Measuring Melanoma Nanomechanical Properties in Relation to Metastatic Ability and Anti-Cancer Drug Treatment Using Scanning Ion Conductance Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Anti-Cancer Drugs and Reagents
2.3. MTT Cell Viability Assay
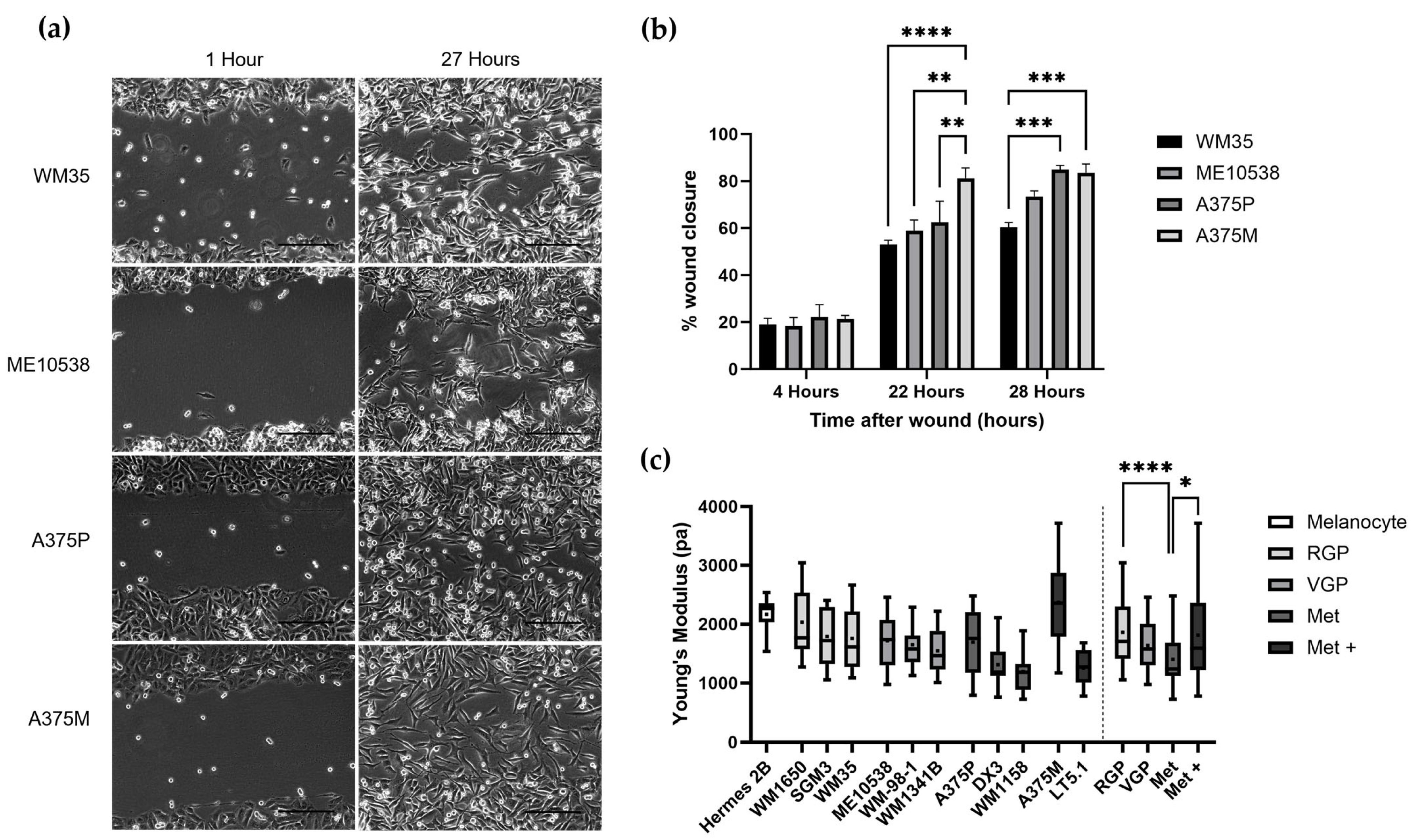
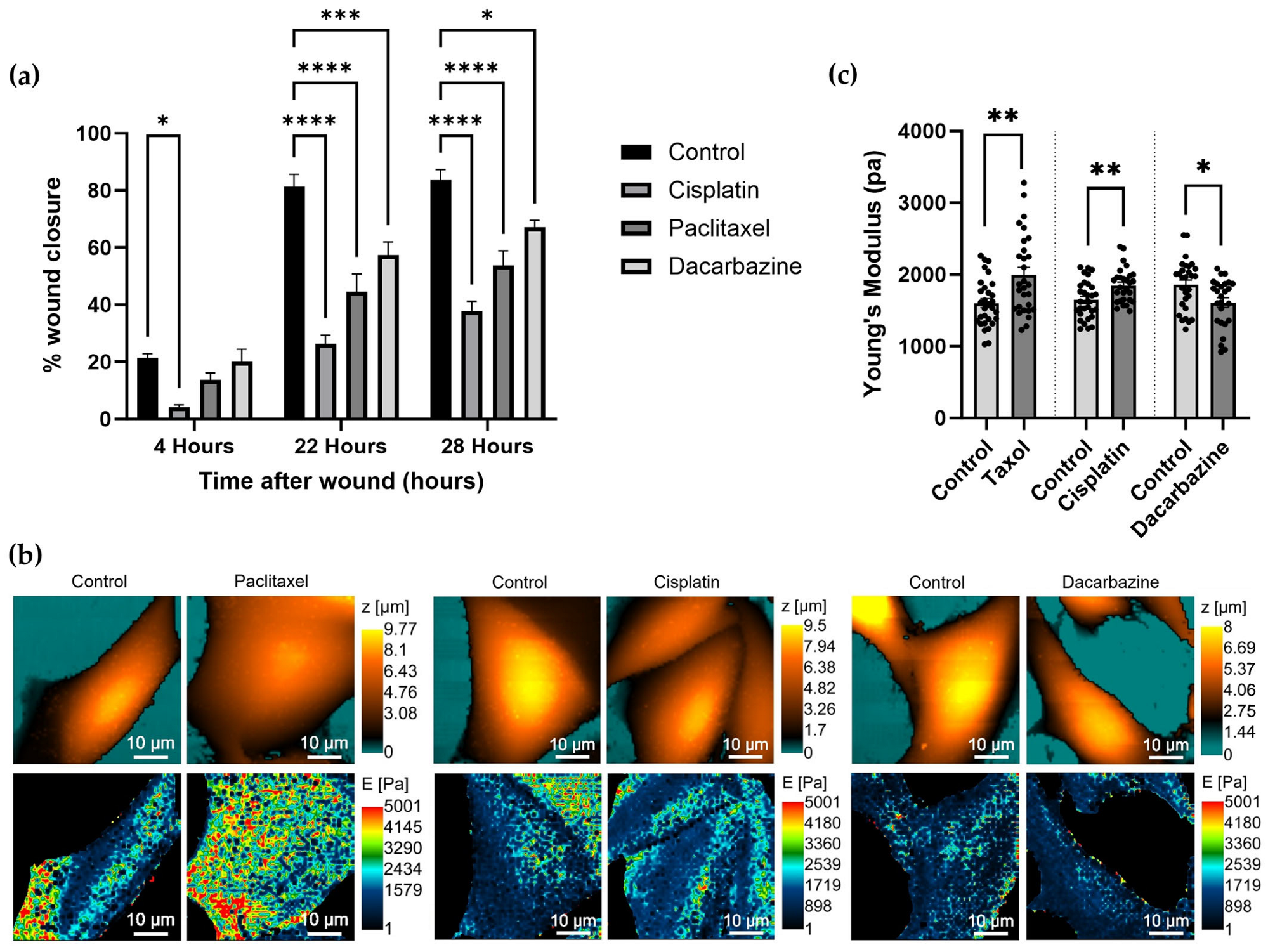
2.4. Wound Healing Assay
2.5. Scanning Ion Conductance Microscopy
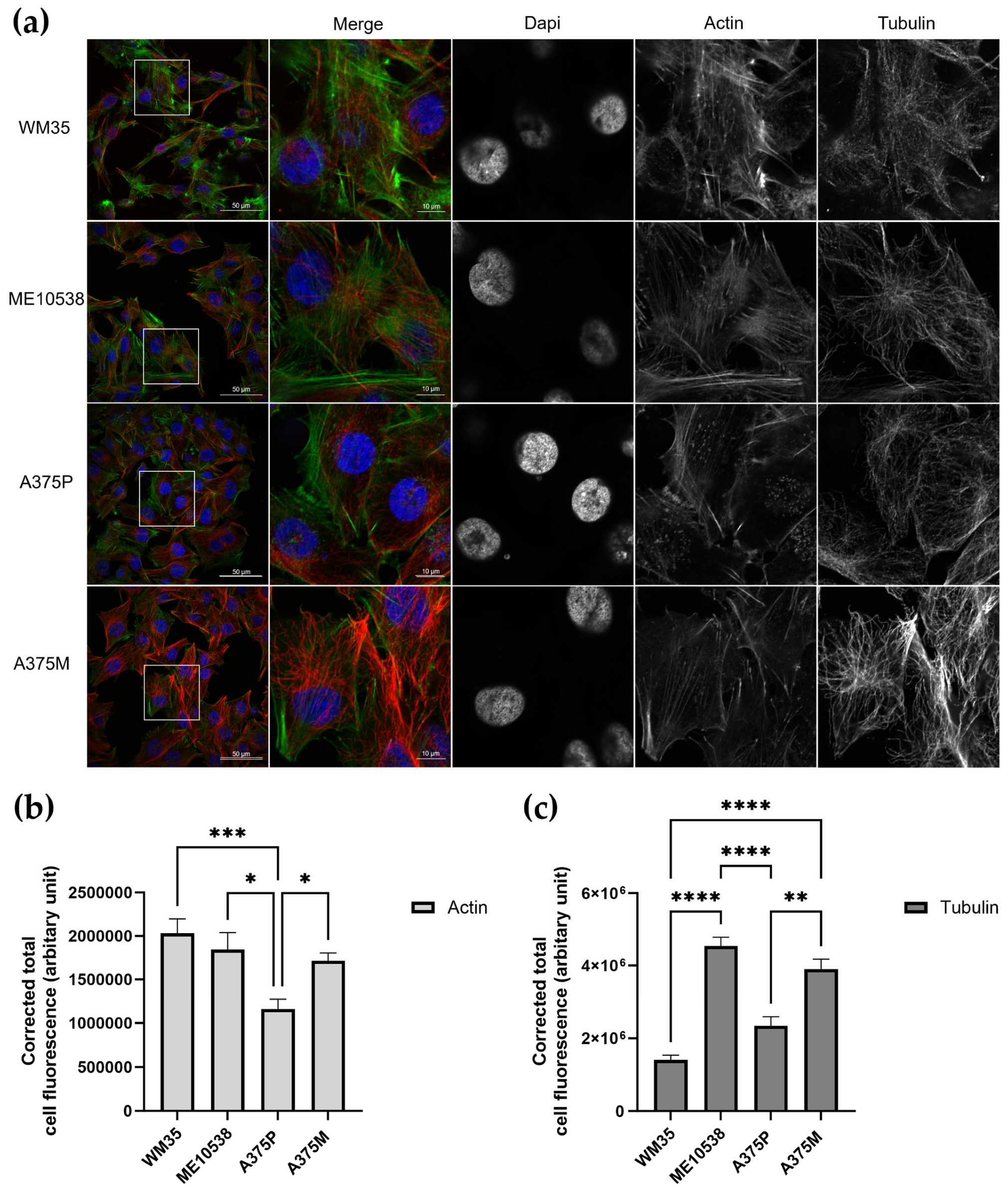
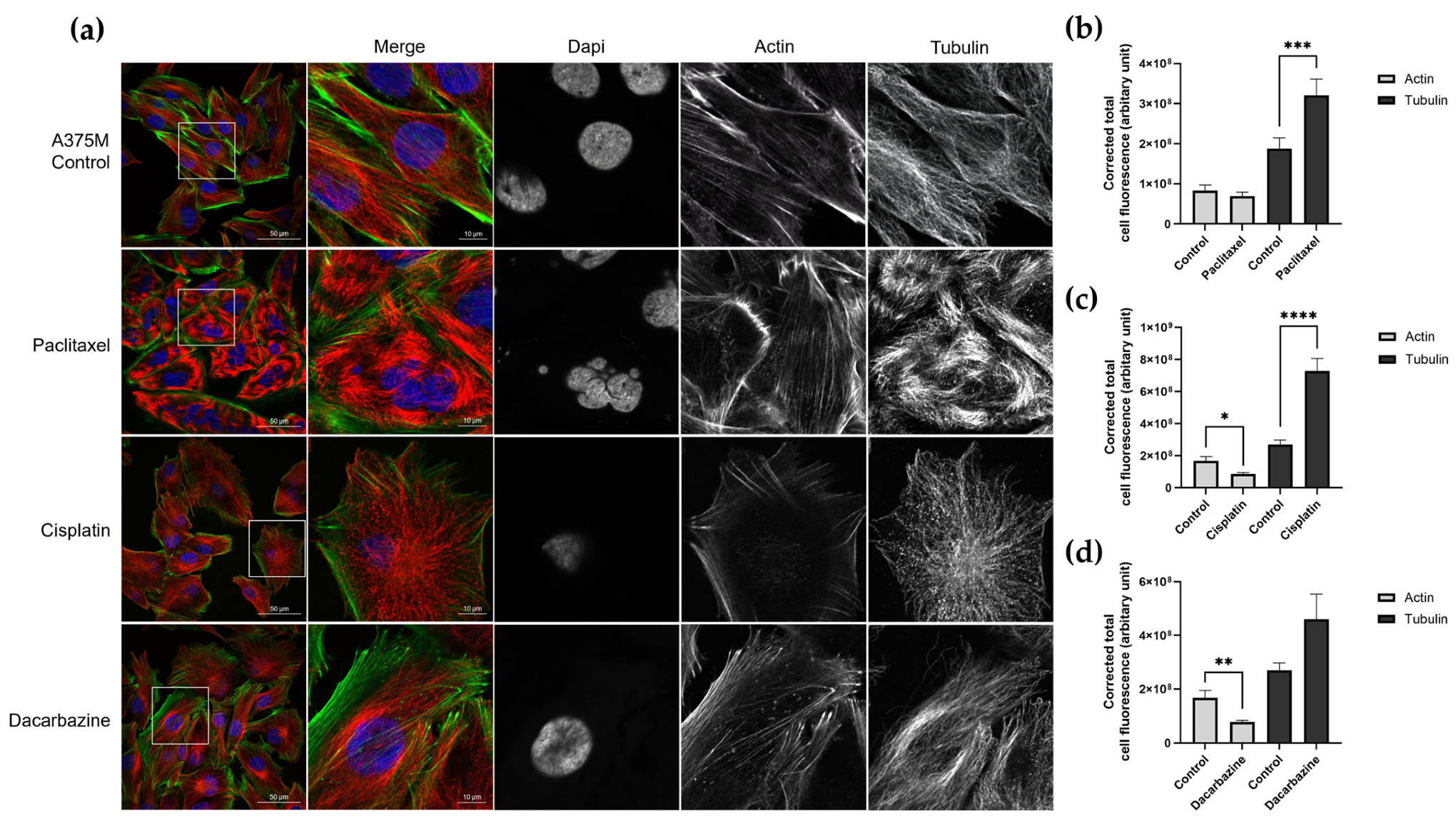
2.6. Immunofluorescence and Confocal Imaging
2.7. Data Analysis and Statistics
3. Results
3.1. Nanomechanical Properties of Melanoma Cell Lines with Increasing Metastatic Ability
3.2. Nanomechanical Properties in Relation to Cytoskeletal Elements
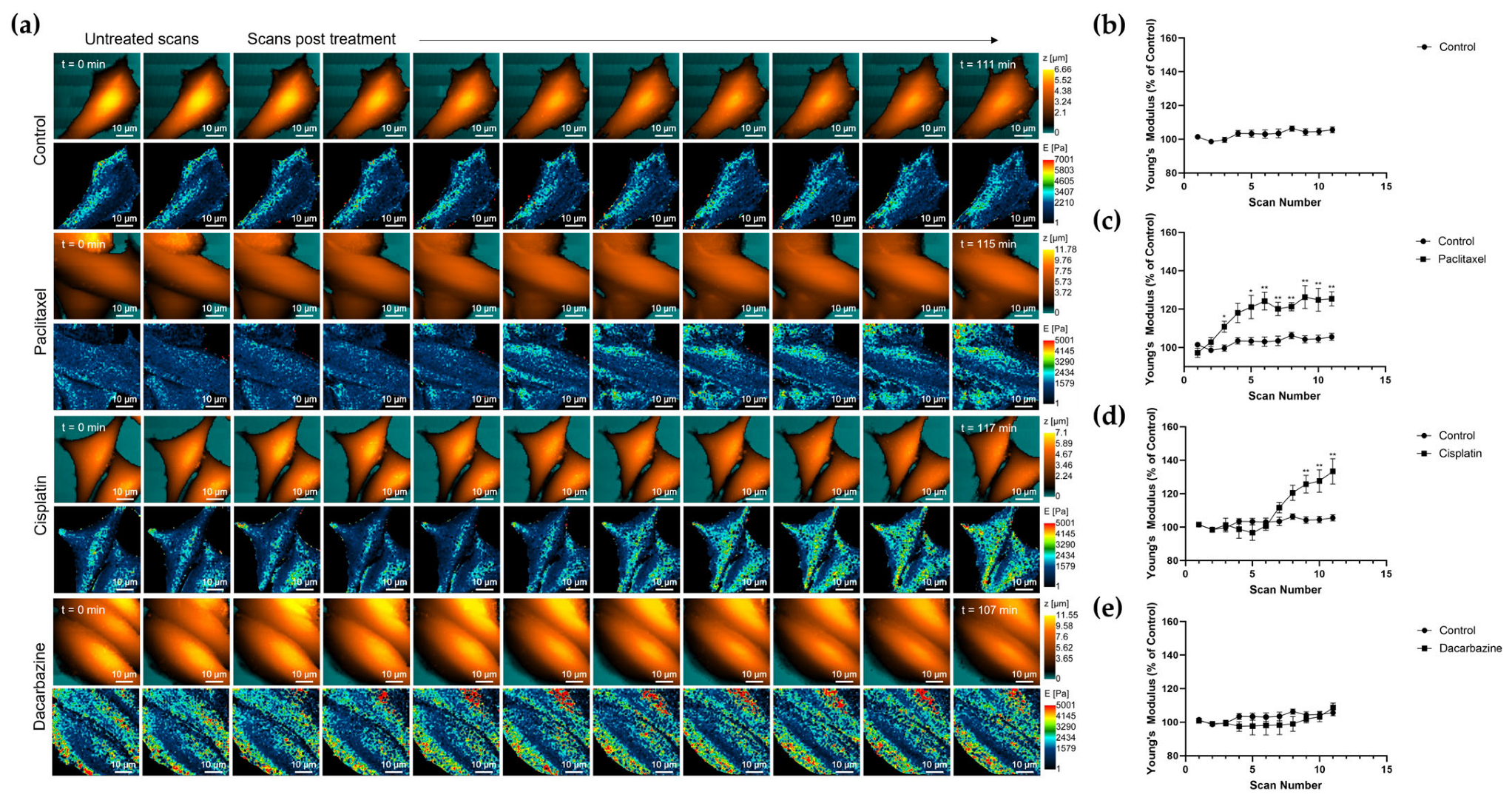
3.3. Investigating Changes in Nanomechanical Properties following 24-Hour Anti-Cancer Drug Treatment
3.4. Changes in Nanomechanical Properties following 2-Hour Anti-Cancer Drug Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Takahashi, Y.; Hong, S.P.; Liu, F.; Bednarska, J.; Goff, P.S.; Novak, P.; Shevchuk, A.; Gopal, S.; Barozzi, I.; et al. High-resolution label-free 3D mapping of extracellular pH of single living cells. Nat. Commun. 2019, 10, 5610. [Google Scholar] [CrossRef] [PubMed]
- Vaneev, A.N.; Gorelkin, P.V.; Garanina, A.S.; Lopatukhina, H.V.; Vodopyanov, S.S.; Alova, A.V.; Ryabaya, O.O.; Akasov, R.A.; Zhang, Y.; Novak, P.; et al. In Vitro and In Vivo Electrochemical Measurement of Reactive Oxygen Species after Treatment with Anticancer Drugs. Anal. Chem. 2020, 92, 8010–8014. