The Roles of Bone Marrow-Derived Stem Cells in Coronary Collateral Growth Induced by Repetitive Ischemia
Abstract
:1. Introduction
2. Materials and Methods
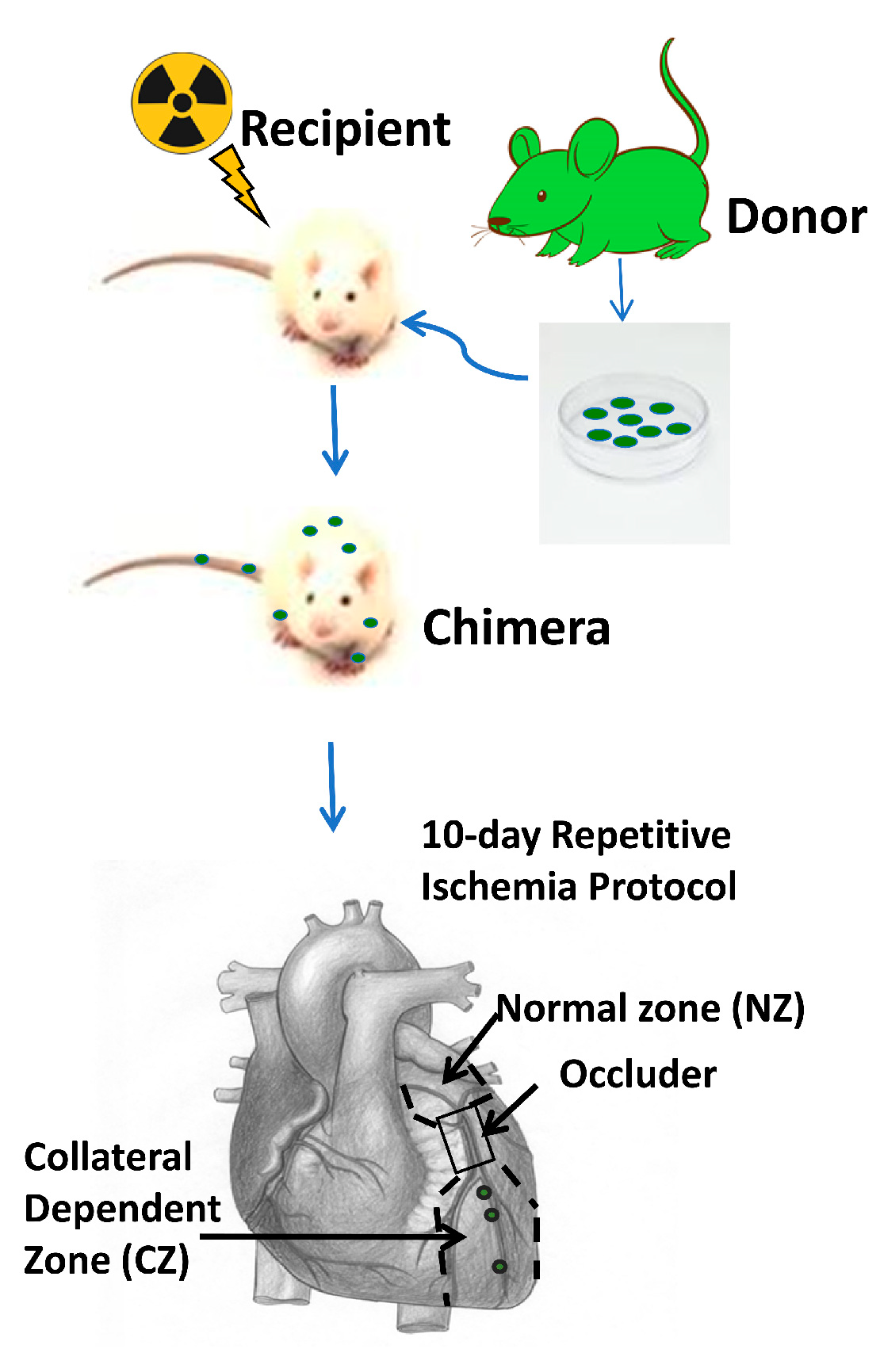
2.1. Animals
2.2. Bone Marrow Collection
2.3. Bone Marrow Transplantation
2.4. Flow Cytometry and Sorting
2.5. Rat Model of Coronary Collateral Growth Induced by Repetitive Ischemia (RI)
2.6. Echocardiographic Analysis of Cardiac Function
2.7. Measurements of Myocardial Blood Flow
2.8. Immunostaining and Imaging
2.9. Retrograde Microfil Perfusion of Rat Hearts and Micro-Computed Tomographic (Micro-CT) Analysis
2.10. Statistics
3. Results
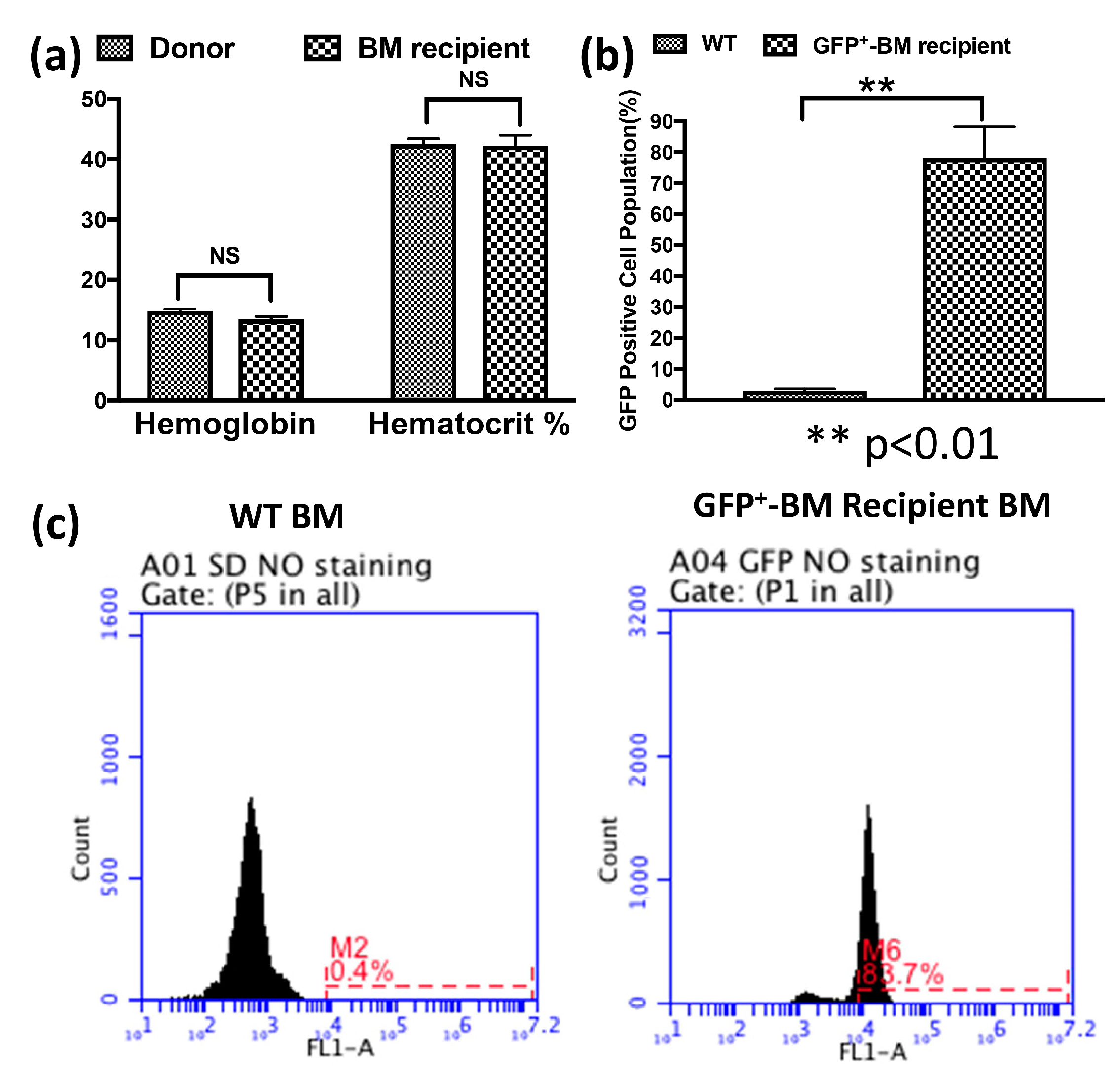
3.1. Bone Marrow Stem Cells (BMSCs) Rescued the Recipient Rats from Lethal Irradiation after Transplantation and Repopulated the Chimeras’ Bone Marrow
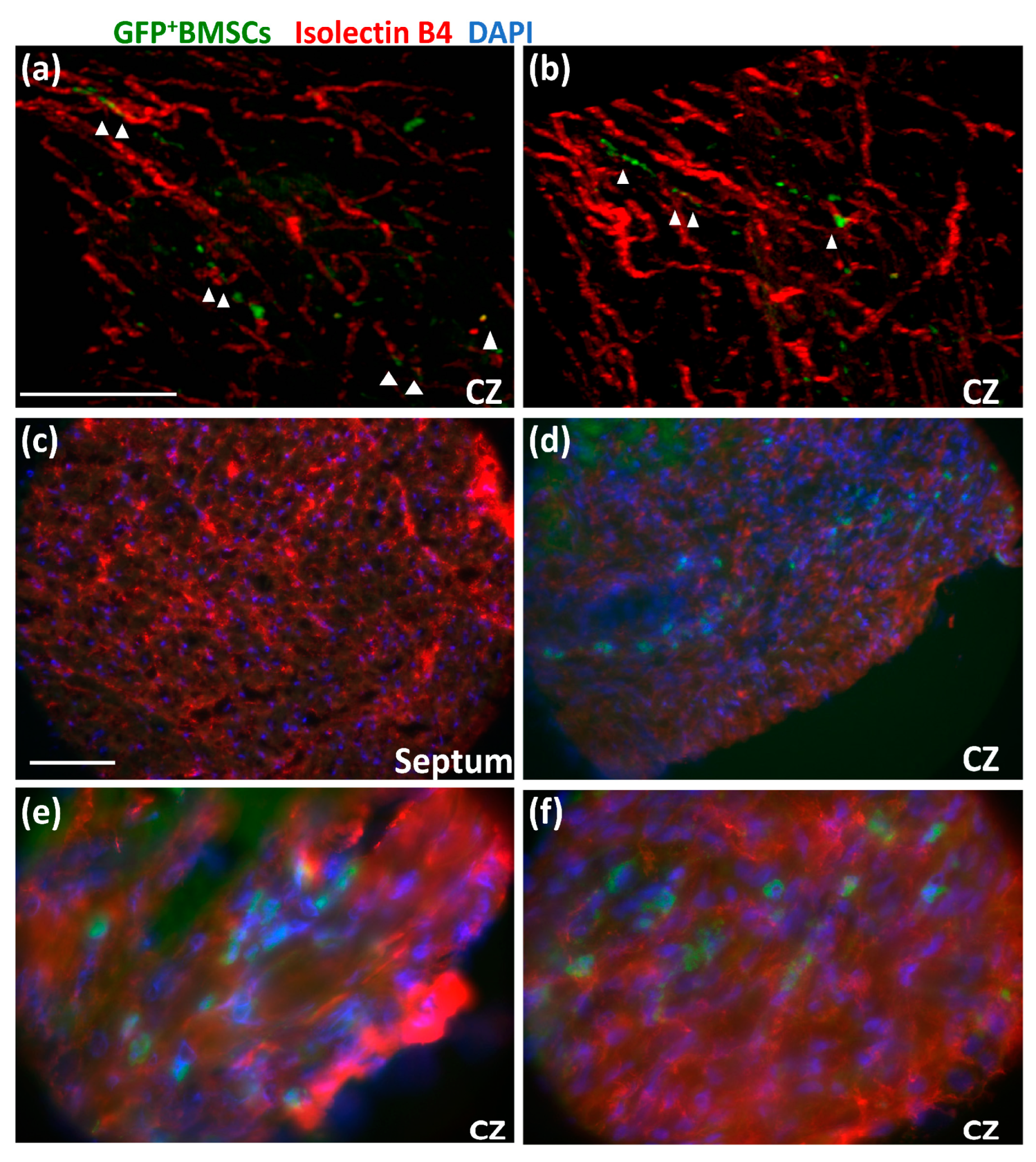
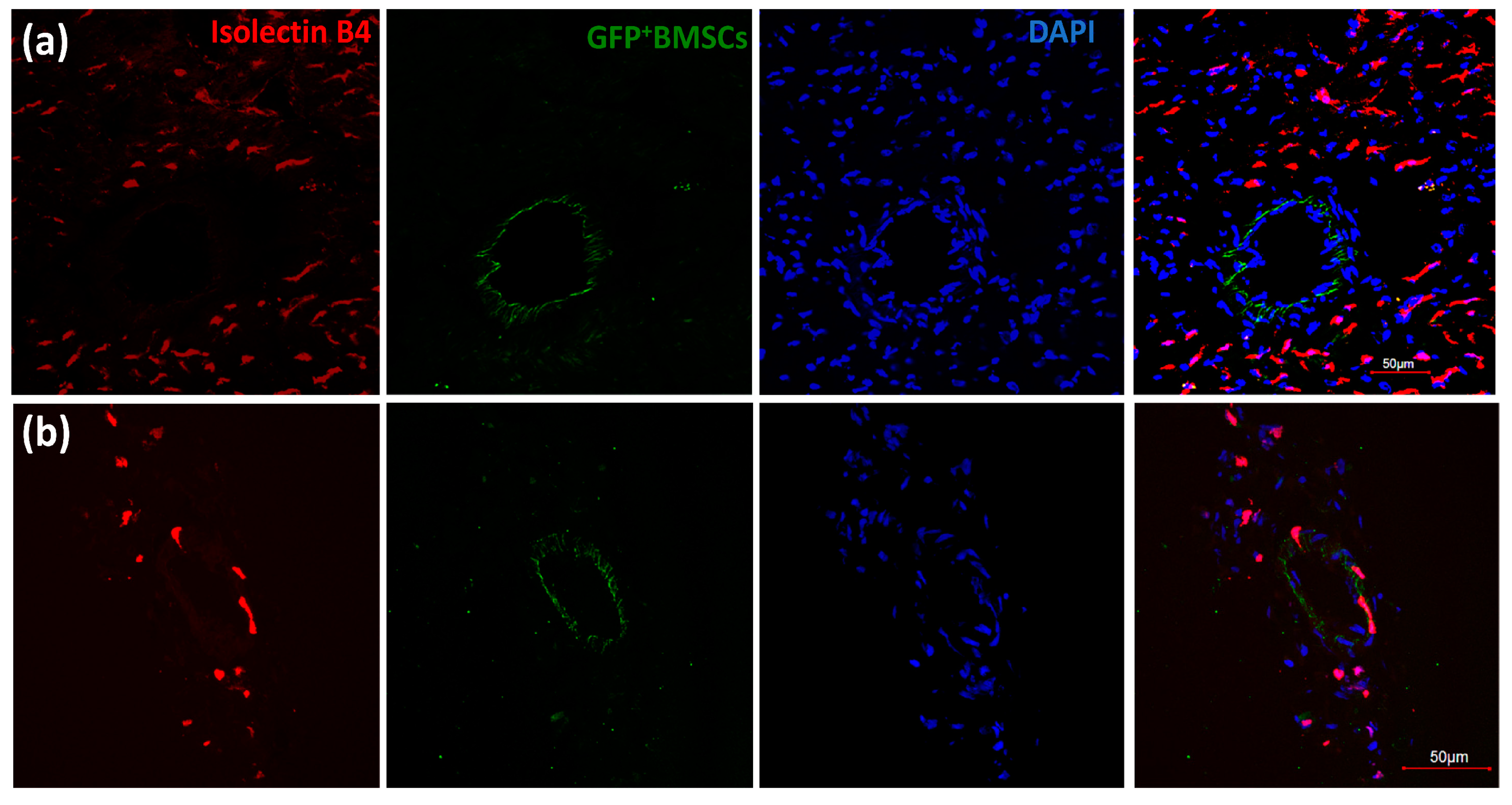
3.2. GFP+ BMSCs Engrafted in the Collateral-Dependent Zone of Chimeric Rat Hearts and Differentiated into Endothelial Cells
3.3. Engrafted GFP+ BMSCs Proliferated during RI
3.4. More c-kit+GFP+ BMSCs Homed for CCG during RI
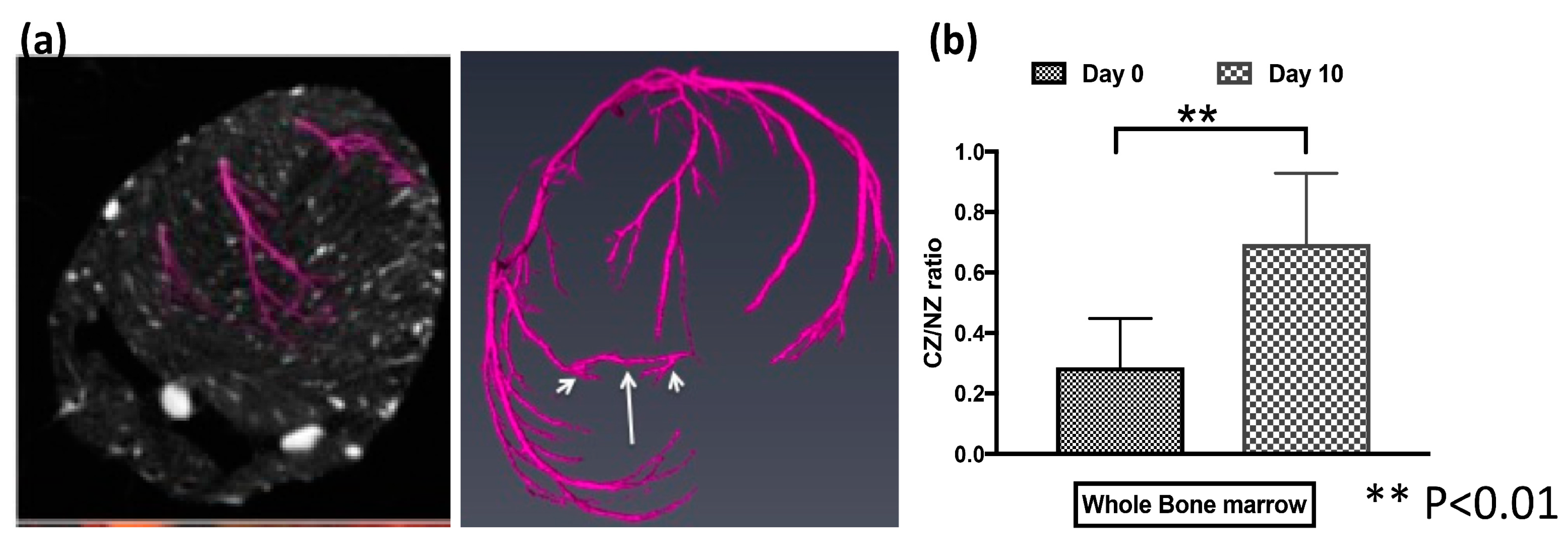
3.5. BMSCs Contributed to CCG
