Madagascar Leaf-Tail Geckos (Uroplatus spp.) Share Independently Evolved Differentiated ZZ/ZW Sex Chromosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Material
2.2. Preparation of Chromosome Suspensions
2.3. Chromosome Staining and Karyotype Reconstruction
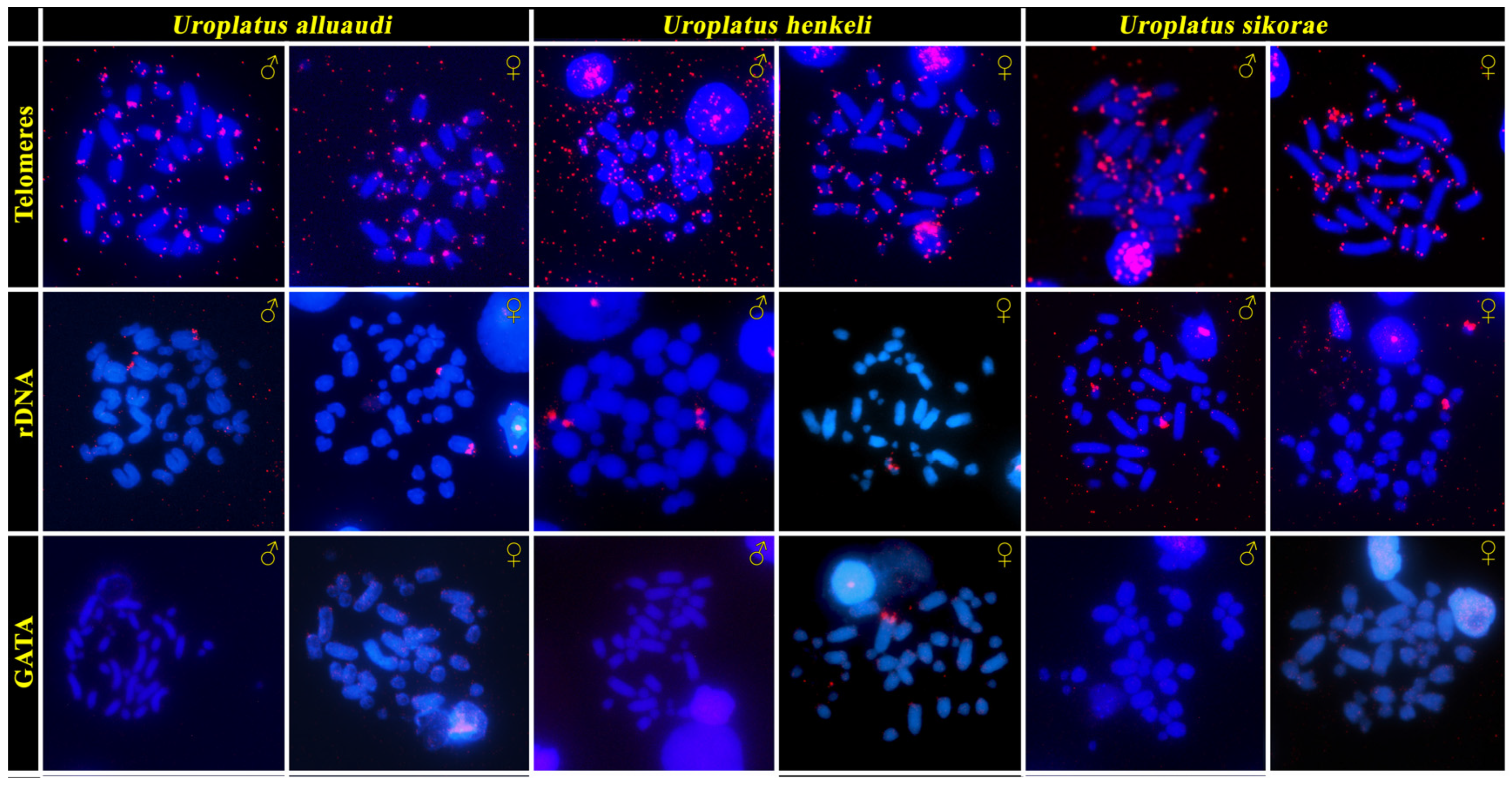
2.4. Fluorescence In Situ hybridization with Probe for Telomeric Sequences, 18S/28S rDNA Loci and GATA Microsatellite Motif
2.5. Comparative Gene Coverage Analysis
2.6. qPCR Validation of Z-Specific Genes and Test of Homology Across Geckos
3. Results
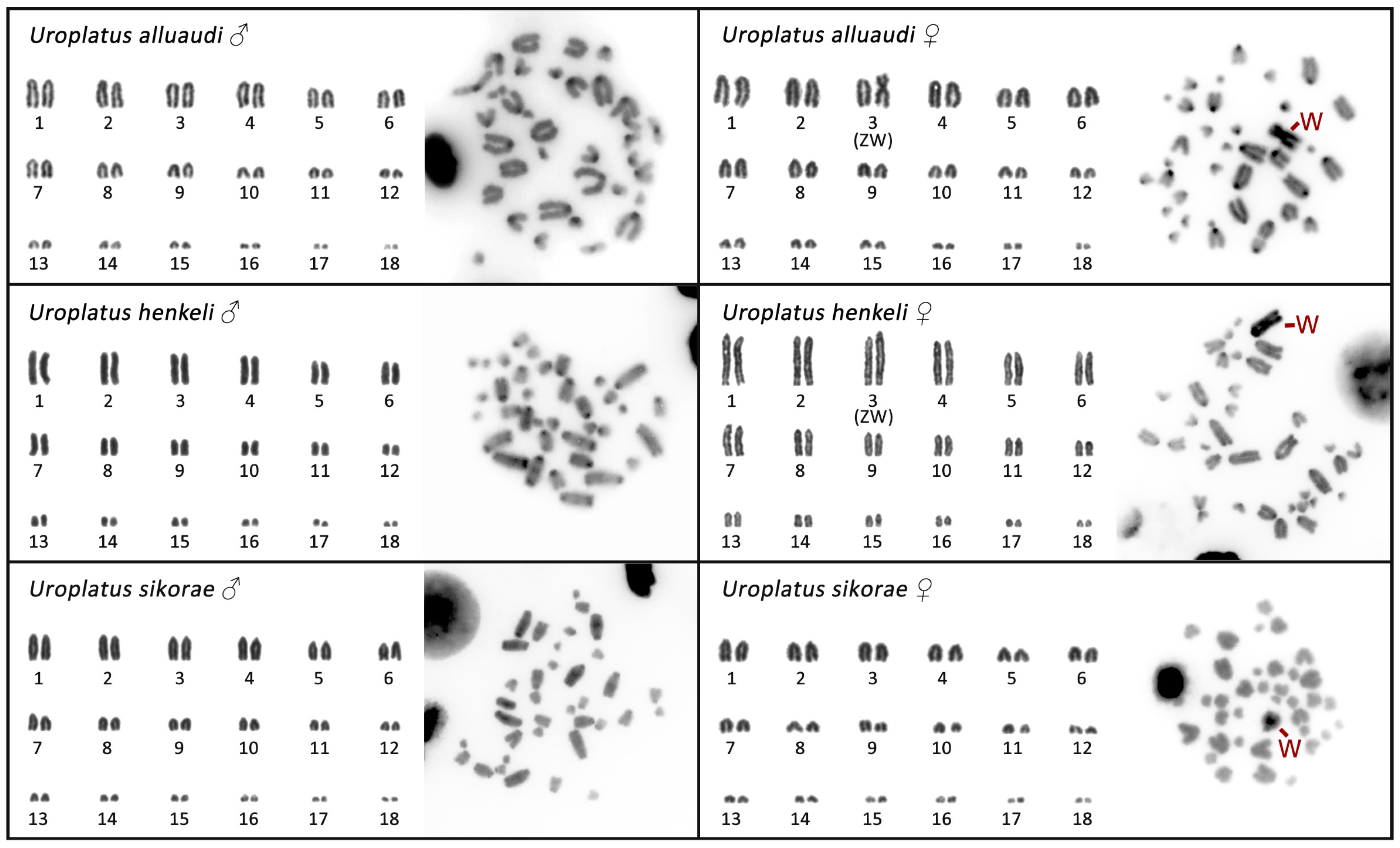
3.1. Cytogenetic Analysis
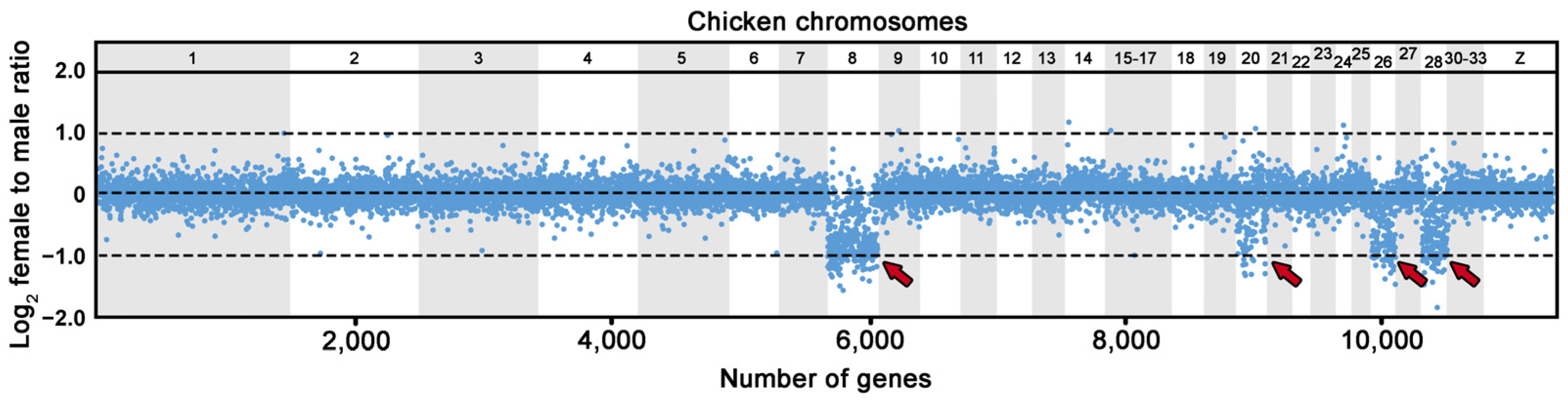
3.2. Comparative Gene Coverage Analysis
3.3. qPCR Validation of Z-Specific Markers and Test of Homology Across Geckos
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bull, J.J. Evolution of Sex Determining Mechanisms; Benjamin/Cumming: Menlo Park, CA, USA, 1983. [Google Scholar]
- Capel, B. Sex determination in vertebrates. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2019; Volume 134. [Google Scholar]
- Kratochvíl, L.; Stöck, M.; Rovatsos, M.; Bullejos, M.; Herpin, A.; Jeffries, D.L.; Peichel, C.L.; Perrin, N.; Valenzuela, N.; Johnson Pokorná, M. Expanding the classical paradigm: What we have learnt from vertebrates about sex chromosome evolution. Philos. Trans. R. Soc. B 2021, 376, 20200097. [Google Scholar] [CrossRef]
- Stöck, M.; Kratochvíl, L.; Kuhl, H.; Rovatsos, M.; Evans, B.J.; Suh, A.; Valenzuela, N.; Veyrunes, F.; Zhou, Q.; Gamble, T.; et al. A brief review of vertebrate sex evolution with a pledge for integrative research: Towards ‘sexomics’. Philos. Trans. R. Soc. B 2021, 376, 20200426. [Google Scholar] [CrossRef] [PubMed]