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.W.; Novak, P.; Zhukov, A.; Tyler, E.J.; Cano-Jaimez, M.; Drews, A.; Richards, O.; Volynski, K.; Bishop, C.; Klenerman, D. Low Stress Ion Conductance Microscopy of Sub-Cellular Stiffness. Soft Matter. 2016, 12, 7953–7958. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Johnson, N.; Li, C.; Novak, P.; Rheinlaender, J.; Zhang, Y.; Anand, U.; Anand, P.; Gorelik, J.; Frolenkov, G.I.; et al. Noncontact measurement of the local mechanical properties of living cells using pressure applied via a pipette. Biophys. J. 2008, 95, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Rheinlaender, J.; Schäffer, T.E. Mapping the mechanical stiffness of live cells with the scanning ion conductance microscope. Soft Matter. 2013, 9, 3230–3236. [Google Scholar] [CrossRef]
- Kolmogorov, V.S.; Erofeev, A.S.; Woodcock, E.; Efremov, Y.M.; Iakovlev, A.P.; Savin, N.A.; Alova, A.V.; Lavrushkina, S.V.; Kireev, I.I.; Prelovskaya, A.O.; et al. Mapping mechanical properties of living cells at nanoscale using intrinsic nanopipette–sample force interactions. Nanoscale 2021, 13, 6558–6568. [Google Scholar] [CrossRef]
- Seifert, J.; Rheinlaender, J.; Novak, P.; Korchev, Y.E.; Schäffer, T.E. Comparison of Atomic Force Microscopy and Scanning Ion Conductance Microscopy for Live Cell Imaging. Langmuir 2015, 31, 6807–6813. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1953–1960. [Google Scholar] [CrossRef]
