The Study of the Caudal Vertebrae of Thick-Toed Geckos after a Prolonged Space Flight by X-ray Phase-Contrast Micro-CT
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Life-Support
2.2. Logistics and Sample Preparation
2.3. Ethical Statement
2.4. X-ray Phase-Contrast (XPCT) Experiment
2.5. Data Processing
2.6. List of Morphometric Parameters and Abbreviations
2.7. Statistical Analysis
2.8. Histological Study
3. Results
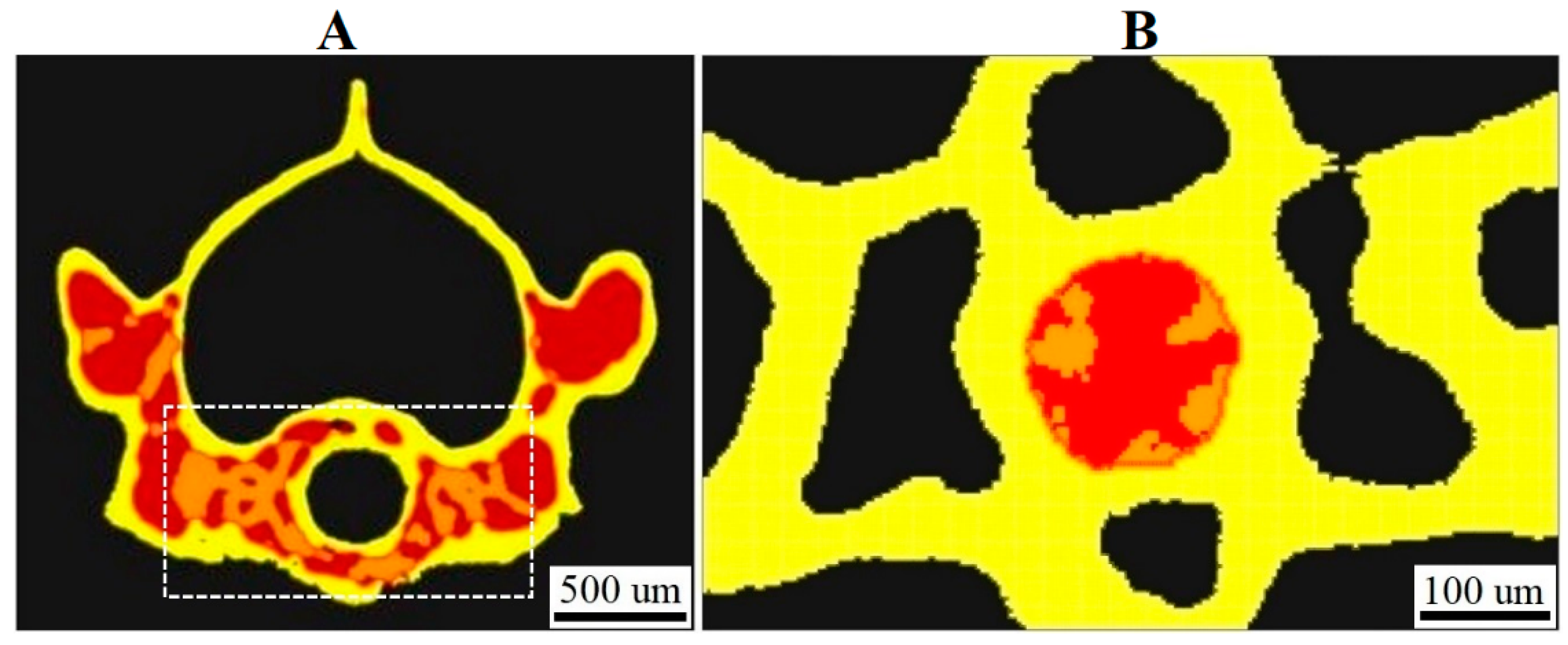
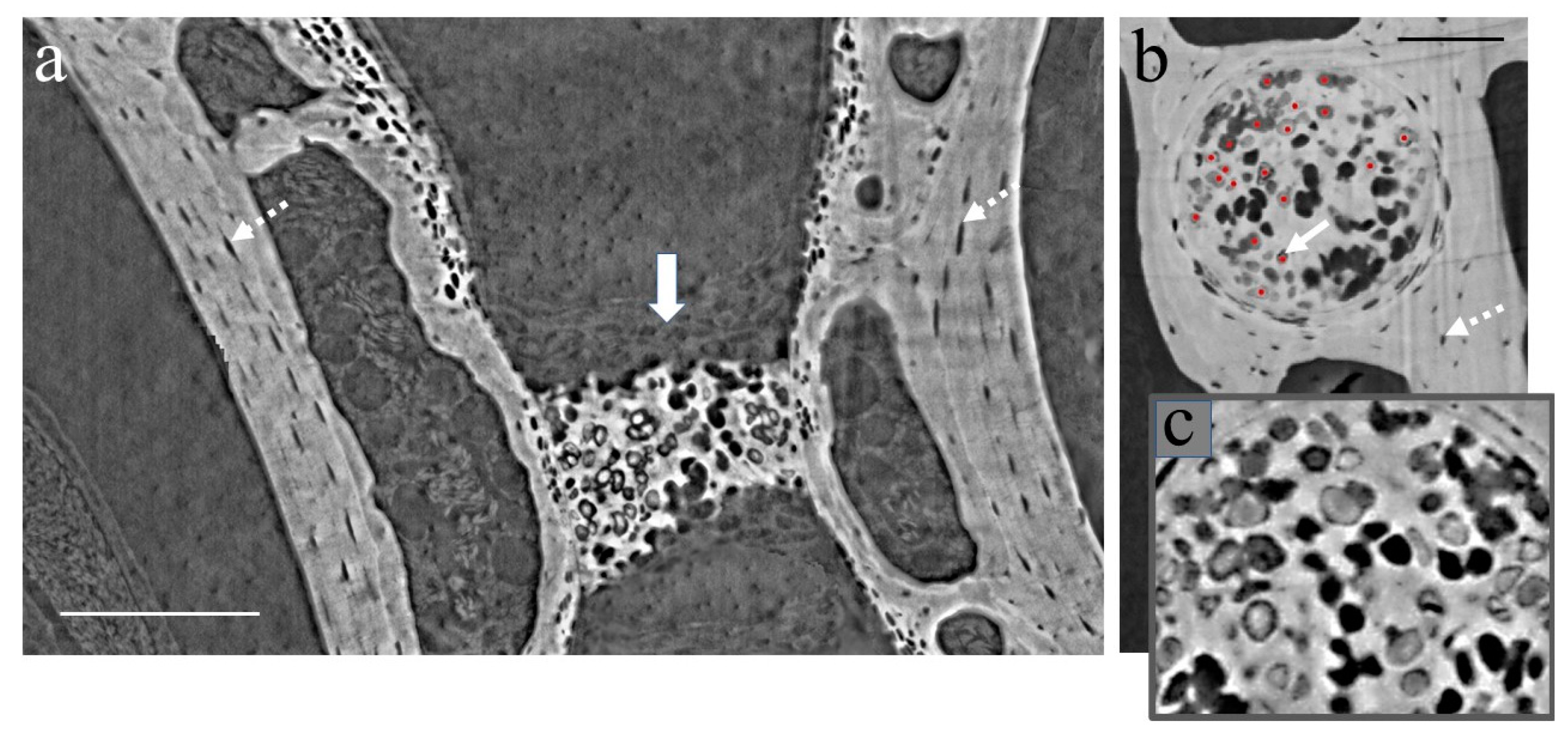
3.1. XPCT Experimental Results and 3D Visualization
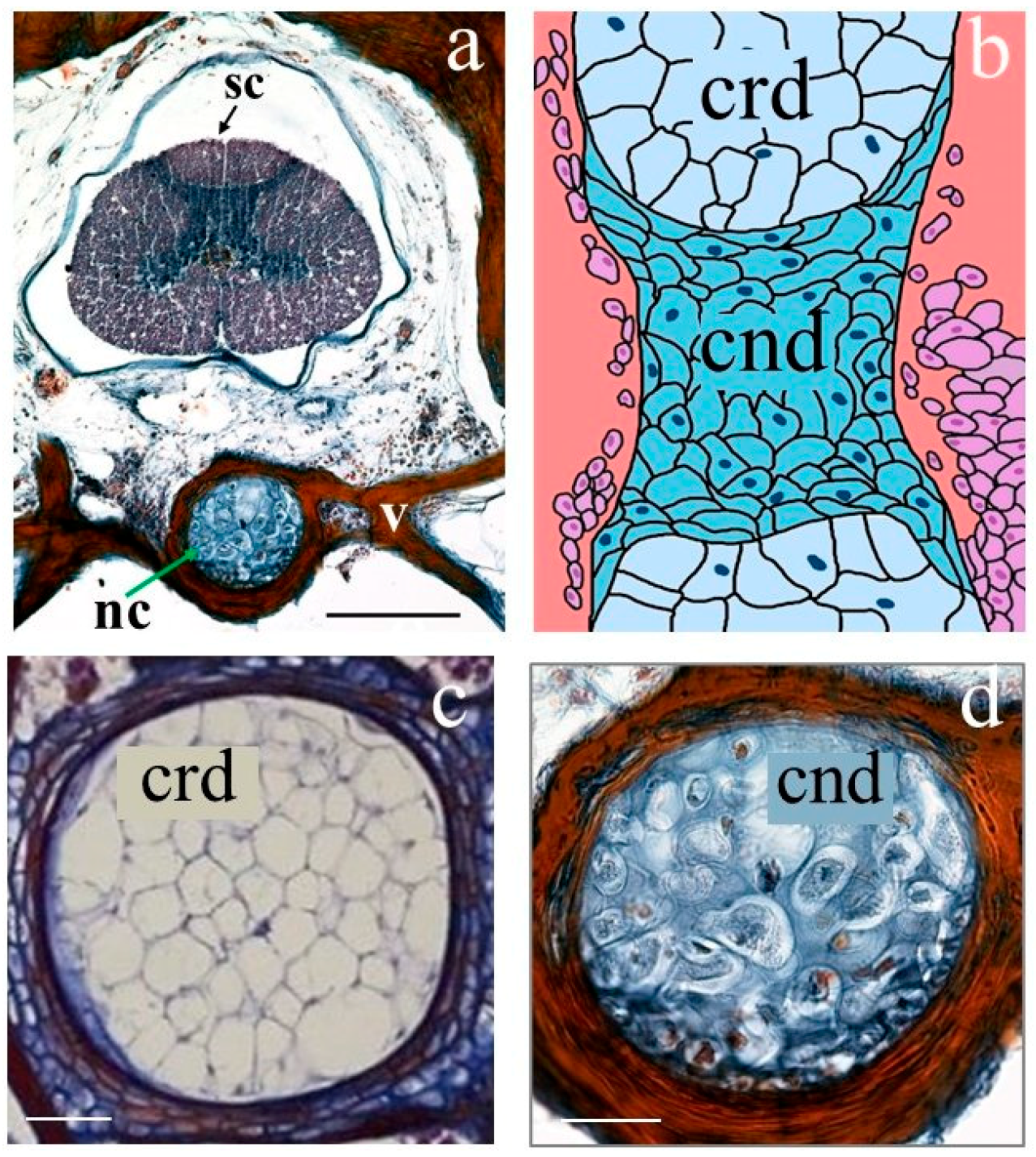
3.2. The Vertebrae and Notochord 3D Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozlovskaya, I.B.; Sayenko, I.V.; Sayenko, D.G.; Miller, T.F.; Khusnutdinova, D.R.; Melnik, K.A. Role of support afferentation in control of the tonic muscle activity. Acta Astronaut. 2007, 60, 285–294. [Google Scholar] [CrossRef]
- Vico, L.; Hargens, A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018, 14, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Saveko, A.; Rukavishnikov, I.; Brykov, V.; Osetsky, N.; Ryazanskiy, S.; Tomilovskaya, E.; Kozlovskaya, I.; Grishin, M.A. Foot-ground reaction force during long-term space flight and after it: Walking in active treadmill mode. Gait Posture 2020, 76, 382–388. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Edgerton, V.R.; Harkema, S.; Kozlovskaya, I. Gravity dependent mechanisms of sensorimotor regulation of posture and locomotion. Aerosp. Environ. Med. 2020, 54, 27–42. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Mirzoev, T.M.; Kozlovskaya, I.B. Tonic activity and gravitational control of the postural muscle. Hum. Physiol. 2021, 47, 744–756. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Marini, M.; Tarantino, U.; Tancredi, V. Microgravity and Musculoskeletal Health: What Strategies Should Be Used for a Great Challenge? Life 2023, 13, 1423. [Google Scholar] [CrossRef] [PubMed]