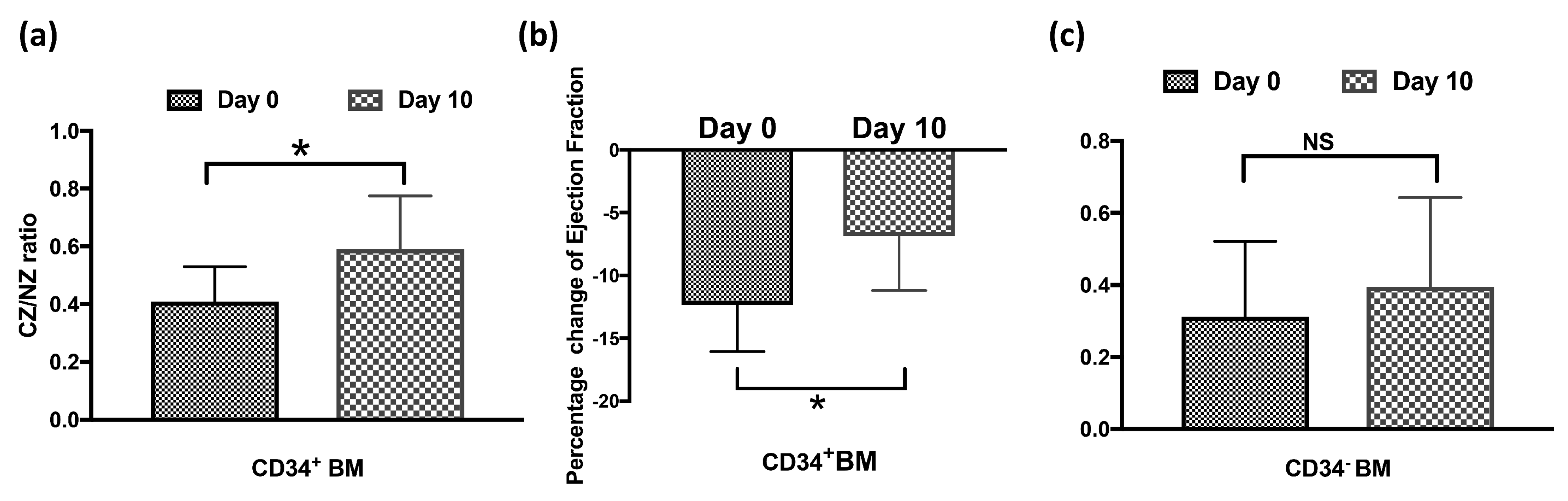
3.6. CD34+GFP+ BMSCs Are Essential to CCG
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaper, W. Collateral circulation: Past and present. Basic Res. Cardiol. 2009, 104, 5–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, P.R.; Smart, N. Vascularizing the heart. Cardiovasc. Res. 2011, 91, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilian, W.M.; Penn, M.S.; Pung, Y.F.; Dong, F.; Mayorga, M.; Ohanyan, V.; Logan, S.; Yin, L. Coronary collateral growth—Back to the future. J. Mol. Cell. Cardiol. 2012, 52, 905–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaper, W.; Gorge, G.; Winkler, B.; Schaper, J. The collateral circulation of the heart. Prog. Cardiovasc. Dis. 1988, 31, 57–77. [Google Scholar] [CrossRef]
- van Royen, N.; Piek, J.J.; Schaper, W.; Fulton, W.F. A critical review of clinical arteriogenesis research. J. Am. Coll. Cardiol. 2009, 55, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Sasayama, S. Reappraisal of functional importance of coronary collateral circulation. Cardiology 2010, 117, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Pung, Y.F.; Chilian, W.M. Corruption of coronary collateral growth in metabolic syndrome: Role of oxidative stress. World J. Cardiol. 2010, 2, 421–427. [Google Scholar] [CrossRef]