- Cortez, D.; Marin, R.; Toledo-Flores, D.; Froidevaux, L.; Liechti, A.; Waters, P.D.; Grützner, F.; Kaessmann, H. Origins and functional evolution of Y chromosomes across mammals. Nature 2014, 508, 488–493. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Bachtrog, D.; An, N.; Huang, Q.; Jarvis, E.D.; Gilbert, M.T.P.; Zhang, G. Complex evolutionary trajectories of sex chromosomes across bird taxa. Science 2014, 346, 1246338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovatsos, M.; Pokorná, M.; Altmanová, M.; Kratochvíl, L. Cretaceous park of sex determination: Sex chromosomes are conserved across iguanas. Biol. Lett. 2014, 10, 20131093. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Vukić, J.; Lymberakis, P.; Kratochvíl, L. Evolutionary stability of sex chromosomes in snakes. Proc. R. Soc. B 2015, 282, 20151992. [Google Scholar] [CrossRef] [Green Version]
- Iannucci, A.; Altmanová, M.; Ciofi, C.; Ferguson-Smith, M.; Milan, M.; Pereira, J.C.; Pether, J.; Rehák, I.; Rovatsos, M.; Stanyon, R.; et al. Conserved sex chromosomes and karyotype evolution in monitor lizards (Varanidae). Heredity 2019, 123, 215–227. [Google Scholar] [CrossRef]
- Kostmann, A.; Kratochvíl, L.; Rovatsos, M. Poorly differentiated XX/XY sex chromosomes are widely shared across skink radiation. Proc. R. Soc. B 2021, 288, 20202139. [Google Scholar] [CrossRef]