- Barabanov, V.M.; Gulimova, V.I.; Berdiev, R.K.; Saveliev, S.V. Individual features of play behavior in thick-toed geckos in weightlessness and normal gravity conditions. Life Sci. Space Res. 2019, 22, 38–46. [Google Scholar] [CrossRef]
- Gulimova, V.; Proshchina, A.; Kharlamova, A.; Krivova, Y.; Barabanov, V.; Berdiev, R.; Asadchikov, V.; Buzmakov, A.; Zolotov, D.; Saveliev, S. Reptiles in Space Missions: Results and Perspectives. Int. J. Mol. Sci. 2019, 20, 3019. [Google Scholar] [CrossRef]
- Wassersug, R.J.; Roberts, L.; Gimian, J.; Hughes, E.; Saunders, R.; Devison, D.; Woodbury, J.; O’Reilly, J.C. The behavioral responses of amphibians and reptiles to microgravity on parabolic flights. Zoology 2005, 108, 107–120. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef]
- Puthoff, J.B.; Prowse, M.S.; Wilkinson, M.; Autumn, K. Changes in materials properties explain the effects of humidity on gecko adhesion. J. Exp. Biol. 2010, 213, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Barabanov, V.; Gulimova, V.; Berdiev, R.; Saveliev, S. Object play in thick-toed geckos during a space experiment. J. Ethol. 2015, 33, 109–115. [Google Scholar] [CrossRef]
- Barabanov, V.M.; Gulimova, V.I.; Berdiev, R.K.; Saveliev, S.V. Attachment of Turner’s thick-toed geckos (Chondrodactylus turneri GRAY 1864) during weightlessness and their responses to flotation. Life Sci. Space Res. 2018, 18, 21–28. [Google Scholar] [CrossRef]
- Nikitin, V.B.; Proshchina, A.E.; Kharlamova, A.S.; Barabanov, V.M.; Krivova, J.S.; Godovalova, O.S.; Savelieva, E.S.; Makarov, A.N.; Gulimova, V.I.; Okshtein, I.L.; et al. Comparative studies of the thick-toed geckoes after the 16 and 12 days spaceflights in “Foton-M” experiments. J. Gravit. Physiol. 2008, 15, 285–288. [Google Scholar]
