A CD19-Anti-ErbB2 scFv Engager Protein Enables CD19-Specific CAR T Cells to Eradicate ErbB2+ Solid Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Preparation of the Human T Cells
2.3. CAR T Cell Generation
2.4. Generation and Expression of the CD19-Fusion Proteins
2.5. Flow Cytometry and Cell Sorting
2.6. Antigen Specific CAR T Cell Activation
2.7. ELISA
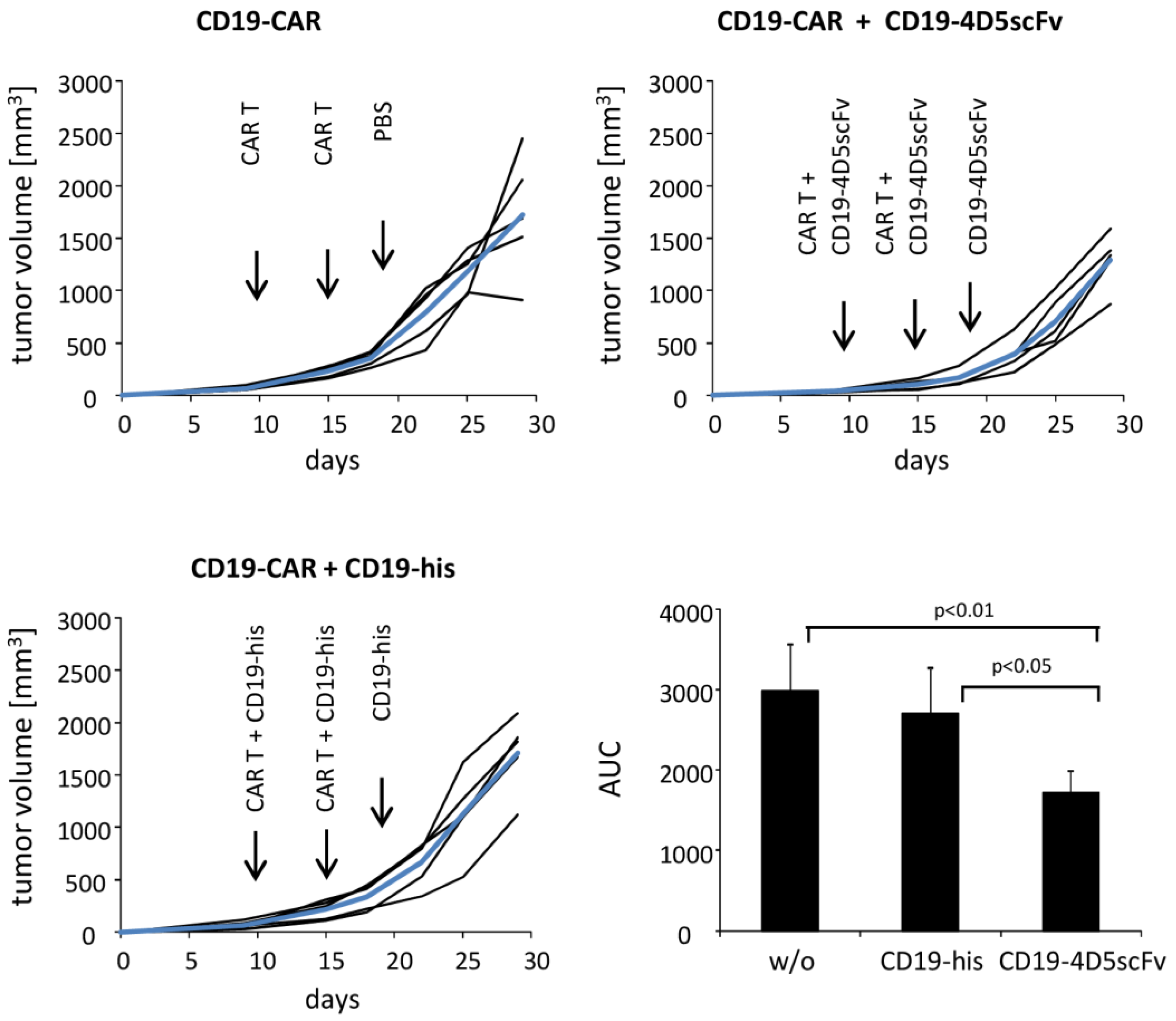
2.8. In Vivo Suppression of the SKOV3 Tumors by the CD19-4D5scFv Redirected Anti-CD19 CAR T Cells
2.9. Statistics
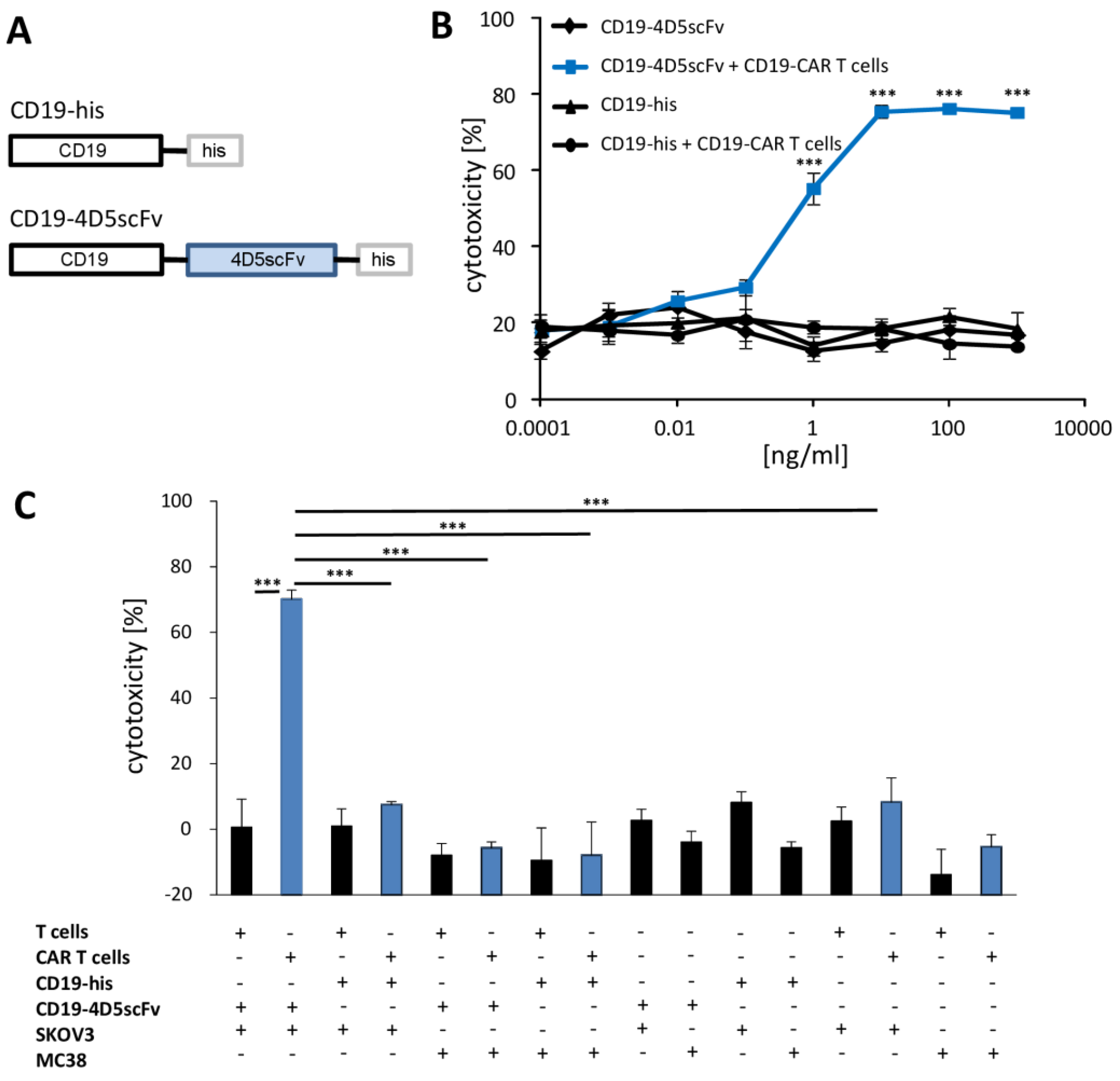
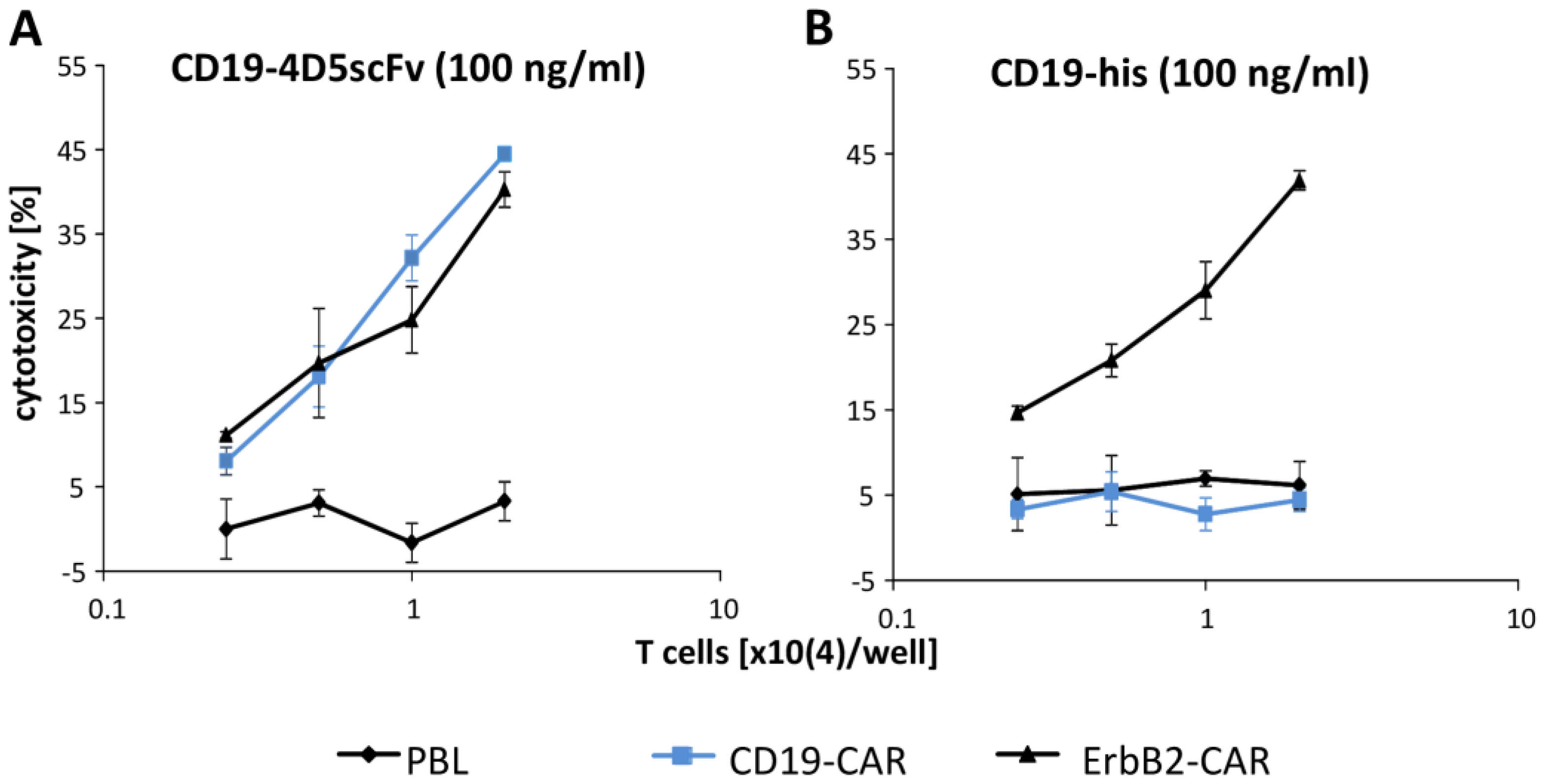
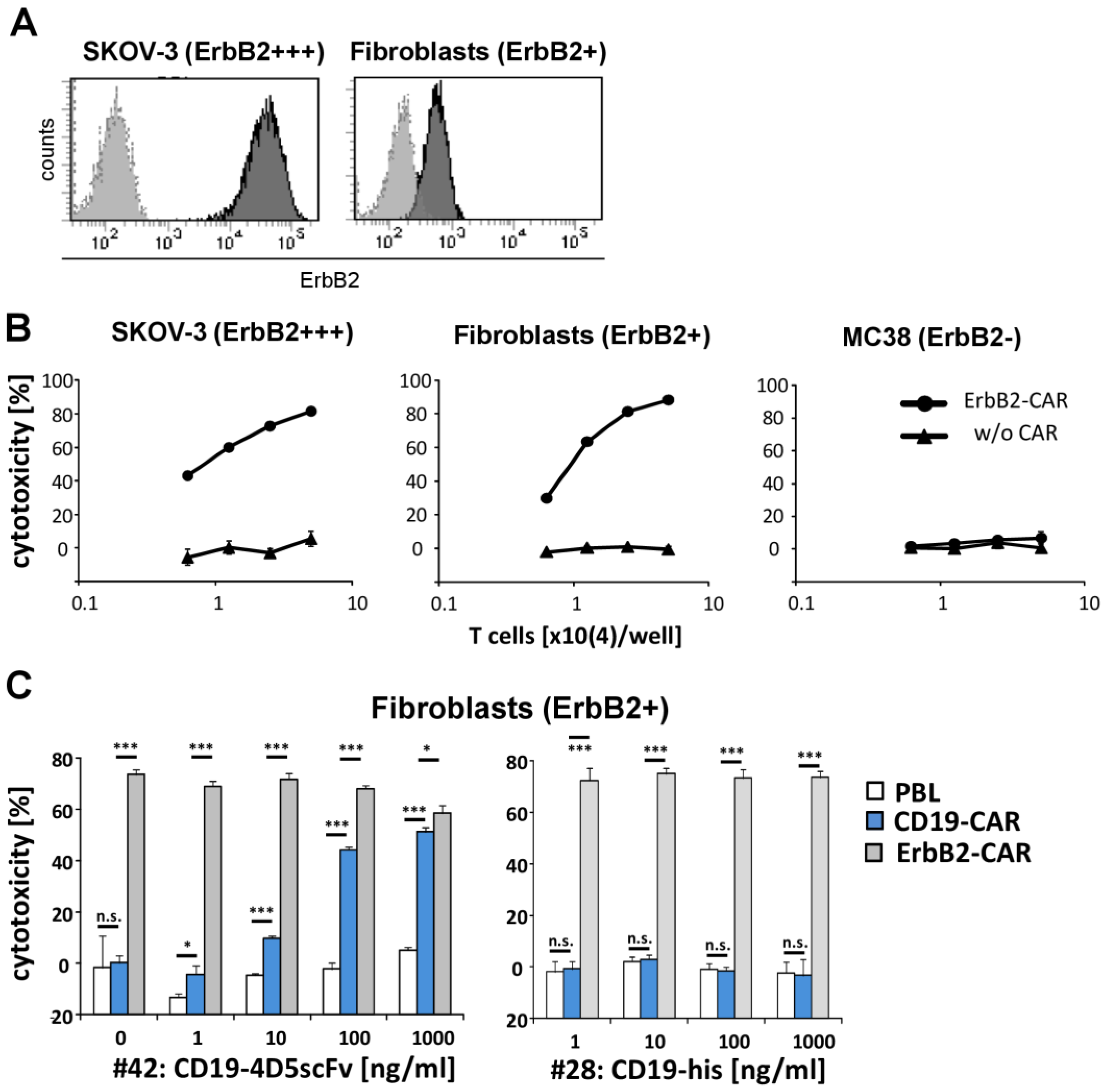
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sermer, D.; Brentjens, R. CAR T-Cell Therapy: Full Speed Ahead. Hematol. Oncol. 2019, 37 (Suppl. S1), 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current Landscape and Future Directions of Bispecific Antibodies in Cancer Immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef] [PubMed]
- Nagorsen, D.; Bargou, R.; Ruttinger, D.; Kufer, P.; Baeuerle, P.A.; Zugmaier, G. Immunotherapy of Lymphoma and Leukemia with T-Cell Engaging BiTE Antibody Blinatumomab. Leuk. Lymphoma 2009, 50, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Crocenzi, T.; Lohr, J.; Bonvini, E.; Johnson, S.; Moore, P.; Wigginton, J. A