- Rovatsos, M.; Vukić, J.; Mrugała, A.; Suwala, G.; Lymberakis, P.; Kratochvíl, L. Little evidence for switches to environmental sex determination and turnover of sex chromosomes in lacertid lizards. Sci. Rep. 2019, 9, 7832. [Google Scholar] [CrossRef] [Green Version]
- Pennell, M.W.; Mank, J.E.; Peichel, C.L. Transitions in sex determination and sex chromosomes across vertebrate species. Mol. Ecol. 2018, 27, 3950–3963. [Google Scholar] [CrossRef]
- Sember, A.; Nguyen, P.; Perez, M.F.; Altmanová, M.; Ráb, P.; Cioffi, M.B. Multiple sex chromosomes in teleost fishes from a cytogenetic perspective: State of the art and future challenges. Philos. Trans. R. Soc. B 2021, 376, 20200098. [Google Scholar] [CrossRef]
- Kuhl, H.; Guiguen, Y.; Höhne, C.; Kreuz, E.; Du, K.; Klopp, C.; Lopez-Roques, C.; Yebra-Pimentel, E.S.; Ciorpac, M.; Gessner, J.; et al. A 180 Myr-old female-specific genome region in sturgeon reveals the oldest known vertebrate sex determining system with undifferentiated sex chromosomes. Philos. Trans. R. Soc. B 2021, 376, 20200089. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, D.L.; Lavanchy, G.; Sermier, R.; Sredl, M.J.; Miura, I.; Borzée, A.; Barrow, L.N.; Canestrelli, D.; Crochet, P.-A.; Dufresnes, C.; et al. A rapid rate of sex-chromosome turnover and non-random transitions in true frogs. Nat. Commun. 2018, 9, 4088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertho, S.; Herpin, A.; Schartl, M.; Guiguen, Y. Lessons from an unusual vertebrate sex-determining gene. Philos. Trans. R. Soc. B 2021, 376, 20200092. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Ieda, R.; Hosoya, S.; Fujikawa, D.; Atsumi, K.; Tajima, S.; Nozawa, A.; Koyama, T.; Hirase, S.; Nakamura, O.; et al. Repeated translocation of a supergene underlying rapid sex chromosome turnover in Takifugu pufferfish. Proc. Natl. Acad. Sci. USA 2022, 119, e2121469119. [Google Scholar] [CrossRef]
- Ross, J.A.; Urton, J.R.; Boland, J.; Shapiro, M.D.; Peichel, C.L. Turnover of sex chromosomes in the stickleback fishes (Gasterosteidae). PLoS Genet. 2009, 5, e1000391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myosho, T.; Takehana, Y.; Hamaguchi, S.; Sakaizumi, M. Turnover of sex chromosomes in celebensis group medaka fishes. G3 2015, 5, 2685–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Taher, A.; Ronco, F.; Matschiner, M.; Salzburger, W.; Böhne, A. Dynamics of sex chromosome evolution in a rapid radiation of cichlid fishes. Sci. Adv. 2021, 7, eabe8215. [Google Scholar] [CrossRef]
- Augstenová, B.; Pensabene, E.; Veselý, M.; Kratochvíl, L.; Rovatsos, M. Are geckos special in sex determination? Independently evolved differentiated ZZ/ZW sex chromosomes in carphodactylid geckos. Genome Biol. Evol. 2021, 13, evab119. [Google Scholar] [CrossRef]
- Gamble, T. A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex. Dev. 2010, 4, 88–103. [Google Scholar] [CrossRef]
- Gamble, T.; Coryell, J.; Ezaz, T.; Lynch, J.; Scantlebury, D.P.; Zarkower, D. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol. Biol. Evol. 2015, 32, 1296–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. (Eds.) The Reptile Database. Available online: https://www.reptile-database.org (accessed on 23 May 2021).