- Tang, K.; Xin, Y.; Li, K.; Chen, X.; Tan, Y. Cell Cytoskeleton and Stiffness Are Mechanical Indicators of Organotropism in Breast Cancer. Biology 2021, 10, 259. [Google Scholar] [CrossRef]
- Tavares, S.; Vieira, A.F.; Taubenberger, A.V.; Araújo, M.; Martins, N.P.; Brás-Pereira, C.; Polónia, A.; Herbig, M.; Barreto, C.; Otto, O.; et al. Actin stress fiber organization promotes cell stiffening and proliferation of pre-invasive breast cancer cells. Nat. Commun. 2017, 8, 15237. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Nyberg, K.D.; Scott, M.B.; Welsh, A.M.; Nguyen, A.H.; Wu, N.; Hohlbauch, S.V.; Geisse, N.A.; Gibb, E.A.; Robertson, A.G.; et al. Stiffness of pancreatic cancer cells is associated with increased invasive potential. Integr. Biol. 2016, 8, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Weder, G.; Hendriks-Balk, M.C.; Smajda, R.; Rimoldi, D.; Liley, M.; Heinzelmann, H.; Meister, A.; Mariotti, A. Increased plasticity of the stiffness of melanoma cells correlates with their acquisition of metastatic properties. Nanomedicine 2014, 10, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef]
- Watanabe, T.; Kuramochi, H.; Takahashi, A.; Imai, K.; Katsuta, N.; Nakayama, T.; Fujiki, H.; Suganuma, M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (2)-epigallocatechin gallate-treated cells. J. Cancer Res. Clin. Oncol. 2012, 138, 859–866. [Google Scholar] [CrossRef]