- Jamaiyar, A.; Juguilon, C.; Wan, W.; Richardson, D.; Chinchilla, S.; Gadd, J.; Enrick, M.; Wang, T.; McCabe, C.; Wang, Y.; et al. The essential role for endothelial cell sprouting in coronary collateral growth. J. Mol. Cell. Cardiol. 2022, 165, 158–171. [Google Scholar] [CrossRef]
- Yin, L.; Ohanyan, V.; Pung, Y.F.; DeLucia, A.; Bailey, E.; Enrick, M.; Stevanov, K.; Kolz, C.L.; Guarini, G.; Chilian, W.M. Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth. Circ. Res. 2012, 110, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Ohanyan, V.; Yin, L.; Bardakjian, R.; Kolz, C.; Enrick, M.; Hakobyan, T.; Luli, J.; Graham, K.; Khayata, M.; Logan, S.; et al. Kv1.3 channels facilitate the connection between metabolism and blood flow in the heart. Microcirculation 2017, 24, e12334. [Google Scholar] [CrossRef]
- Jamaiyar, A.; Wan, W.; Ohanyan, V.; Enrick, M.; Janota, D.; Cumpston, D.; Song, H.; Stevanov, K.; Kolz, C.L.; Hakobyan, T.; et al. Alignment of inducible vascular progenitor cells on a micro-bundle scaffold improves cardiac repair following myocardial infarction. Basic Res. Cardiol. 2017, 112, 41. [Google Scholar] [CrossRef]