- Kharlamova, A.; Proschina, A.; Gulimova, V.; Krivova, Y.; Soldatov, P.; Saveliev, S. Cerebellar morphology and behavioural correlations of the vestibular function alterations in weightlessness. Neurosci. Biobehav. Rev. 2021, 126, 314–328. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Martinez, D.A.; Boudreaux, R.D.; Mantri, A.V. Microgravity stress: Bone and connective tissue. Compr. Physiol. 2016, 6, 645–686. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Van Loon, J.J.W.A.; Bloomfield, S.; Vico, L.; Chopard, A.; Rittweger, J.; Kyparos, A.; Blottner, D.; Vuori, I.; Gerzer, R.; et al. Towards human exploration of space: The THESEUS review series on muscle and bone research priorities. NPJ Microgravity 2017, 3, 8. [Google Scholar] [CrossRef]
- Hart, D.A. Regulation of Bone by Mechanical Loading, Sex Hormones, and Nerves: Integration of Such Regulatory Complexity and Implications for Bone Loss during Space Flight and Post-Menopausal Osteoporosis. Biomolecules 2023, 13, 1136. [Google Scholar] [CrossRef]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar]
- Blaber, E.A.; Dvorochkin, N.; Lee, C.; Alwood, J.S.; Yousuf, R.; Pianetta, P.; Globus, R.K.; Burns, B.P.; Almeida, E.A. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS ONE 2013, 8, e61372. [Google Scholar] [CrossRef]
- Gerbaix, M.; Gnyubkin, V.; Farlay, D.; Olivier, C.; Ammann, P.; Courbon, G.; Laroche, N.; Genthial, R.; Follet, H.; Peyrin, F.; et al. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci. Rep. 2017, 7, 13078. [Google Scholar] [CrossRef]
- Giuliani, A.; Mazzoni, S.; Ruggiu, A.; Canciani, B.; Cancedda, R.; Tavella, S. High-Resolution X-Ray Tomography: A 3D Exploration Into the Skeletal Architecture in Mouse Models Submitted to Microgravity Constraints. Front. Physiol. 2018, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, M.P.; Risin, D. The current state of bone loss research: Data from spaceflight and microgravity simulators. J. Cell. Biochem. 