Non-Psychoactive Phytocannabinoids Inhibit Inflammation-Related Changes of Human Coronary Artery Smooth Muscle and Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cellular Viability Assays
2.4. BrdU Proliferation Assay
2.5. Scratch Wound Assay
2.6. Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.7. Total Cellular Protein Isolation
2.8. Nuclear Protein Isolation
2.9. Western Blot Analysis
2.10. Monocyte Adhesion Assay
2.11. Statistics
3. Results
3.1. Nontoxic Concentrations of CBD and THCV Inhibit PDGF-Induced Migration of HCASMC with Additional Inhibition of PDGF-Induced Proliferation by CBD
3.2. CBD and THCV Do Not Impair the Viability of HCAEC at Low Concentrations
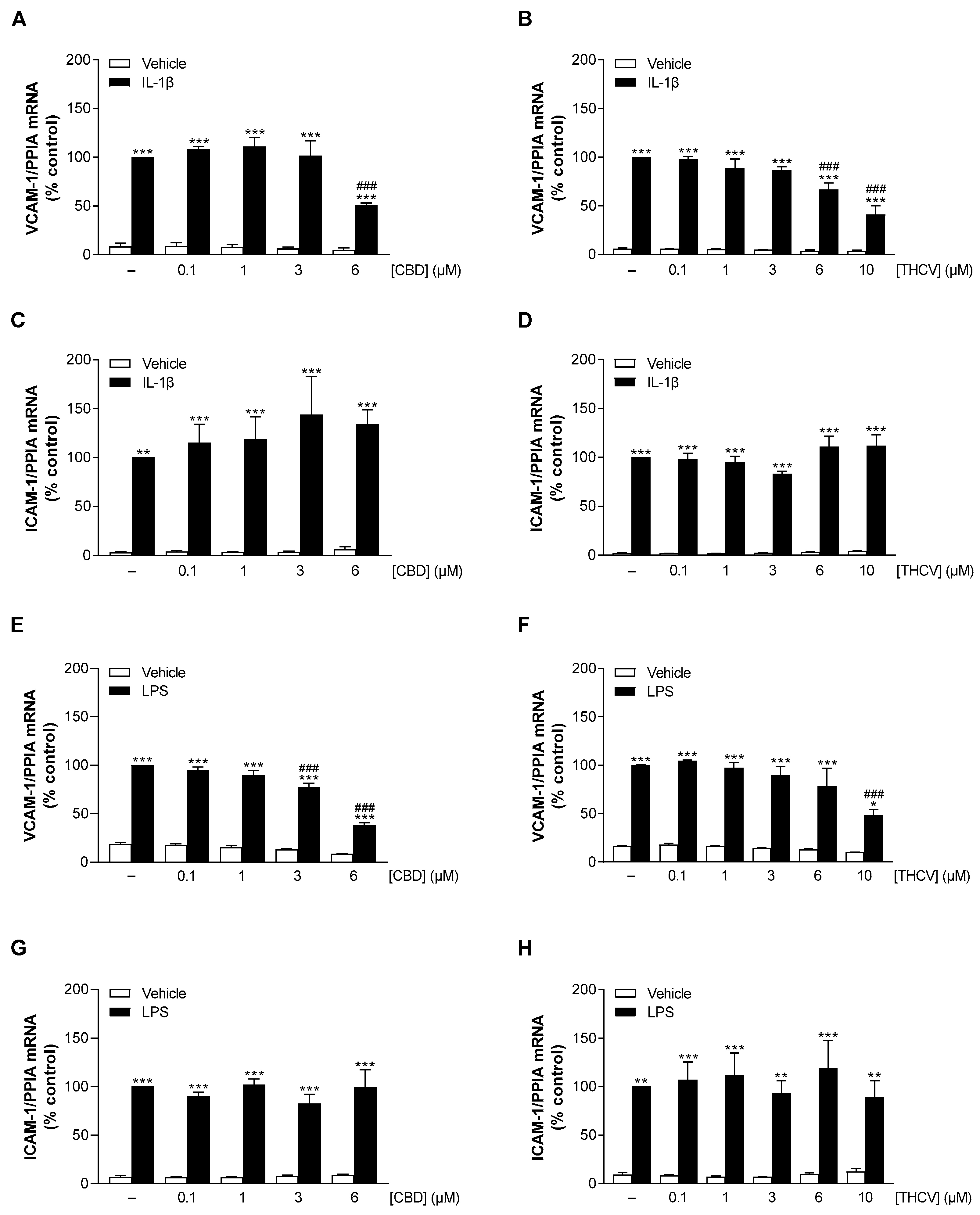
3.3. CBD and THCV Elicit Concentration-Dependent Inhibition of IL-1β- and LPS-Induced VCAM-1 but Not ICAM-1 mRNA Levels in HCAEC
3.4. CBD and THCV Cause a Concentration-Dependent Decrease in VCAM-1 but Not ICAM-1 Protein Levels in HCAEC under IL-1β- and LPS-Induced Conditions
3.5. CBD and THCV Reduce VCAM-1 Protein Levels in HCAEC Independently of Cannabinoid Receptors CB1 and CB2, and TRPV1
3.6. p38 MAPK, NF-κB, HDAC, and ROS Mediate IL-1β- and LPS-Induced VCAM-1 Expression in HCAEC, Whereas p42/44 MAPK and JNK Are Not Involved
3.7. CBD and THCV Do Not Cause Inhibition of IL-1β- and LPS-Induced Activation of p38 MAPK
3.8. CBD and THCV Inhibit LPS-, but Not IL-1β-Induced Activation of NF-κB in HCAEC
3.9. CBD Induces Protein Expression of HDAC5 under Stimulated and Unstimulated Conditions in HCAEC
3.10. CBD and THCV Do Not Cause Significant Inhibition of IL-1β- and LPS-Induced Monocyte Adhesion to HCAEC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 9 February 2023).
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primer 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Kutyna, M.; Cornelissen, A.; Kuntz, S.; Guo, L.; Mori, H.; Harari, E.; Paek, K.H.; et al. Drug-Eluting Coronary Stents: Insights from Preclinical and Pathology Studies. Nat. Rev. Cardiol. 2020, 17, 37–51. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Marx, S.O.; Totary-Jain, H.; Marks, A.R. Vascular smooth muscle cell proliferation in restenosis. Circ. Cardiovasc. Interv. 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Otsuka, F.; Finn, A.V.; Yazdani, S.K.; Nakano, M.; Kolodgie, F.D.; Virmani, R. The Importance of the Endothelium in Atherothrombosis and Coronary Stenting. Nat. Rev. Cardiol. 2012, 9, 439–453. [Google Scholar] [CrossRef]
- Cornelissen, A.; Vogt, F.J. The Effects of Stenting on Coronary Endothelium from a Molecular Biological View: Time for Improvement? J. Cell. Mol. Med. 2019, 23, 39–46. [Google Scholar] [CrossRef]
