkworm and spider silk electrospinning: A review. Environ. Chem. Lett. 2021, 19, 1737–1763. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, T.; Lang, G.; Scheibel, T. Biomimetic polymer fibers-function by design. Bioinspir Biomim. 2023, 18, 014003. [Google Scholar] [CrossRef]
- Wang, Q.; Han, G.; Yan, S.; Zhang, Q. 3D Printing of Silk Fibroin for Biomedical Applications. Materials 2019, 12, 504. [Google Scholar] [CrossRef]
- DeBari, M.K.; Keyser, M.N.; Bai, M.A.; Abbott, R.D. 3D printing with silk: Considerations and applications. Connect. Tissue Res. 2020, 61, 163–173. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Palazzi, V.; Salvati, R.; Chiesa, I.; De Maria, C.; Bonafoni, S.; Mezzanotte, P.; Codini, M.; Pacini, L.; Errante, F.; et al. Biomaterial Inks from Peptide-Functionalized Silk Fibers for 3D Printing of Futuristic Wound-Healing and Sensing Materials. Int. J. Mol. Sci. 2023, 24, 947. [Google Scholar] [CrossRef]
- Wei, L.; Wu, S.; Kuss, M.; Jiang, X.; Sun, R.; Reid, P.; Qin, X.; Duan, B. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioact. Mater. 2019, 4, 256–260. [Google Scholar] [CrossRef]
- Vyas, C.; Zhang, J.; Ovrebo, O.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D printing of silk microparticle reinforced polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111433. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Martin-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef]
- Goegele, C.; Hahn, J.; Elschner, C.; Breier, A.; Schroepfer, M.; Prade, I.; Meyer, M.; Schulze-Tanzil, G. Enhanced Growth of Lapine Anterior Cruciate Ligament-Derived Fibroblasts on Scaffolds Embroidered from Poly(l-lactide-co-epsilon-caprolactone) and Polylactic Acid Threads Functionalized by Fluorination and Hexamethylene Diisocyanate Cross-Linked Collagen Foams. Int. J. Mol. Sci. 2020, 21, 1132. [Google Scholar]
- Farokhi, M.; Aleemardani, M.; Solouk, A.; Mirzadeh, H.; Teuschl, A.H.; Redl, H. Crosslinking strategies for silk fibroin hydrogels: Promising biomedical materials. Biomed. Mater. 2021, 16, 022004. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Costa, J.B.; Carneiro, S.M.; Pina, S.; Veloso, A.C.A.; Reis, R.L.; Oliveira, J.M. Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction. Pharmaceutics 2022, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Lau, K.; Kim, H.N.; Och, Z.; Lim, K.S.; Whitelock, J.; Lord, M.; Rnjak-Kovacina, J. Surface Biofunctionalization of Silk Biomaterials Using Dityrosine Cross-Linking. ACS Appl. Mater. Interfaces 2022, 14, 31551–31566. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Fan, Q.; Yan, K.; Zhang, Q.; Wang, D.; You, R. Silk fibroin scaffolds with stable silk I crystal and tunable properties. Int. J. Biol. Macromol. 2023, 248, 125910. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Ho, M.-P.; Mosallam, A. Study on the mechanical properties of different silkworm silk fibers. J. Compos. Mater. 2009, 43, 2521–2531. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef]
- Sharma, R.; Malviya, R. Utilization of Bioactive Silk Protein in the Development of Optical Devices: Recent Advancements and Applications. Curr. Protein Pept. Sci. 2023, 24, 404–422. [Google Scholar] [CrossRef]
- Lyu, Y.; Liu, Y.; He, H.; Wang, H. Application of Silk-Fibroin-Based Hydrogels in Tissue Engineering. Gels 2023, 9, 431. [Google Scholar] [CrossRef]