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokorná, M.; Kratochvíl, L. Phylogeny of sex-determining mechanisms in squamate reptiles: Are sex chromosomes an evolutionary trap? Zool. J. Linn. Soc. 2009, 156, 168–183. [Google Scholar] [CrossRef] [Green Version]
- Johnson Pokorná, M.; Kratochvíl, L. What was the ancestral sex-determining mechanism in amniote vertebrates? Biol. Rev. 2016, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, E.; Kratochvíl, L.; Rovatsos, M. Independent evolution of sex chromosomes in eublepharid geckos, a lineage with environmental and genotypic sex determination. Life 2020, 10, 342. [Google Scholar] [CrossRef]
- Keating, S.E.; Blumer, M.; Grismer, L.L.; Lin, A.; Nielsen, S.V.; Thura, M.K.; Wood, P.L., Jr.; Quah, E.S.H.; Gamble, T. Sex chromosome turnover in bent-toed geckos (Cyrtodactylus). Genes 2021, 12, 116. [Google Scholar] [CrossRef]
- Keating, S.E.; Greenbaum, E.; Johnson, J.D.; Gamble, T. Identification of a cis-sex chromosome transition in banded geckos (Coleonyx, Eublepharidae, Gekkota). J. Evol. Biol. 2022, 35, 1675–1682. [Google Scholar] [CrossRef]
- Pinto, B.J.; Keating, S.E.; Nielsen, S.V.; Scantlebury, D.P.; Daza, J.D.; Gamble, T. Chromosome-level genome assembly reveals dynamic sex chromosomes in neotropical leaf-litter geckos (Sphaerodactylidae: Sphaerodactylus). J. Hered. 2022, 113, 272–287. [Google Scholar] [CrossRef]
- Rovatsos, M.; Gamble, T.; Nielsen, S.V.; Georges, A.; Ezaz, T.; Kratochvíl, L. Do male and female heterogamety really differ in expression regulation? Lack of global dosage balance in pygopodid geckos. Philos. Trans. R. Soc. B 2021, 376, 20200102. [Google Scholar] [CrossRef]
- Ratsoavina, F.M.; Raminosoa, N.R.; Louis, E.E., Jr.; Raselimanana, A.P.; Glaw, F.; Vences, M. An overview of Madagascar’s leaf tailed geckos (genus Uroplatus): Species boundaries, candidate species and review of geographical distribution based on molecular data. Salamandra 2013, 49, 115–148. [Google Scholar]
- Solleder, E.; Schmid, M. XX/XY-sex chromosomes in Gekko gecko (Sauria, Reptilia). Amphib.-Reptil. 1984, 5, 339–345. [Google Scholar] [CrossRef]
- Tokunaga, S. Temperature-dependent sex determination in Gekko japonicus (Gekkonidae, Reptilia). Dev. Growth Differ. 1985, 27, 117–120. [Google Scholar] [CrossRef]
- Yoshida, M.; Itoh, M. Karyotype of the gecko, Gekko japonicus. Chrom. Info. Serv. 1974, 17, 29–31. [Google Scholar]
- Kawai, A.; Ishijima, J.; Nishida, C.; Kosaka, A.; Ota, H.; Kohno, S.; Matsuda, Y. The ZW sex chromosomes of Gekko hokouensis (Gekkonidae, Squamata) represent highly conserved homology with those of avian species. Chromosoma 2009, 118, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.H.; Yang, J.; Wang, J.; Ji, X. Offspring sex in a TSD gecko correlates with an interaction between incubation temperature and yolk steroid hormones. Naturwissenschaften 2012, 99, 999–1006. [Google Scholar] [CrossRef]
- Koubová, M.; Johnson Pokorná, M.; Rovatsos, M.; Farkačová, K.; Altmanová, M.; Kratochvíl, L. Sex determination in Madagascar geckos of the genus Paroedura (Squamata: Gekkonidae): Are differentiated sex chromosomes indeed so evolutionary stable? Chromosome Res. 2014, 22, 441–452. [Google Scholar] [CrossRef]