- Hernandez, D.R.; Artiles, A.; Duque, J.C.; Martinez, L.; Pinto, M.T.; Webster, K.A.; Velazquez, O.C.; Vazquez-Padron, R.I.; Lassance-Soares, R.M. Loss of c-Kit function impairs arteriogenesis in a mouse model of hindlimb ischemia. Surgery 2018, 163, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-H.; Chen, Y.-H.; Wang, C.-H.; Chen, J.-S.; Tsai, H.-Y.; Lin, F.-Y.; Lo, W.-Y.; Wu, T.-C.; Sata, M.; Chen, J.-W.; et al. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arter. Thromb. Vasc. Biol. 2009, 29, 1179–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maliken, B.D.; Kanisicak, O.; Karch, J.; Khalil, H.; Fu, X.; Boyer, J.G.; Prasad, V.; Zheng, Y.; Molkentin, J.D. Gata4-Dependent Differentiation of c-Kit+-Derived Endothelial Cells Underlies Artefactual Cardiomyocyte Regeneration in the Heart. Circulation 2018, 138, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ge, J.; Zhao, L.; Qian, J.; Huang, Z.; Shen, L.; Sun, A.; Wang, K.; Zou, Y. Host vascular niche contributes to myocardial repair induced by intracoronary transplantation of bone marrow CD34+ progenitor cells in infarcted swine heart. Stem Cells 2007, 25, 1195–1203. [Google Scholar] [CrossRef]