- Hu, Y.; Ran, J.; Zheng, Z.; Jin, Z.; Chen, X.; Yin, Z.; Tang, C.; Chen, Y.; Huang, J.; Le, H.; et al. Exogenous stromal derived factor-1 releasing silk scaffold combined with intra-articular injection of progenitor cells promotes bone-ligament-bone regeneration. Acta Biomater. 2018, 71, 168–183. [Google Scholar] [CrossRef]
- Shen, W.; Chen, X.; Hu, Y.; Yin, Z.; Zhu, T.; Hu, J.; Chen, J.; Zheng, Z.; Zhang, W.; Ran, J.; et al. Long-term effects of knitted silk-collagen sponge scaffold on anterior cruciate ligament reconstruction and osteoarthritis prevention. Biomaterials 2014, 35, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kojima, K.; Tamada, Y. Higher Gene Expression Related to Wound Healing by Fibroblasts on Silk Fibroin Biomaterial than on Collagen. Molecules 2020, 25, 1939. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Zhang, G.; Zhang, Y. Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine. Polymers 2023, 15, 2941. [Google Scholar] [CrossRef] [PubMed]
- Widhe, M.; Shalaly, N.D.; Hedhammar, M. A fibronectin mimetic motif improves integrin mediated cell biding to recombinant spider silk matrices. Biomaterials 2016, 74, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Trossmann, V.T.; Scheibel, T. Design of Recombinant Spider Silk Proteins for Cell Type Specific Binding. Adv. Healthc. Mater. 2023, 12, e2202660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, X.; Liu, Y.; Liu, J.; He, W.; Dong, Y. Synthesis of silver @hydroxyapatite nanoparticles based biocomposite and their assessment for viability of Osseointegration for rabbit knee joint anterior cruciate ligament rehabilitation. J. Photochem. Photobiol. B 2020, 202, 111677. [Google Scholar] [CrossRef]
- Kajave, N.S.; Schmitt, T.; Patrawalla, N.Y.; Kishore, V. Design-Build-Validate Strategy to 3D Print Bioglass Gradients for Anterior Cruciate Ligament Enthesis Reconstruction. Tissue Eng. Part. C Methods 2022, 28, 158–167. [Google Scholar] [CrossRef]
- Midha, S.; Kumar, S.; Sharma, A.; Kaur, K.; Shi, X.; Naruphontjirakul, P.; Jones, J.R.; Ghosh, S. Silk fibroin-bioactive glass based advanced biomaterials: Towards patient-specific bone grafts. Biomed. Mater. 2018, 13, 055012. [Google Scholar] [CrossRef]
- Yang, M.; Yu, S.; Zhao, P.; Shi, G.; Guo, Y.; Xie, L.; Lyu, G.; Yu, J. Fabrication of biologically inspired electrospun collagen/silk fibroin/bioactive glass composited nanofibrous to accelerate the treatment efficiency of wound repair. Int. Wound J. 2023, 20, 687–698. [Google Scholar] [CrossRef]
- Dong, Q.; Cai, J.; Wang, H.; Chen, S.; Liu, Y.; Yao, J.; Shao, Z.; Chen, X. Artificial ligament made from silk protein/Laponite hybrid fibers. Acta Biomater. 2020, 106, 102–113. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Qu, L.; Zhu, R.; Fan, X.; Xue, Y.; Xie, Z.; Fan, H. Immobilized lentivirus vector on chondroitin sulfate-hyaluronate acid-silk fibroin hybrid scaffold for tissue-engineered ligament-bone junction. Biomed. Res. Int. 2014, 2014, 816979. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Sun, L.; Chen, X.; Qu, L.; Li, H.; Liu, X.; Zhang, Y.; Cheng, P.; Fan, H. Implementation of a stratified approach and gene immobilization to enhance the osseointegration of a silk-based ligament graft. J. Mater. Chem. B 2017, 5, 7035–7050. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Sheng, D.; Chen, J.; Cai, J.; Wang, S.; Jiang, J.; Chen, S. Surface modification of vascular endothelial growth factor-loaded silk fibroin to improve biological performance of ultra-high-molecular-weight polyethylene via promoting angiogenesis. Int. J. Nanomedicine 2017, 12, 7737–7750. [Google Scholar] [CrossRef] [PubMed]