- Rovatsos, M.; Farkačová, K.; Altmanová, M.; Johnson Pokorná, M.; Kratochvíl, L. The rise and fall of differentiated sex chromosomes in geckos. Mol. Ecol. 2019, 28, 3042–3052. [Google Scholar] [CrossRef]
- Ota, H.; Hikida, T.; Matsui, M.; Mori, A. Karyotypes of two species of the genus Cyrtodactylus (Squamata: Gekkonidae) from Sarawak, Malaysia. Caryologia 1992, 45, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Hikida, T.; Nabhitabhata, J.; Panha, S. Cryptic taxonomic diversity in two broadly distributed lizards of Thailand (Mabuya macularia and Dixonius siamensis) as revealed by chromosomal investigations (Reptilia: Lacertilia). Nat. Hist. J. Chulalongkorn Univ. 2001, 1, 1–7. [Google Scholar]
- Trifonov, V.A.; Giovannotti, M.; O’Brien, P.C.; Wallduck, M.; Lovell, F.; Rens, W.; Parise-Maltempi, P.P.; Caputo, V.; Ferguson-Smith, M.A. Chromosomal evolution in Gekkonidae. I. Chromosome painting between Gekko and Hemidactylus species reveals phylogenetic relationships within the group. Chromosome Res. 2011, 19, 843–855. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Brunelli, E.; Odierna, G.; Guarino, F.M. First insights on the karyotype diversification of the endemic malagasy leaf-toed geckos (Squamata: Gekkonidae: Uroplatus). Animals 2022, 12, 2054. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Augstenová, B.; Clemente, L.; Auer, M.; Fritz, U.; Praschag, P.; Protiva, T.; Velenský, P.; Kratochvíl, L.; Rovatsos, M. Turtles of the genera Geoemyda and Pangshura (Testudines: Geoemydidae) lack differentiated sex chromosomes: The end of a 40-year error cascade for Pangshura. PeerJ 2019, 7, e6241. [Google Scholar] [CrossRef] [Green Version]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Ijdo, J.W.; Wells, R.A.; Baldini, A.; Reeders, S.T. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res. 1991, 19, 4780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovatsos, M.; Johnson Pokorná, M.; Altmanová, M.; Kratochvíl, L. Female heterogamety in Madagascar chameleons (Squamata: Chamaeleonidae: Furcifer): Differentiation of sex and neo-sex chromosomes. Sci. Rep. 2015, 5, 13196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endow, S.A. Polytenization of the ribosomal genes on the X and Y chromosomes of Drosophila melanogaster. Genetics 1982, 100, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, Q.; Wang, Y.; Luo, L.; Yang, J.; Yang, L.; Liu, M.; Li, Y.R.; Qian, T.M.; Zheng, Y.; et al. Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nat. Commun. 2015, 24, 10033. [Google Scholar] [CrossRef] [Green Version]
- Rovatsos, M.; Kratochvíl, L. Molecular sexing applicable in 4000 species of lizards and snakes? From dream to real possibility. Methods Ecol. Evol. 2017, 8, 902–906. [Google Scholar] [CrossRef] [Green Version]
- Pyron, R.A.; Burbrink, F.T. Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol. Lett. 2014, 17, 13–21. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochvíl, L.; Gamble, T.; Rovatsos, M. Sex chromosome evolution among amniotes: Is the origin of sex chromosomes non-random? Philos. Trans. R. Soc. B 2021, 376, 20200108. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Rehák, I.; Velenský, P.; Kratochvíl, L. Shared ancient sex chromosomes in varanids, beaded lizards, and alligator lizards. Mol. Biol. Evol. 2019, 36, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Kay, T.; Depincé, A.; Adolfi, M.; Schartl, M.; Guiguen, Y.; Herpin, A. Evolution of master sex determiners: TGF-β signalling pathways at regulatory crossroads. Philos. Trans. R. Soc. B 2021, 376, 20200091. [Google Scholar] [CrossRef]