2013, 114, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Berg-Johansen, B.; Liebenberg, E.C.; Li, A.; Macias, B.R.; Hargens, A.R.; Lotz, J.C. Spaceflight-induced bone loss alters failure mode and reduces bending strength in murine spinal segments. J. Orthop. Res. 2015, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wassersug, R.J. Vertebrate biology in microgravity: Basic questions about how complex organisms respond to spaceflight and microgravity can only be answered by long-term study. Am. Sci. 2001, 89, 46–53. [Google Scholar] [CrossRef]
- Clément, G.; Slenzka, K. (Eds.) Fundamentals of Space Biology: Research on Cells, Animals, and Plants in Space; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 18, p. 376. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Hill, E.L.; Souza, K.A. Animals and spaceflight: From survival to understanding. J. Musculoskelet. Neuronal Interact. 2007, 7, 17. [Google Scholar]
- Fu, J.; Goldsmith, M.; Crooks, S.D.; Condon, S.F.; Morris, M.; Komarova, S.V. Bone health in spacefaring rodents and primates: Systematic review and meta-analysis. NPJ Microgravity 2021, 7, 19. [Google Scholar] [CrossRef]
- Grigoryan, E.N. Impact of Microgravity and Other Spaceflight Factors on Retina of Vertebrates and Humans In Vivo and In Vitro. Life 2023, 13, 1263. [Google Scholar] [CrossRef]
- Santucci, D.; Kawano, F.; Ohira, T.; Terada, M.; Nakai, N.; Francia, N.; Alleva, E.; Aloe, L.; Ochiai, T.; Cancedda, R.; et al. Evaluation of gene, protein and neurotrophin expression in the brain of mice exposed to space environment for 91 days. PLoS ONE 2012, 7, e40112. [Google Scholar] [CrossRef]
- Cancedda, R.; Liu, Y.; Ruggiu, A.; Tavella, S.; Biticchi, R.; Santucci, D.; Schwartz, S.; Ciparelli, P.; Falcetti, G.; Tenconi, C.; et al. The Mice Drawer System (MDS) experiment and the space endurance record-breaking mice. PLoS ONE 2012, 7, e32243. [Google Scholar] [CrossRef]