- Dal Pra, I.; Petrini, P.; Chiarini, A.; Bozzini, S.; Fare, S.; Armato, U. Silk fibroin-coated three-dimensional polyurethane scaffolds for tissue engineering: Interactions with normal human fibroblasts. Tissue Eng. 2003, 9, 1113–1121. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, R.; Chen, J.; Wang, H.; Zhu, Y.; Cao, Z.; Zhao, X.; Wang, Z.; Liu, C.; Chen, Z.; et al. Silk Fibroin and Sericin Differentially Potentiate the Paracrine and Regenerative Functions of Stem Cells Through Multiomics Analysis. Adv. Mater. 2023, 35, e2210517. [Google Scholar] [CrossRef]
- Teuschl, A.; Heimel, P.; Nuernberger, S.; van Griensven, M.; Redl, H.; Nau, T. A Novel Silk Fiber-Based Scaffold for Regeneration of the Anterior Cruciate Ligament: Histological Results From a Study in Sheep. Am. J. Sports Med. 2016, 44, 1547–1557. [Google Scholar] [CrossRef]
- Tsubouchi, K.; Nakao, H.; Igarashi, Y.; Takasu, Y.; Yamada, H. Bombyx mori fibroin enhanced the proliferation of cultured human skin fibroblasts. J. Insect Biotechnol. Sericol. 2003, 72, 65–69. [Google Scholar]
- Chu, J.; Lu, M.; Pfeifer, C.G.; Alt, V.; Docheva, D. Rebuilding Tendons: A Concise Review on the Potential of Dermal Fibroblasts. Cells 2020, 9, 2047. [Google Scholar] [CrossRef]
- Unger, R.E.; Wolf, M.; Peters, K.; Motta, A.; Migliaresi, C.; James Kirkpatrick, C. Growth of human cells on a non-woven silk fibroin net: A potential for use in tissue engineering. Biomaterials 2004, 25, 1069–1075. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Toh, S.L.; Goh, J.C. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials 2008, 29, 1443–1453. [Google Scholar] [CrossRef]
- Seo, Y.K.; Yoon, H.H.; Song, K.Y.; Kwon, S.Y.; Lee, H.S.; Park, Y.S.; Park, J.K. Increase in cell migration and angiogenesis in a composite silk scaffold for tissue-engineered ligaments. J. Orthop. Res. 2009, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Musson, D.S.; Naot, D.; Chhana, A.; Matthews, B.G.; McIntosh, J.D.; Lin, S.T.; Choi, A.J.; Callon, K.E.; Dunbar, P.R.; Lesage, S.; et al. In vitro evaluation of a novel non-mulberry silk scaffold for use in tendon regeneration. Tissue Eng. Part. A 2015, 21, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, J.; Qi, Y.; Cai, J.; Zhao, J.; Duan, B.; Chen, S. Tendon-bioinspired wavy nanofibrous scaffolds provide tunable anisotropy and promote tenogenesis for tendon tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112181. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, B.; Gumusderelioglu, M. Aligned silk fibroin/poly-3-hydroxybutyrate nanofibrous scaffolds seeded with adipose-derived stem cells for tendon tissue engineering. Int. J. Biol. Macromol. 2021, 193, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.H.; Chang, S.J.; Chiu, Y.K.; Chiu, Y.H.; Fang, T.J.; Chen, H.C. Low Molecular Weight Sericin Enhances the In Vitro of Immunological Modulation and Cell Migration. Front. Bioeng. Biotechnol. 2022, 10, 925197. [Google Scholar] [CrossRef]
- Chen, J.; Horan, R.L.; Bramono, D.; Moreau, J.E.; Wang, Y.; Geuss, L.R.; Collette, A.L.; Volloch, V.; Altman, G.H. Monitoring mesenchymal stromal cell developmental stage to apply on-time mechanical stimulation for ligament tissue engineering. Tissue Eng. 2006, 12, 3085–3095. [Google Scholar] [CrossRef]
- Park, H.; Nazhat, S.N.; Rosenzweig, D.H. Mechanical activation drives tenogenic differentiation of human mesenchymal stem cells in aligned dense collagen hydrogels. Biomaterials 2022, 286, 121606. [Google Scholar] [CrossRef]