- Wahid, F.S.A.; Ismail, N.A.; Wan Jamaludin, W.F.; Muhamad, N.A.; Mohamad Idris, M.A.; Lai, N.M. Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis. Curr. Stem Cell Res. Ther. 2018, 13, 265–283. [Google Scholar] [CrossRef]
- Tokgozoglu, L.; Yorgun, H.; Gurses, K.M.; Canpolat, U.; Ateş, A.H.; Tulumen, E.; Kaya, E.B.; Aytemir, K.; Kabakcı, G.; Tuncer, M.; et al. The association between circulating endothelial progenitor cells and coronary collateral formation. Atherosclerosis 2011, 219, 851–854. [Google Scholar] [CrossRef]
- Kocaman, S.A.; Yalcin, M.R.; Yagci, M.; Sahinarslan, A.; Turkoglu, S.; Arslan, U.; Kurşunluoğlu, N.; Özdemir, M.; Timurkaynak, T.; Cemri, M.; et al. Endothelial progenitor cells (CD34+KDR+) and monocytes may provide the development of good coronary collaterals despite the vascular risk factors and extensive atherosclerosis. Anatol. J. Cardiol. 2011, 11, 290–299. [Google Scholar] [CrossRef]
- Aghajanian, A.; Zhang, H.; Buckley, B.K.; Wittchen, E.S.; Ma, W.Y.; Faber, J.E. Decreased inspired oxygen stimulates de novo formation of coronary collaterals in adult heart. J. Mol. Cell. Cardiol. 