- Fatima, I.; Safdar, N.; Akhtar, W.; Munir, A.; Saqib, S.; Ayaz, A.; Bahadur, S.; Alrefaei, A.F.; Ullah, F.; Zaman, W. Evaluation of potential inhibitory effects on acetylcholinesterase, pancreatic lipase, and cancer cell lines using raw leaves extracts of three fabaceae species. Heliyon 2023, 9, e15909. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.; Wang, S.; Wang, Z.; Qu, L.; Wang, C.; Xu, K. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine 2023, 118, 154940. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, L.; Wu, X.; Peng, Y. Review on near-field detection technology in the biomedical field. Adv. Photonics Nexus 2023, 2, 044002. [Google Scholar] [CrossRef]
- Sharma, S.; Santiskulvong, C.; Rao, J.; Gimzewski, J.K.; Dorigo, O. The role of Rho GTPase in cell stiffness and cisplatin resistance in ovarian cancer cells. Integr. Biol. 2014, 6, 611–617. [Google Scholar] [CrossRef]
- Raudenska, M.; Kratochvilova, M.; Vicar, T.; Gumulec, J.; Balvan, J.; Polanska, H.; Pribyl, J.; Masarik, M. Cisplatin enhances cell stiffness and decreases invasiveness rate in prostate cancer cells by actin accumulation. Sci. Rep. 2019, 9, 1660. [Google Scholar] [CrossRef]
- Islam, M.; Mezencev, R.; McFarland, B.; Brink, H.; Campbell, B.; Tasadduq, B.; Waller, E.K.; Lam, W.; Alexeev, A.; Sulchek, T. Microfluidic cell sorting by stiffness to examine heterogenic responses of cancer cells to chemotherapy. Cell Death Dis. 2018, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.C.; Bar-Eli, M. Regulation of gene expression in melanoma: New approaches for treatment. J. Cell Biochem. 