- Singh, N.; Rahatekar, S.S.; Koziol, K.K.; Ng, T.S.; Patil, A.J.; Mann, S.; Hollander, A.P.; Kafienah, W. Directing chondrogenesis of stem cells with specific blends of cellulose and silk. Biomacromolecules 2013, 14, 1287–1298. [Google Scholar] [CrossRef]
- Kwansa, A.L.; Empson, Y.M.; Ekwueme, E.C.; Walters, V.I.; Freeman, J.W.; Laurencin, C.T. Novel matrix based anterior cruciate ligament (ACL) regeneration. Soft Matter 2010, 6, 5016–5025. [Google Scholar] [CrossRef]
- Goegele, C.; Hahn, J.; Schulze-Tanzil, G. Anatomical Tissue Engineering of the Anterior Cruciate Ligament Entheses. Int. J. Mol. Sci. 2023, 24, 9745. [Google Scholar] [CrossRef]
- He, P.; Ng, K.S.; Toh, S.L.; Goh, J.C. In vitro ligament-bone interface regeneration using a trilineage coculture system on a hybrid silk scaffold. Biomacromolecules 2012, 13, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, J.; Sun, L.; Liu, X.; Cheng, P.; Fan, H. Functional regeneration of ligament-bone interface using a triphasic silk-based graft. Biomaterials 2016, 106, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cui, J.; Wu, S.; Geng, Z.; Su, J. Silk fibroin-based biomaterials for cartilage/osteochondral repair. Theranostics 2022, 12, 5103–5124. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.Y.; Kweon, H.; Kim, D.W.; Baek, K.; Chae, W.S.; Kang, Y.J.; Oh, J.H.; Kim, S.G.; Garagiola, U. Silk sericin application increases bone morphogenic protein-2/4 expression via a toll-like receptor-mediated pathway. Int. J. Biol. Macromol. 2021, 190, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Koop, F.; Strauss, S.; Peck, C.T.; Aper, T.; Wilhelmi, M.; Hartmann, C.; Hegermann, J.; Schipke, J.; Vogt, P.M.; Bucan, V. Preliminary application of native Nephila edulis spider silk and fibrin implant causes granulomatous foreign body reaction in vivo in rat’s spinal cord. PLoS ONE 2022, 17, e0264486. [Google Scholar] [CrossRef]
- Dong, L.; Li, L.; Song, Y.; Fang, Y.; Liu, J.; Chen, P.; Wang, S.; Wang, C.; Xia, T.; Liu, W.; et al. MSC-derived immunomodulatory extracellular matrix functionalized electrospun fibers for mitigating foreign-body reaction and tendon adhesion. Acta Biomater. 2021, 133, 280–296. [Google Scholar] [CrossRef]
- Qian, Y.; Li, L.; Song, Y.; Dong, L.; Chen, P.; Li, X.; Cai, K.; Germershaus, O.; Yang, L.; Fan, Y. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials 2018, 164, 22–37. [Google Scholar] [CrossRef]
- Ma, X.Y.; Cui, D.; Wang, Z.; Liu, B.; Yu, H.L.; Yuan, H.; Xiang, L.B.; Zhou, D.P. Silk Fibroin/Hydroxyapatite Coating Improved Osseointegration of Porous Titanium Implants under Diabetic Conditions via Activation of the PI3K/Akt Signaling Pathway. ACS Biomater. Sci. Eng. 2022, 8, 2908–2919. [Google Scholar] [CrossRef]
- Jung, S.R.; Song, N.J.; Hwang, H.S.; An, J.J.; Cho, Y.J.; Kweon, H.Y.; Kang, S.W.; Lee, K.G.; Yoon, K.; Kim, B.J.; et al. Silk peptides inhibit adipocyte differentiation through modulation of the Notch pathway in C3H10T1/2 cells. Nutr. Res. 2011, 31, 723–730. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, D.; Li, L.; Qian, Z.; He, W.; Ding, R.; Liu, H.; Fan, Y. Effect of Electrospun Silk Fibroin-Silk Sericin Films on Macrophage Polarization and Vascularization. ACS Biomater. Sci. Eng. 2020, 6, 3502–3512. [Google Scholar] [CrossRef]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Koprivec, S.; Novak, M.; Bernik, S.; Voga, M.; Mohorič, L.; Majdič, G. Treatment of cranial cruciate ligament injuries in dogs using a combination of tibial tuberosity advancement procedure and autologous mesenchymal stem cells/multipotent mesenchymal stromal cells–A pilot study. Acta Vet. Hung. 2021, 68, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, W.; Jin, W.; Sun, Y.; Cai, J.; Gu, K.; Mi, R.; Chen, N.; Chen, S.; Shao, Z. An interference screw made using a silk fibroin-based bulk material with high content of hydroxyapatite for anterior cruciate ligament reconstruction in a rabbit model. J. Mater. Chem. B 2021, 9, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Goegele, C.; Vogt, J.; Hahn, J.; Breier, A.; Bernhardt, R.; Meyer, M.; Schroepfer, M.; Schaefer-Eckart, K.; Schulze-Tanzil, G. Co-Culture of Mesenchymal Stem Cells and Ligamentocytes on Triphasic Embroidered Poly(L-lactide-co-epsilon-caprolactone) and Polylactic Acid Scaffolds for Anterior Cruciate Ligament Enthesis Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 6714. [Google Scholar] [CrossRef] [PubMed]


| Issue | Advantage | Drawback | Reference |
|---|---|---|---|
| Source | Natural | Natural: scarcity, variability, batch dependent | [12] |
| Costs | Gene technology: recombinant protein expression systems | Natural source: high, e.g., spider silk | [11] |
| Processing | Versatile: many techniques can be applied, Section 3.4, tunable properties, can be influenced by cross-linking | Processing changes properties | [104,107] |
| Biomechanics | Stable, suitable for ACL reconstruction | Influenced by processing, etc. | [20,62,108] |
| Degradation | Long, tunable by processing | Influenced by processing (e.g., sericin removal) and secondary structure | [15,41,109] |
| Shape stability | High durability, reversible swelling and shrinking | [110] | |
| Preparation, purification | Easy | Sericin removal necessary (immunogenicity) without damage | [5,50] |
| Conformation | Changeable, fibers | pH-dependent | [40] |
| Bioadsorbility | Adsorption properties: adsorption of proteins (particularly hydrophobic [beta sheet] fibroin part | Wetting required Adsorption of proteins also leads to inflammatory cell recruitment | [15,50,111] |
| Functionalization of silk | Multiple strategies possible, Section 3.5 | Biomechanics changed | [19,106,112,113] |
| Cell interaction | Bioactivity | [50,110] | |
| Biocompatibility | If cleaned, high | Higher than synthetic polymers | [17] |
| Immunogenicity | Fibroin: low | Sericin: high | [111] |
| Properties | Sericin: hydrophilic | Fibroin: hydrophobic, antimicrobial properties questionable | [15,48] |
| Sterilization | Heat dry sterilization (180 °C, 30 min): no structural changes in fibroin, no effect on fibroblast adhesion | Autoclaving: structural changes in fibroin | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, J.; Gögele, C.; Schulze-Tanzil, G. Could an Anterior Cruciate Ligament Be Tissue-Engineered from Silk? Cells 2023, 12, 2350. https://doi.org/10.3390/cells12192350
Hahn J, Gögele C, Schulze-Tanzil G. Could an Anterior Cruciate Ligament Be Tissue-Engineered from Silk? Cells. 2023; 12(19):2350. https://doi.org/10.3390/cells12192350
Chicago/Turabian StyleHahn, Judith, Clemens Gögele, and Gundula Schulze-Tanzil. 2023. "Could an Anterior Cruciate Ligament Be Tissue-Engineered from Silk?" Cells 12, no. 19: 2350. https://doi.org/10.3390/cells12192350
APA StyleHahn, J., Gögele, C., & Schulze-Tanzil, G. (2023). Could an Anterior Cruciate Ligament Be Tissue-Engineered from Silk? Cells, 12(19), 2350. https://doi.org/10.3390/cells12192350