Phase I, First-in-Human, Open Label, Dose Escalation Study of MGD007, a Humanized GpA33 × CD3 Dual-Affinity Re-Targeting (DART®) Protein in Patients with Relapsed/Refractory Metastatic Colorectal Carcinoma. J. Immunother. Cancer 2014, 2, P86. [Google Scholar] [CrossRef] [Green Version]
- Gedeon, P.C.; Schaller, T.H.; Chitneni, S.K.; Choi, B.D.; Kuan, C.-T.; Suryadevara, C.M.; Snyder, D.J.; Schmittling, R.J.; Szafranski, S.E.; Cui, X.; et al. A Rationally Designed Fully Human EGFRvIII:CD3-Targeted Bispecific Antibody Redirects Human T Cells to Treat Patient-Derived Intracerebral Malignant Glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3611–3631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, M.; Raum, T.; Lutterbuese, R.; Voelkel, M.; Deegen, P.; Rau, D.; Kischel, R.; Hoffmann, P.; Brandl, C.; Schuhmacher, J.; et al. Regression of Human Prostate Cancer Xenografts in Mice by AMG 212/BAY2010112, a Novel PSMA/CD3-Bispecific BiTE Antibody Cross-Reactive with Non-Human Primate Antigens. Mol. Cancer Ther. 2012, 11, 2664–2673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, T.; Sano, Y.; Komatsu, S.-I.; Kamata-Sakurai, M.; Kaneko, A.; Kinoshita, Y.; Shiraiwa, H.; Azuma, Y.; Tsunenari, T.; Kayukawa, Y.; et al. An Anti-Glypican 3/CD3 Bispecific T Cell-Redirecting Antibody for Treatment of Solid Tumors. Sci. Transl. Med. 2017, 9, eaal4291. [Google Scholar] [CrossRef] [Green Version]