2005, 94, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667. [Google Scholar] [CrossRef]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Li, R.H.; Sun, C.; Liu, Y.Q.; Zheng, J.N. Dacarbazine Combined Targeted Therapy versus Dacarbazine Alone in Patients with Malignant Melanoma: A Meta-Analysis. PLoS ONE 2014, 9, e111920. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Murashko, A.V.; Frolova, A.A.; Akovantseva, A.A.; Kotova, S.L.; Timashev, P.S.; Efremov, Y.M. The cell softening as a universal indicator of cell damage during cytotoxic effects. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2023, 1867, 130348. [Google Scholar] [CrossRef]
- Park, S.; Koch, D.; Cardenas, R.; Käs, J.; Shih, C.K. Cell Motility and Local Viscoelasticity of Fibroblasts. Biophys. J. 2005, 89, 4330–4342. [Google Scholar] [CrossRef]
- Lekka, M.; Laidler, P.; Gil, D.; Lekki, J.; Stachura, Z.; Hrynkiewicz, A.Z. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J. 1999, 28, 312–316. [Google Scholar] [CrossRef]
- Sobiepanek, A.; Milner-Krawczyk, M.; Lekka, M.; Kobiela, T. AFM and QCM-D as tools for the distinction of melanoma cells with a different metastatic potential. Biosens. Bioelectron. 2017, 93, 274–281. [Google Scholar] [CrossRef]
- Bobrowska, J.; Awsiuk, K.; Pabijan, J.; Bobrowski, P.; Lekki, J.; Sowa, K.M.; Rysz, J.; Budkowski, A.; Lekka, M. Biophysical and biochemical characteristics as complementary indicators of melanoma progression. Anal. Chem. 2019, 91, 9885–9892. [Google Scholar] [CrossRef] [PubMed]