- Brunner, H.; Cockcroft, J.R.; Deanfield, J.; Donald, A.; Ferrannini, E.; Halcox, J.; Kiowski, W.; Lüscher, T.F.; Mancia, G.; Natali, A.; et al. Endothelial Function and Dysfunction. Part II: Association with Cardiovascular Risk Factors and Diseases. A Statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 233–246. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; García, L.; Gabrielli, L.; Corbalán, R.; et al. VCAM-1 as a Predictor Biomarker in Cardiovascular Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A Master Regulator of Cellular Responses in Inflammation, Injury Resolution, and Tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, C.; Ridley, A.J. Endothelial Cell-Cell Adhesion and Signaling. Exp. Cell Res. 2017, 358, 31–38. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.-C.; Connelly, P.W.; Milstone, D.S. A Major Role for VCAM-1, but Not ICAM-1, in Early Atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Bourdillon, M.-C.; Poston, R.N.; Covacho, C.; Chignier, E.; Bricca, G.; McGregor, J.L. ICAM-1 Deficiency Reduces Atherosclerotic Lesions in Double-Knockout Mice (ApoE−/−/ICAM-1−/−) Fed a Fat or a Chow Diet. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2630–2635. [Google Scholar] [CrossRef]
- Schwartz, M.; Böckmann, S.; Hinz, B. Up-Regulation of Heme Oxygenase-1 Expression and Inhibition of Disease-Associated Features by Cannabidiol in Vascular Smooth Muscle Cells. Oncotarget 2018, 9, 34595–34616. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol Attenuates High Glucose-Induced Endothelial Cell Inflammatory Response and Barrier Disruption. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol Causes Endothelium-Dependent Vasorelaxation of Human Mesenteric Arteries via CB1 Activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol Improves Lung Function and Inflammation in Mice Submitted to LPS-Induced Acute Lung Injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–41. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, and Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Wargent, E.T.; Zaibi, M.S.; Silvestri, C.; Hislop, D.C.; Stocker, C.J.; Stott, C.G.; Guy, G.W.; Duncan, M.; Di Marzo, V.; Cawthorne, M.A. The Cannabinoid Δ9-Tetrahydrocannabivarin (THCV) Ameliorates Insulin Sensitivity in Two Mouse Models of Obesity. Nutr. Diabetes 2013, 3, e68. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A Commentary on Potential Therapeutic Benefit for the Management of Obesity and Diabetes. J. Cannabis Res. 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol Protects against Hepatic Ischemia/Reperfusion Injury by Attenuating Inflammatory Signaling and Response, Oxidative/Nitrative Stress, and Cell Death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Bátkai, S.; Mukhopadhyay, P.; Horváth, B.; Rajesh, M.; Gao, R.Y.; Mahadevan, A.; Amere, M.; Battista, N.; Lichtman, A.H.; Gauson, L.A.; et al. Δ8-Tetrahydrocannabivarin Prevents Hepatic Ischaemia/Reperfusion Injury by Decreasing Oxidative Stress and Inflammatory Responses through Cannabinoid CB2 Receptors: Δ8-Tetrahydrocannabivarin for Reperfusion Injury. Br. J. Pharmacol. 2012, 165, 2450–2461. [Google Scholar] [CrossRef]