- Andreev-Andrievskiy, A.; Popova, A.; Boyle, R.; Alberts, J.; Shenkman, B.; Vinogradova, O.; Dolgov, O.; Anokhin, K.; Tsvirkun, D.; Soldatov, P.; et al. Mice in Bion-M 1 space mission: Training and selection. PLoS ONE 2014, 9, e104830. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.E.; Moyer, E.L.; Talyansky, Y.; Lowe, M.; Padmanabhan, S.; Choi, S.; Gong, C.; Cadena, S.M.; Stodieck, L.; Globus, R.K. Behavior of mice aboard the International Space Station. Sci. Rep. 2019, 9, 4717. [Google Scholar] [CrossRef] [PubMed]
- von Kroge, S.; Wölfel, E.M.; Buravkova, L.B.; Atiakshin, D.A.; Markina, E.A.; Schinke, T.; Rolvien, T.; Busse, B.; Jähn-Rickert, K. Bone loss recovery in mice following microgravity with concurrent bone-compartment-specific osteocyte characteristics. Eur. Cells Mater. 2021, 42, 220–231. [Google Scholar] [CrossRef]
- Jonasson, K.A.; Russell, A.P.; Vickaryous, M.K. Histology and histochemistry of the gekkotan notochord and their bearing on the development of notochordal cartilage. J. Morphol. 2012, 273, 596–603. [Google Scholar] [CrossRef]
- Witten, P.E.; Hall, B.K. The Notochord: Development, Evolution and Contributions to the Vertebral Column, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Stefanutti, E.; Sierra, A.; Miocchi, P.; Massimi, L.; Brun, F.; Maugeri, L.; Bukreeva, I.; Nurmi, A.; Provinciali, G.B.; Tromba, G.; et al. Assessment of the effects of different sample perfusion procedures on phase-contrast tomographic images of mouse spinal cord. J. Instrum. 2018, 13, C03027. [Google Scholar] [CrossRef]