2021, 150, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Faber, J.E. De-novo collateral formation following acute myocardial infarction: Dependence on CCR2+ bone marrow cells. J. Mol. Cell. Cardiol. 2015, 87, 4–16. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, J.; Chen, Y.; Chang, S.; Li, C.; Lu, H.; Ren, D.; Zhang, F.; Huang, Z.; Qian, J.; et al. Circulating CD34+VEGFR-2+ endothelial progenitor cells correlate with revascularization-mediated long-term improvement of cardiac function in patients with coronary chronic total occlusions. Int. J. Cardiol. 2021, 322, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Koyama, H.; Nishiyama, A.; Shigematsu, K.; Miyata, T.; Watanabe, T. Adequate Selection of a Therapeutic Site Enables Efficient Development of Collateral Vessels in Angiogenic Treatment with Bone Marrow Mononuclear Cells. J. Am. Hear. Assoc. 2015, 4, e002287. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Tao, J.; Yang, L.; Zhang, E.; Boriboun, C.; Zhou, J.; Sun, T.; Cheng, M.; Huang, K.; Shi, J.; et al. E2F1 Suppresses Oxidative Metabolism and Endothelial Differentiation of Bone Marrow Progenitor Cells. Circ. Res. 2018, 122, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhong, J.; Ye, J.; He, Y.; Liang, Z.; Cheng, Y.; Zheng, J.; Chen, H.; Chen, C. miR-324-5p protects against oxidative stress-induced endothelial progenitor cell injury by targeting Mtfr1. J. Cell. Physiol. 