- Thomas, A.; Stevenson, L.A.; Wease, K.N.; Price, M.R.; Baillie, G.; Ross, R.A.; Pertwee, R.G. Evidence That the Plant Cannabinoid Δ9-Tetrahydrocannabivarin Is a Cannabinoid CB1 and CB2 Receptor Antagonist. Br. J. Pharmacol. 2005, 146, 917–926. [Google Scholar] [CrossRef]
- Pertwee, R.G. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Δ9-Tetrahydrocannabinol, Cannabidiol and Δ9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Bolognini, D.; Costa, B.; Maione, S.; Comelli, F.; Marini, P.; Di Marzo, V.; Parolaro, D.; Ross, R.A.; Gauson, L.A.; Cascio, M.G.; et al. The Plant Cannabinoid Δ9-Tetrahydrocannabivarin Can Decrease Signs of Inflammation and Inflammatory Pain in Mice: Δ9-Tetrahydrocannabivarin and Inflammation. Br. J. Pharmacol. 2010, 160, 677–687. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes: Novel Pharmacology of Minor Plant Cannabinoids. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Peng, H.; Zhang, J.; Bo, L.; Wen, L.; Liu, W.; Bai, W.; Zhang, H. Histone Deacetylase Inhibitor Trichostatin A Reduces Endothelial Cell Proliferation by Suppressing STAT5A-Related Gene Transcription. Front. Oncol. 2021, 11, 746266. [Google Scholar] [CrossRef]
- Ono, K.; Han, J. The P38 Signal Transduction Pathway Activation and Function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hoefen, R.J.; Berk, B.C. The Role of MAP Kinases in Endothelial Activation. Vascul. Pharmacol. 2002, 38, 271–273. [Google Scholar] [CrossRef] [PubMed]
- May, M.J.; Ghosh, S. Signal Transduction through NF-κB. Immunol. Today 1998, 19, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Kobayashi, M.; Yano, K.; Miura, M.; Izumi, A.; Mataki, C.; Doi, T.; Hamakubo, T.; Reid, P.C.; Hume, D.A.; et al. Histone Deacetylase Inhibitor Reduces Monocyte Adhesion to Endothelium Through the Suppression of Vascular Cell Adhesion Molecule-1 Expression. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.F.; Chang, C.C.; Lee, I.T.; Lee, C.W.; Lin, W.N.; Lin, C.C.; Yang, C.M. Activation of ROS/NF-κB and Ca2+/CaM kinase II are necessary for VCAM-1 induction in IL-1β-treated human tracheal smooth muscle cells. Toxicol. Appl. Pharmacol. 2009, 237, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Li, R.; Liu, Y.; Liu, J.; Pan, T.; Zhang, R.; Liu, B.; Chen, E.; Tang, Y.; Qu, H. Metformin Ameliorates Endotoxemia-Induced Endothelial pro-Inflammatory Responses via AMPK-Dependent Mediation of HDAC5 and KLF2. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1701–1712. [Google Scholar] [CrossRef]
- Hu, C.; Peng, K.; Wu, Q.; Wang, Y.; Fan, X.; Zhang, D.M.; Passerini, A.G.; Sun, C. HDAC1 and 2 regulate endothelial VCAM-1 expression and atherogenesis by suppressing methylation of the GATA6 promoter. Theranostics 2021, 11, 5605–5619. [Google Scholar] [CrossRef]