- Slaga, D.; Ellerman, D.; Lombana, T.N.; Vij, R.; Li, J.; Hristopoulos, M.; Clark, R.; Johnston, J.; Shelton, A.; Mai, E.; et al. Avidity-Based Binding to HER2 Results in Selective Killing of HER2-Overexpressing Cells by Anti-HER2/CD3. Sci. Transl. Med. 2018, 10, eaat5775. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, J.; Bae, J.; Li, H.; Sun, Z.; Moore, C.; Hsu, E.; Han, C.; Qiao, J.; Fu, Y.-X. Rejuvenation of Tumour-Specific T Cells through Bispecific Antibodies Targeting PD-L1 on Dendritic Cells. Nat. Biomed. Eng. 2021, 5, 1261–1273. [Google Scholar] [CrossRef]
- Seung, E.; Xing, Z.; Wu, L.; Rao, E.; Cortez-Retamozo, V.; Ospina, B.; Chen, L.; Beil, C.; Song, Z.; Zhang, B.; et al. A Trispecific Antibody Targeting HER2 and T Cells Inhibits Breast Cancer Growth via CD4 Cells. Nature 2022, 603, 328–334. [Google Scholar] [CrossRef]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977, 36, 59–74. [Google Scholar] [CrossRef]
- Martyniszyn, A.; Krahl, A.-C.; André, M.C.; Hombach, A.A.; Abken, H. CD20-CD19 Bispecific CAR T Cells for the Treatment of B-Cell Malignancies. Hum. Gene Ther. 2017, 28, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Schmidt, P.; Hombach, A.A.; Hallek, M.; Abken, H. Engineered T Cells for the Adoptive Therapy of B-Cell Chronic Lymphocytic Leukaemia. Adv. Hematol. 