- Alibert, C.; Goud, B.; Manneville, J.B. Are cancer cells really softer than normal cells? Biol. Cell. 2017, 109, 167–189. [Google Scholar] [CrossRef] [PubMed]
- Molter, C.W.; Muszynski, E.F.; Tao, Y.; Trivedi, T.; Clouvel, A.; Ehrlicher, A.J. Prostate cancer cells of increasing metastatic potential exhibit diverse contractile forces, cell stiffness, and motility in a microenvironment stiffness-dependent manner. Front. Cell Dev. Biol. 2022, 10, 932510. [Google Scholar] [CrossRef] [PubMed]
- Kraning-Rush, C.M.; Califano, J.P.; Reinhart-King, C.A. Cellular Traction Stresses Increase with Increasing Metastatic Potential. PLoS ONE 2012, 7, e32572. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhao, C.; Xu, B.; Huang, Y.; Liu, C. Effect of docetaxel on mechanical properties of ovarian cancer cells. Exp. Cell Res. 2021, 408, 112853. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Huang, H.; Liu, Y.; Zheng, X.; Zou, Q. An Atomic Force Microscope Study Revealed Two Mechanisms in the Effect of Anticancer Drugs on Rate-Dependent Young’s Modulus of Human Prostate Cancer Cells. PLoS ONE 2015, 10, e0126107. [Google Scholar] [CrossRef]
- Huang, X.; He, J.; Zhang, H.T.; Sun, K.; Yang, J.; Wang, H.; Zhang, H.; Guo, Z.; Zha, Z.-G.; Zhou, C. Effect of dacarbazine on CD44 in live melanoma cells as measured by atomic force microscopy-based nanoscopy. Int. J. Nanomed. 2017, 12, 8867–8886. [Google Scholar] [CrossRef]