- Mestas, J.; Ley, K. Monocyte-Endothelial Cell Interactions in the Development of Atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Kong, D.; Li, S.; Li, C.; Wang, H.; Sun, Y. Atorvastatin Calcium Inhibits Phenotypic Modulation of PDGF-BB-Induced VSMCs via Down-Regulation the Akt Signaling Pathway. PLoS ONE 2015, 10, e0122577. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, J.; Liu, H.; Ma, W.; Yu, L.; Tan, X.; Wang, S.; Ren, F.; Li, X.; Li, X. Cannabidiol Attenuates Pulmonary Arterial Hypertension by Improving Vascular Smooth Muscle Cells Mitochondrial Function. Theranostics 2021, 11, 5267–5278. [Google Scholar] [CrossRef]
- Böckmann, S.; Hinz, B. Cannabidiol Promotes Endothelial Cell Survival by Heme Oxygenase-1-Mediated Autophagy. Cells 2020, 9, 1703. [Google Scholar] [CrossRef]
- Muller, W.A. Leukocyte-Endothelial Cell Interactions in the Inflammatory Response. Lab. Investig. 2002, 82, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Hwa, J.S.; Mun, L.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Kwak, J.H.; Lee, D.-U.; Chang, K.C. Genipin Selectively Inhibits TNF-α-Activated VCAM-1 But Not ICAM-1 Expression by Upregulation of PPAR-γ in Human Endothelial Cells. Korean J. Physiol. Pharmacol. 2011, 15, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Kim, Y.M.; Jin, H.; Son, K.H.; Lee, J.H.; Chang, K.C.; Kim, H.J. Tanshinone IIA Inhibits TNF-α-Mediated Induction of VCAM-1 but Not ICAM-1 through the Regulation of GATA-6 and IRF-1. Int. Immunopharmacol. 2012, 14, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two Non-Psychoactive Cannabinoids Reduce Intracellular Lipid Levels and Inhibit Hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rossi, E.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Choublier, N.; Smirnova, M.; Glacial, F.; Perrière, N.; Bourdoulous, S.; et al. Cannabidiol Increases Proliferation, Migration, Tubulogenesis, and Integrity of Human Brain Endothelial Cells through TRPV2 Activation. Mol. Pharm. 2019, 16, 1312–1326. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol Provides Long-Lasting Protection against the Deleterious Effects of Inflammation in a Viral Model of Multiple Sclerosis: A Role for A2A Receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489–501. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Qi, W.; Zhang, Y.; Li, J.; Li, Z.; Lin, Y.; Bai, X.; Liu, X.; Chen, X.; et al. Nicotine Promotes Atherosclerosis via ROS-NLRP3-Mediated Endothelial Cell Pyroptosis. Cell Death Dis. 2018, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Habas, K.; Shang, L. Alterations in Intercellular Adhesion Molecule 1 (ICAM-1) and Vascular Cell Adhesion Molecule 1 (VCAM-1) in Human Endothelial Cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Bae, Y.H.; Bae, S.K.; Choi, K.S.; Yoon, K.H.; Koo, T.H.; Jang, H.O.; Yun, I.; Kim, K.W.; Kwon, Y.G.; et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim. Biophys. Acta 2008, 1783, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Ye, J.; Bai, S.; Huang, F.; Guo, Y.-L. NF-ΚB, but Not P38 MAP Kinase, Is Required for TNF-α-Induced Expression of Cell Adhesion Molecules in Endothelial Cells. J. Cell. Biochem. 