2012, 2012, 595060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Textor, A.; Listopad, J.J.; Wührmann, L.L.; Perez, C.; Kruschinski, A.; Chmielewski, M.; Abken, H.; Blankenstein, T.; Charo, J. Efficacy of CAR T-Cell Therapy in Large Tumors Relies upon Stromal Targeting by IFNγ. Cancer Res. 2014, 74, 6796–6805. [Google Scholar] [CrossRef] [Green Version]
- Hombach, A.; Wieczarkowiecz, A.; Marquardt, T.; Heuser, C.; Usai, L.; Pohl, C.; Seliger, B.; Abken, H. Tumor-Specific T Cell Activation by Recombinant Immunoreceptors: CD3 Zeta Signaling and CD28 Costimulation Are Simultaneously Required for Efficient IL-2 Secretion and Can Be Integrated into One Combined CD28/CD3 Zeta Signaling Receptor Molecule. J. Immunol. 2001, 167, 6123–6131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golumba-Nagy, V.; Kuehle, J.; Abken, H. Genetic Modification of T Cells with Chimeric Antigen Receptors: A Laboratory Manual. Hum. Gene Ther. Methods 2017, 28, 302–309. [Google Scholar] [CrossRef]
- Hombach, A.A.; Görgens, A.; Chmielewski, M.; Murke, F.; Kimpel, J.; Giebel, B.; Abken, H. Superior Therapeutic Index in Lymphoma Therapy: CD30(+) CD34(+) Hematopoietic Stem Cells Resist a Chimeric Antigen Receptor T-Cell Attack. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, C.; Su, L.; Wu, L.; Dufort, F.J.; Sanford, T.; Birt, A.; Hackel, B.J.; Hombach, A.; Abken, H.; Lobb, R.R.; et al. Anti-CD19 CAR T Cells Potently Redirected to Kill Solid Tumor Cells. PLoS ONE 2021, 16, e0247701. [Google Scholar] [CrossRef]
- Hombach, A.A.; Schildgen, V.; Heuser, C.; Finnern, R.; Gilham, D.E.; Abken, H. T Cell Activation by Antibody-like Immunoreceptors: The Position of the Binding Epitope within the Target Molecule Determines the Efficiency of Activation of Redirected T Cells. J. Immunol. 2007, 178, 4650–4657. [Google Scholar] [CrossRef] [Green Version]
- Jost, L.M.; Kirkwood, J.M.; Whiteside, T.L. Improved Short- and Long-Term XTT-Based Colorimetric Cellular Cytotoxicity Assay for Melanoma and Other Tumor Cells. J. Immunol. Methods 1992, 147, 153–165. [Google Scholar] [CrossRef]
- Duan, F.; Simeone, S.; Wu, R.; Grady, J.; Mandoiu, I.; Srivastava, P.K. Area under the Curve as a Tool to Measure Kinetics of Tumor Growth in Experimental Animals. J. Immunol. Methods 2012, 382, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Haso, W.M.; Shern, J.F.; Wanhainen, K.M.; Murgai, M.; Ingaramo, M.; Smith, J.P.; Walker, A.J.; Kohler, M.E.; Venkateshwara, V.R.; et al. 4-1BB Costimulation Ameliorates T Cell Exhaustion Induced by Tonic Signaling of Chimeric Antigen Receptors. Nat. Med. 2015, 21, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef] [Green Version]