- Massimi, L.; Bukreeva, I.; Santamaria, G.; Fratini, M.; Corbelli, A.; Brun, F.; Fumagalli, S.; Maugeri, L.; Pacureanu, A.; Cloetens, P.; et al. Exploring Alzheimer’s disease mouse brain through X-ray phase contrast tomography: From the cell to the organ. NeuroImage 2018, 184, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Quenot, L.; Bohic, S.; Brun, E. X-ray Phase Contrast Imaging from Synchrotron to Conventional Sources: A Review of the Existing Techniques for Biological Applications. Appl. Sci. 2022, 12, 9539. [Google Scholar] [CrossRef]
- Mayo, S.C.; Davis, T.J.; Gureyev, T.E.; Miller, P.R.; Paganin, D.; Pogany, A.; Stevenson, A.W.; Wilkins, S.W. X-ray phase-contrast microscopy and microtomography. Opt. Express 2003, 11, 2289–2302. [Google Scholar] [CrossRef]
- Bukreeva, I.; Junemann, O.; Cedola, A.; Palermo, F.; Maugeri, L.; Begani Provinciali, G.; Pieroni, N.; Sanna, A.; Otlyga, D.A.; Buzmakov, A.; et al. Investigation of the human pineal gland 3D organization by X-ray phase contrast tomography. J. Struct. Biol. 2020, 212, 107659. [Google Scholar] [CrossRef]
- Di Fonzo, S.; Jark, W.; Soullié, G.; Cedola, A.; Lagomarsino, S.; Cloetens, P.; Riekel, C. Submicrometre resolution phase-contrast radiography with the beam from an X-ray waveguide. J. Synchrotron Radiat. 1998, 5 Pt 3, 376–378. [Google Scholar] [CrossRef]
- Mittone, A.; Fardin, L.; Di Lillo, F.; Fratini, M.; Requardt, H.; Mauro, A.; Homs-Regojo, R.A.; Douissard, P.A.; Barbone, G.E.; Stroebel, J.; et al. Multiscale pink-beam microCT imaging at the ESRF-ID17 biomedical beamline. J. Synchrotron Radiat. 2020, 27 Pt 5, 1347–1357. [Google Scholar] [CrossRef]
- van Aarle, W.; Palenstijn, W.J.; Cant, J.; Janssens, E.; Bleichrodt, F.; Dabravolski, A.; De Beenhouwer, J.; Batenburg, K.J.; Sijbers, J. Fast and flexible X-ray tomography using the ASTRA toolbox. Opt. Express 2016, 24, 25129. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E.; Eddins, S.L. Frequency domain processing. In Digital Image Processing Using MATLAB; Pearson Prentice Hall: Hoboken, NJ, USA, 2004. [Google Scholar]