2019, 234, 22082–22092. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhao, J.; Zhang, Z.J.; Zhao, Q. MiR-183-5p overexpression in bone mesenchymal stem cell-derived exosomes protects against myocardial ischemia/reperfusion injury by targeting FOXO1. Immunobiology 2022, 227, 152204. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Q.; Liu, X.; Zhang, T.; Wang, S.; Zhou, L.; Zou, L.; Fan, F.; Chi, H.; Sun, J.; et al. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int. Immunopharmacol. 2021, 101, 107592. [Google Scholar] [CrossRef]
- Hakimzadeh, N.; Elias, J.; Wijntjens, G.W.; Theunissen, R.; van Weert, A.; Smulders, M.W.; van den Akker, N.; Moerland, P.D.; Verberne, H.J.; Hoebers, L.P.; et al. Monocytic microRNA profile associated with coronary collateral artery function in chronic total occlusion patients. Sci. Rep. 2017, 7, 1532. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enrick, M.; Jamaiyar, A.; Ohanyan, V.; Juguilon, C.; Kolz, C.; Shi, X.; Janota, D.; Wan, W.; Richardson, D.; Stevanov, K.; et al. The Roles of Bone Marrow-Derived Stem Cells in Coronary Collateral Growth Induced by Repetitive Ischemia. Cells 2023, 12, 242. https://doi.org/10.3390/cells12020242