2008, 105, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.W.; Schoenleber, R.; Jesmok, G.; Best, J.; Moore, S.A.; Collins, T.; Gerritsen, M.E. Novel Inhibitors of Cytokine-Induced IκBα Phosphorylation and Endothelial Cell Adhesion Molecule Expression Show Anti-Inflammatory Effects in Vivo. J. Biol. Chem. 1997, 272, 21096–21103. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, M.H.; Kim, E.; Cho, J.Y. BAY 11-7082 Is a Broad-Spectrum Inhibitor with Anti-Inflammatory Activity against Multiple Targets. Mediat. Inflamm. 2012, 2012, 416036. [Google Scholar] [CrossRef]
- Muthumalage, T.; Rahman, I. Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [Google Scholar] [CrossRef]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The Family of the Interleukin-1 Receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Marui, N.; Offermann, M.K.; Swerlick, R.; Kunsch, C.; Rosen, C.A.; Ahmad, M.; Alexander, R.W.; Medford, R.M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J. Clin. Investig. 1993, 92, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB Activation by Small Molecules as a Therapeutic Strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Kamata, H.; Kamata, K.; Yagisawa, H.; Hirata, H. N-Acetylcysteine Suppresses TNF-Induced NF-κB Activation through Inhibition of IκB Kinases. FEBS Lett. 2000, 472, 196–202. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Li, W.; Zhan, D.; Wang, M. N-acetyl cysteine inhibits lipopolysaccharide-mediated induction of interleukin-6 synthesis in MC3T3-E1 cells through the NF-kB signaling pathway. Arch. Oral Biol. 2018, 93, 149–154. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhang, Z.X.; Xu, Y.J.; Ni, W.; Chen, S.X.; Yang, Z.; Ma, D. N-Acetyl-L-cysteine and pyrrolidine dithiocarbamate inhibited nuclear factor-κB activation in alveolar macrophages by different mechanisms. Acta Pharmacol. Sin. 2006, 27, 339–346. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Vanhaecke, T.; Papeleu, P.; Elaut, G.; Rogiers, V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: Toxicological point of view. Curr. Med. Chem. 2004, 11, 1629–1643. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Rafehi, H.; Balcerczyk, A.; Lunke, S.; Kaspi, A.; Ziemann, M.; Kn, H.; Okabe, J.; Khurana, I.; Ooi, J.; Khan, A.W.; et al. Vascular histone deacetylation by pharmacological HDAC inhibition. Genome Res. 2014, 24, 1271–1284. [Google Scholar] [CrossRef]
- Hebbel, R.P.; Vercellotti, G.M.; Pace, B.S.; Solovey, A.N.; Kollander, R.; Abanonu, C.F.; Nguyen, J.; Vineyard, J.V.; Belcher, J.D.; Abdulla, F.; et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood 2010, 115, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Nam, K.H.; Kim, J.; Baek, M.W.; Park, J.E.; Park, H.Y.; Kwon, H.J.; Kwon, O.S.; Kim, D.Y.; Oh, G.T. Trichostatin A exacerbates atherosclerosis in low density lipoprotein receptor-deficient mice. Arter. Thromb. Vasc. Biol. 2005, 25, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Fischle, W.; Dequiedt, F.; Hendzel, M.J.; Guenther, M.G.; Lazar, M.A.; Voelter, W.; Verdin, E. Enzymatic Activity Associated with Class II HDACs Is Dependent on a Multiprotein Complex Containing HDAC3 and SMRT/N-CoR. Mol. Cell 2002, 9, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Verdin, E. Regulatory Signal Transduction Pathways for Class IIa Histone Deacetylases. Curr. Opin. Pharmacol. 