- Hildebrand, T.; Ruegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Gostick, J.T.; Khan, Z.A.; Tranter, T.G.; Kok, M.D.; Agnaou, M.; Sadeghi, M.; Jervis, R. PoreSpy: A python toolkit for quantitative analysis of porous media images. J. Open Source Softw. 2019, 4, 1296. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Varga, P.; Hesse, B.; Langer, M.; Schrof, S.; Männicke, N.; Suhonen, H.; Pacureanu, A.; Pahr, D.; Peyrin, F.; Raum, K. Synchrotron X-ray phase nano-tomography-based analysis of the lacunar-canalicular network morphology and its relation to the strains experienced by osteocytes in situ as predicted by case-specific finite element analysis. Biomech. Model. Mechanobiol. 2015, 14, 267–282. [Google Scholar] [CrossRef] [PubMed]


| Index | Units | Control Group | Flight Group | Difference,% | p-Value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Total Body | |||||||
| Total length, mm | mm | 158.0 | 2.78 | 150.67 | 7.52 | −4.64 | 0.2275 (NS) |
| Snout-vent length, mm | mm | 78.33 | 0.58 | 79.50 | 3.50 | 1.49 | 0.6579 (NS) |
| Tail length, mm | mm | 79.67 | 3.33 | 71.17 | 6.25 | −10.67 | 0.1277 (NS) |
| Launch body mass, g | g | 20.45 | 0.80 | 19.69 | 2.17 | −3.72 | 0.6156 (NS) |
| Landing body mass, g | g | 19.54 | 1.25 | 20.11 | 1.94 | 2.90 | 0.6955 (NS) |
| Delta body mass at landing, g | g | −0.91 | 1.11 | 0.42 | 1.14 | − | 0.2232 (NS) |
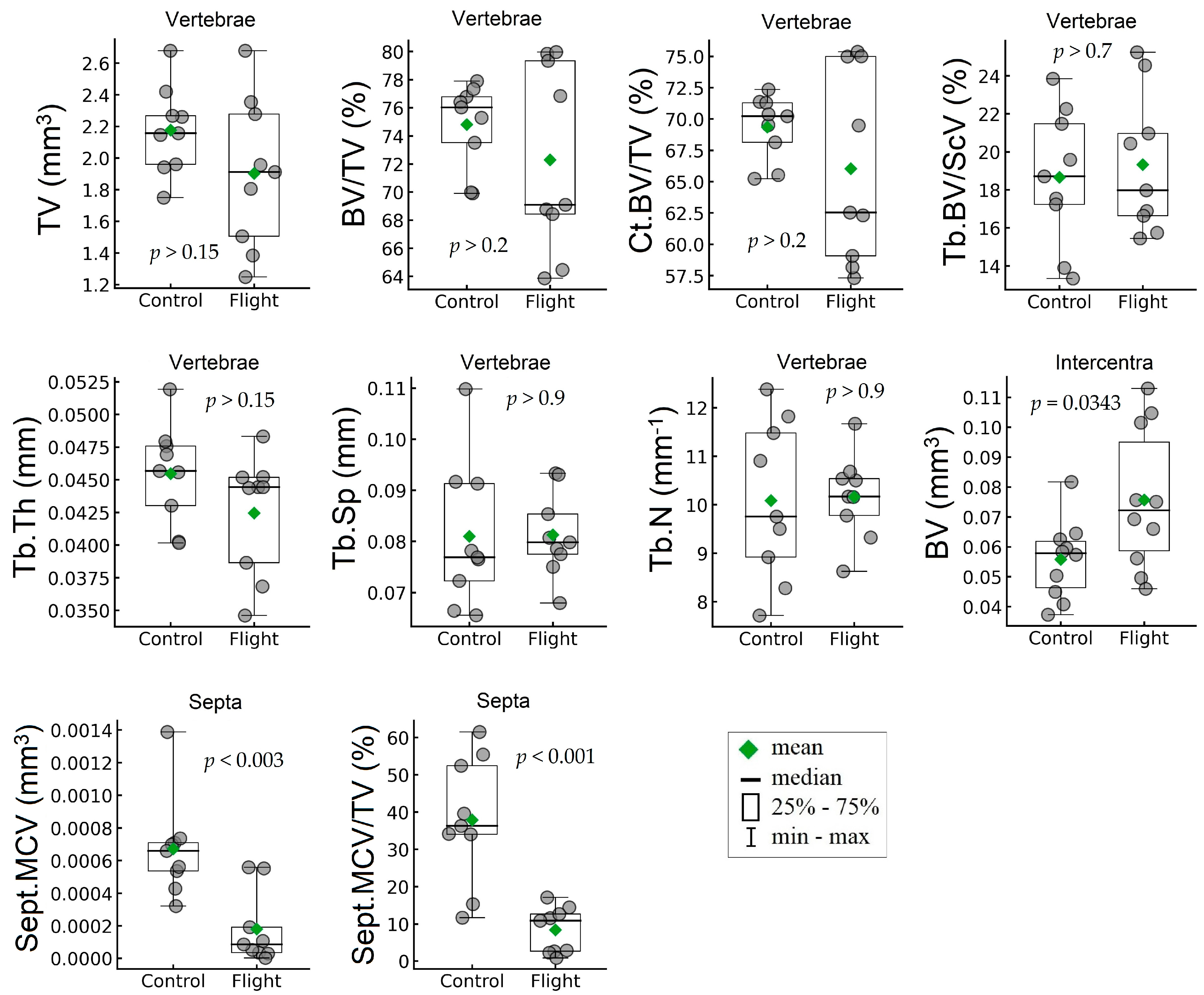
| Whole Vertebrae | |||||||
| TV, mm3 | mm3 | 2.18 | 0.28 | 1.90 | 0.48 | −12.55 | 0.1608 (NS) |
| BV/TV, % | % | 74.80 | 3.020 | 72.30 | 6.67 | −3.35 | 0.3266 (NS) |
| Ct.BV/TV, % | % | 69.34 | 2.54 | 66.033 | 7.68 | −4.77 | 0.2486 (NS) |
| Tb.BV/Sc.V, % | % | 18.66 | 3.58 | 19.33 | 3.69 | 3.57 | 0.7028 (NS) |
| Ventral side of vertebrae | |||||||
| Tb.Th, mm | mm | 0.046 | 0.0038 | 0.043 | 0.0046 | −6.61 | 0.1505 (NS) |
| Tb.Sp, mm | mm | 0.081 | 0.014 | 0.081 | 0.0082 | 0.36 | 0.9580 (NS) |