Enrick M, Jamaiyar A, Ohanyan V, Juguilon C, Kolz C, Shi X, Janota D, Wan W, Richardson D, Stevanov K, et al. The Roles of Bone Marrow-Derived Stem Cells in Coronary Collateral Growth Induced by Repetitive Ischemia. Cells. 2023; 12(2):242. https://doi.org/10.3390/cells12020242
Chicago/Turabian StyleEnrick, Molly, Anurag Jamaiyar, Vahagn Ohanyan, Cody Juguilon, Christopher Kolz, Xin Shi, Danielle Janota, Weiguo Wan, Devan Richardson, Kelly Stevanov, and et al. 2023. "The Roles of Bone Marrow-Derived Stem Cells in Coronary Collateral Growth Induced by Repetitive Ischemia" Cells 12, no. 2: 242. https://doi.org/10.3390/cells12020242
APA StyleEnrick, M., Jamaiyar, A., Ohanyan, V., Juguilon, C., Kolz, C., Shi, X., Janota, D., Wan, W., Richardson, D., Stevanov, K., Hakobyan, T., Shockling, L., Diaz, A., Usip, S., Dong, F., Zhang, P., Chilian, W. M., & Yin, L. (2023). The Roles of Bone Marrow-Derived Stem Cells in Coronary Collateral Growth Induced by Repetitive Ischemia. Cells, 12(2), 242. https://doi.org/10.3390/cells12020242