2010, 10, 454–460. [Google Scholar] [CrossRef]
- Wang, W.; Ha, C.H.; Jhun, B.S.; Wong, C.; Jain, M.K.; Jin, Z.-G. Fluid Shear Stress Stimulates Phosphorylation-Dependent Nuclear Export of HDAC5 and Mediates Expression of KLF2 and eNOS. Blood 2010, 115, 2971–2979. [Google Scholar] [CrossRef]
- Napoli, C.; de Nigris, F.; Williams-Ignarro, S.; Pignalosa, O.; Sica, V.; Ignarro, L.J. Nitric Oxide and Atherosclerosis: An Update. Nitric Oxide 2006, 15, 265–279. [Google Scholar] [CrossRef]
- Kiernan, R.; Brès, V.; Ng, R.W.M.; Coudart, M.-P.; El Messaoudi, S.; Sardet, C.; Jin, D.-Y.; Emiliani, S.; Benkirane, M. Post-Activation Turn-off of NF-κB-Dependent Transcription Is Regulated by Acetylation of P65. J. Biol. Chem. 2003, 278, 2758–2766. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Fu, W.; Sahebkar, A.; Tan, Y.; Xu, S.; Li, H. Histone Deacetylases (HDACs) and Atherosclerosis: A Mechanistic and Pharmacological Review. Front. Cell Dev. Biol. 2020, 8, 581015. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, J.; Xu, C.; Zhao, H.; Zhou, Y.; Wang, Y.; Yang, M.; Chen, X.; Chen, J. Histone deacetylase 4 inhibits NF-κB activation by facilitating IκBα sumoylation. J. Mol. Cell. Biol. 2020, 12, 933–945. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte Trafficking across the Vessel Wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Ley, K.; McEver, R.P.; Hidalgo, A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011, 118, 6743–6751. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teichmann, E.; Blessing, E.; Hinz, B. Non-Psychoactive Phytocannabinoids Inhibit Inflammation-Related Changes of Human Coronary Artery Smooth Muscle and Endothelial Cells. Cells 2023, 12, 2389. https://doi.org/10.3390/cells12192389
Teichmann E, Blessing E, Hinz B. Non-Psychoactive Phytocannabinoids Inhibit Inflammation-Related Changes of Human Coronary Artery Smooth Muscle and Endothelial Cells. Cells. 2023; 12(19):2389. https://doi.org/10.3390/cells12192389
Chicago/Turabian StyleTeichmann, Elisa, Elane Blessing, and Burkhard Hinz. 2023. "Non-Psychoactive Phytocannabinoids Inhibit Inflammation-Related Changes of Human Coronary Artery Smooth Muscle and Endothelial Cells" Cells 12, no. 19: 2389. https://doi.org/10.3390/cells12192389
APA StyleTeichmann, E., Blessing, E., & Hinz, B. (2023). Non-Psychoactive Phytocannabinoids Inhibit Inflammation-Related Changes of Human Coronary Artery Smooth Muscle and Endothelial Cells. Cells, 12(19), 2389. https://doi.org/10.3390/cells12192389