- Adolfi, M.C.; Herpin, A.; Schartl, M. The replaceable master of sex determination: Bottom-up hypothesis revisited. Philos. Trans. R. Soc. B 2021, 376, 20200090. [Google Scholar] [CrossRef]
- Kurokawa, H.; Saito, D.; Nakamura, S.; Katoh-Fukui, Y.; Ohta, K.; Baba, T.; Morohashi, K.; Tanaka, M. Germ cells are essential for sexual dimorphism in the medaka gonad. Proc. Natl. Acad. Sci. USA 2007, 104, 16958–16963. [Google Scholar] [CrossRef] [Green Version]
- King, M. Chromosomal and immunogenetic data: A new perspective on the origin of Australia’s reptiles. In Cytogenetics of Amphibians and Reptiles; Olmo, E., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1990; pp. 153–180. [Google Scholar]
- Gorman, G.C. The chromosomes of the Reptilia, a cytotaxonomic interpretation. In Cytotaxonomy and Vertebrate Evolution; Chiarelli, A.B., Capanna, E., Eds.; Academic Press: London, UK, 1973; pp. 349–424. [Google Scholar]
- Johnson Pokorná, M.; Trifonov, V.A.; Rens, W.; Ferguson-Smith, M.A.; Kratochvíl, L. Low rate of interchromosomal rearrangements during old radiation of gekkotan lizards (Squamata: Gekkota). Chromosome Res. 2015, 23, 299–309. [Google Scholar] [CrossRef]
- Suwala, G.; Altmanová, M.; Mazzoleni, S.; Karameta, E.; Pafilis, P.; Kratochvíl, L.; Rovatsos, M. Evolutionary variability of W-linked repetitive content in lacertid lizards. Genes 2020, 11, 531. [Google Scholar] [CrossRef]
- De Smet, W.H. Description of the orcein stained karyotypes of 36 lizard species (Lacertilia, Reptilia) belonging to the families Teiidae, Scincidae, Lacertidae, Cordylidae and Varanidae (Autarchoglossa). Acta Zool. Pathol. Antverp. 1981, 76, 73–118. [Google Scholar]
- Odierna, G.; Caprigilone, T.; Kupriyanova, L.A.; Olmo, E. Further data on sex chromosomes of Lacertidae and a hypothesis on their evolutionary trend. Amphibia-Reptilia 1993, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rovatsos, M.; Marchal, J.A.; Giagia-Athanasopoulou, E.; Sánchez, A. Molecular composition of heterochromatin and its contribution to chromosome variation in the Microtus thomasi/Microtus atticus species complex. Genes 2021, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.J.; Marchal, J.A.; Fernández-Espartero, C.; Romero-Fernández, I.; Rovatsos, M.T.; Giagia-Athanasopoulou, E.B.; Gornung, E.; Castiglia, R.; Sánchez, A. Characterization of the satellite DNA Msat-160 from species of Terricola (Microtus) and Arvicola (Rodentia, Arvicolinae). Genetica 2010, 138, 1085–1098. [Google Scholar] [CrossRef]
- Hughes, J.F.; Skaletsky, H.; Pyntikova, T.; Graves, T.A.; van Daalen, S.K.M.; Minx, P.J.; Fulton, R.S.; McGrath, S.D.; Locke, D.P.; Friedman, C.; et al. Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature 2010, 463, 536–539. [Google Scholar] [CrossRef]