- Gavara, N.; Chadwick, R.S. Relationship between cell stiffness and stress fiber amount, assessed by simultaneous atomic force microscopy and live-cell fluorescence imaging. Biomech. Model. Mechanobiol. 2016, 15, 511–523. [Google Scholar] [CrossRef]
- Li, Q.S.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 609–613. [Google Scholar] [CrossRef]
- Ferrandina, G.; Zannoni, G.F.; Martinelli, E.; Paglia, A.; Gallotta, V.; Mozzetti, S.; Scambia, G.; Ferlini, C. Class III β-Tubulin Overexpression Is a Marker of Poor Clinical Outcome in Advanced Ovarian Cancer Patients. Clin. Cancer Res. 2006, 12, 2774–2779. [Google Scholar] [CrossRef]
- Grady, M.E.; Composto, R.J.; Eckmann, D.M. Cell elasticity with altered cytoskeletal architectures across multiple cell types. J. Mech. Behav. Biomed. Mater. 2016, 61, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kung, M.L.; Hsieh, C.W.; Tai, M.H.; Weng, C.-H.; Wu, D.-C.; Wu, W.-J.; Yeh, B.-W.; Hsieh, S.-L.; Kuo, C.-H.; Hung, H.-S.; et al. Nanoscale characterization illustrates the cisplatin-mediated biomechanical changes of B16-F10 melanoma cells. Phys. Chem. Chem. Phys. 2016, 18, 7124–7131. [Google Scholar] [CrossRef]
- Schäfer, A.; Radmacher, M. Influence of myosin II activity on stiffness of fibroblast cells. Acta Biomater. 2005, 1, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Carvalho, F.A.; Figueiredo, J.; Carvalho, R.; Mestre, T.; Monteiro, J.; Guedes, A.F.; Fonseca, M.; Sanches, J.; Seruca, R.; et al. Atomic force microscopy and graph analysis to study the P-cadherin/SFK mechanotransduction signalling in breast cancer cells. Nanoscale 2016, 8, 19390–19401. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodcock, E.; Gorelkin, P.V.; Goff, P.S.; Edwards, C.R.W.; Zhang, Y.; Korchev, Y.; Sviderskaya, E.V. Measuring Melanoma Nanomechanical Properties in Relation to Metastatic Ability and Anti-Cancer Drug Treatment Using Scanning Ion Conductance Microscopy. Cells 2023, 12, 2401. https://doi.org/10.3390/cells12192401
Woodcock E, Gorelkin PV, Goff PS, Edwards CRW, Zhang Y, Korchev Y, Sviderskaya EV. Measuring Melanoma Nanomechanical Properties in Relation to Metastatic Ability and Anti-Cancer Drug Treatment Using Scanning Ion Conductance Microscopy. Cells. 2023; 12(19):2401. https://doi.org/10.3390/cells12192401
Chicago/Turabian StyleWoodcock, Emily, Peter V. Gorelkin, Philip S. Goff, Christopher R. W. Edwards, Yanjun Zhang, Yuri Korchev, and Elena V. Sviderskaya. 2023. "Measuring Melanoma Nanomechanical Properties in Relation to Metastatic Ability and Anti-Cancer Drug Treatment Using Scanning Ion Conductance Microscopy" Cells 12, no. 19: 2401. https://doi.org/10.3390/cells12192401
APA StyleWoodcock, E., Gorelkin, P. V., Goff, P. S., Edwards, C. R. W., Zhang, Y., Korchev, Y., & Sviderskaya, E. V. (2023). Measuring Melanoma Nanomechanical Properties in Relation to Metastatic Ability and Anti-Cancer Drug Treatment Using Scanning Ion Conductance Microscopy. Cells, 12(19), 2401. https://doi.org/10.3390/cells12192401