- Finney, O.C.; Brakke, H.M.; Rawlings-Rhea, S.; Hicks, R.; Doolittle, D.; Lopez, M.; Futrell, R.B.; Orentas, R.J.; Li, D.; Gardner, R.A.; et al. CD19 CAR T Cell Product and Disease Attributes Predict Leukemia Remission Durability. J. Clin. Investig. 2019, 130, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.V.; Turtle, C.J. Toxicities of CD19 CAR-T Cell Immunotherapy. Am. J. Hematol. 2019, 94, S42–S49. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hombach, A.A.; Ambrose, C.; Lobb, R.; Rennert, P.; Abken, H. A CD19-Anti-ErbB2 scFv Engager Protein Enables CD19-Specific CAR T Cells to Eradicate ErbB2+ Solid Cancer. Cells 2023, 12, 248. https://doi.org/10.3390/cells12020248
Hombach AA, Ambrose C, Lobb R, Rennert P, Abken H. A CD19-Anti-ErbB2 scFv Engager Protein Enables CD19-Specific CAR T Cells to Eradicate ErbB2+ Solid Cancer. Cells. 2023; 12(2):248. https://doi.org/10.3390/cells12020248
Chicago/Turabian StyleHombach, Andreas A., Christine Ambrose, Roy Lobb, Paul Rennert, and Hinrich Abken. 2023. "A CD19-Anti-ErbB2 scFv Engager Protein Enables CD19-Specific CAR T Cells to Eradicate ErbB2+ Solid Cancer" Cells 12, no. 2: 248. https://doi.org/10.3390/cells12020248