| Tb.N, mm−1 | mm−1 | 10.085 | 1.64 | 10.16 | 0.87 | 0.75 | 0.9045 (NS) |
| Mineralized cartilage of septa | |||||||
| Sept.MCV, mm3 | mm3 | 0.00067 | 0.0003 | 0.00018 | 0.00022 | −73.19 | 0.0027 |
| Sept.MCV/TV, % | % | 37.86 | 16.98 | 8.40 | 6.17 | −77.83 | 0.0006 |
| Intercentra | |||||||
| BV, mm3 | mm3 | 0.056 | 0.013 | 0.076 | 0.024 | 35.73 | 0.0343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukreeva, I.; Gulimova, V.I.; Krivonosov, Y.S.; Buzmakov, A.V.; Junemann, O.; Cedola, A.; Fratini, M.; Maugeri, L.; Begani Provinciali, G.; Palermo, F.; et al. The Study of the Caudal Vertebrae of Thick-Toed Geckos after a Prolonged Space Flight by X-ray Phase-Contrast Micro-CT. Cells 2023, 12, 2415. https://doi.org/10.3390/cells12192415
Bukreeva I, Gulimova VI, Krivonosov YS, Buzmakov AV, Junemann O, Cedola A, Fratini M, Maugeri L, Begani Provinciali G, Palermo F, et al. The Study of the Caudal Vertebrae of Thick-Toed Geckos after a Prolonged Space Flight by X-ray Phase-Contrast Micro-CT. Cells. 2023; 12(19):2415. https://doi.org/10.3390/cells12192415
Chicago/Turabian StyleBukreeva, Inna, Victoria I. Gulimova, Yuri S. Krivonosov, Alexey V. Buzmakov, Olga Junemann, Alessia Cedola, Michela Fratini, Laura Maugeri, Ginevra Begani Provinciali, Francesca Palermo, and et al. 2023. "The Study of the Caudal Vertebrae of Thick-Toed Geckos after a Prolonged Space Flight by X-ray Phase-Contrast Micro-CT" Cells 12, no. 19: 2415. https://doi.org/10.3390/cells12192415
APA StyleBukreeva, I., Gulimova, V. I., Krivonosov, Y. S., Buzmakov, A. V., Junemann, O., Cedola, A., Fratini, M., Maugeri, L., Begani Provinciali, G., Palermo, F., Sanna, A., Pieroni, N., Asadchikov, V. E., & Saveliev, S. V. (2023). The Study of the Caudal Vertebrae of Thick-Toed Geckos after a Prolonged Space Flight by X-ray Phase-Contrast Micro-CT. Cells, 12(19), 2415. https://doi.org/10.3390/cells12192415


.png)