- Rovatsos, M.T.; Marchal, J.A.; Romero-Fernández, I.; Giagia-Athanasopoulou, E.B.; Sánchez, A. Molecular and physical characterization of the complex pericentromeric heterochromatin of the vole species Microtus thomasi. Cytogenet. Genome Res. 2014, 144, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Shirleen Soh, Y.Q.; Alföldi, J.; Pyntikova, T.; Brown, L.G.; Graves, T.; Minx, P.J.; Fulton, R.S.; Kremitzki, C.; Koutseva, N.; Mueller, J.L.; et al. Sequencing the mouse Y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell 2014, 159, 800–813. [Google Scholar] [CrossRef] [Green Version]
- Altmanová, M.; Rovatsos, M.; Kratochvíl, L.; Johnson Pokorná, M. Minute Y chromosomes and karyotype evolution in Madagascan iguanas (Squamata: Iguania: Opluridae). Biol. J. Linn. Soc. 2016, 118, 618–633. [Google Scholar] [CrossRef]
- Morgan, A.P.; Pardo-Manuel de Villena, F. Sequence and structural diversity of mouse Y chromosomes. Mol. Biol. Evol. 2017, 34, 3186–3204. [Google Scholar] [CrossRef] [Green Version]
- Augstenová, B.; Mazzoleni, S.; Kratochvíl, L.; Rovatsos, M. Evolutionary dynamics of the W chromosome in caenophidian snakes. Genes 2017, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Rovatsos, M.; Altmanová, M.; Augstenová, B.; Mazzoleni, S.; Velenský, P.; Kratochvíl, L. ZZ/ZW sex determination with multiple neo-sex chromosomes is common in Madagascan chameleons of the genus Furcifer (Reptilia: Chamaeleonidae). Genes 2019, 10, 1020. [Google Scholar] [CrossRef] [Green Version]
- Marshall Graves, J.A. Sex chromosome specialization and degeneration in mammals. Cell 2006, 124, 901–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, D.K. Is the Y chromosome disappearing? Both sides of the argument. Chromosome Res. 2012, 20, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.-L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Sex determination: Why so many ways of doing it? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovatsos, M.; Kratochvíl, L. Evolution of dosage compensation does not depend on genomic background. Mol. Ecol. 2021, 30, 1836–1845. [Google Scholar] [CrossRef] [PubMed]

| Family | Species | ♂ | ♀ |
|---|---|---|---|
| Carphodactylidae | Underwoodisaurus milii | 1 | 1 |
| Carphodactylidae | Saltuarius cornutus | 1 | 1 |
| Eublepharidae | Coleonyx elegans | 1 | 1 |
| Gekkonidae | Cnemaspis psychedelica | 1 | 1 |
| Gekkonidae | Ebenavia inunguis | 1 | 1 |
| Gekkonidae | Paroedura oviceps | 1 | 1 |
| Gekkonidae | Paroedura androyensis | 1 | 1 |
| Gekkonidae | Paroedura vazimba | 1 | 1 |
| Gekkonidae | Paroedura ibityensis | 1 | 1 |
| Gekkonidae | Uroplatus phantasticus | 1 | 1 |
| Gekkonidae | Uroplatus alluaudi | 1 | 1 |
| Gekkonidae | Uroplatus lineatus | 1 | 1 |
| Gekkonidae | Uroplatus henkeli | 2 | 3 |
| Gekkonidae | Uroplatus sikorae | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pensabene, E.; Yurchenko, A.; Kratochvíl, L.; Rovatsos, M. Madagascar Leaf-Tail Geckos (Uroplatus spp.) Share Independently Evolved Differentiated ZZ/ZW Sex Chromosomes. Cells 2023, 12, 260. https://doi.org/10.3390/cells12020260
Pensabene E, Yurchenko A, Kratochvíl L, Rovatsos M. Madagascar Leaf-Tail Geckos (Uroplatus spp.) Share Independently Evolved Differentiated ZZ/ZW Sex Chromosomes. Cells. 2023; 12(2):260. https://doi.org/10.3390/cells12020260
Chicago/Turabian StylePensabene, Eleonora, Alona Yurchenko, Lukáš Kratochvíl, and Michail Rovatsos. 2023. "Madagascar Leaf-Tail Geckos (Uroplatus spp.) Share Independently Evolved Differentiated ZZ/ZW Sex Chromosomes" Cells 12, no. 2: 260. https://doi.org/10.3390/cells12020260
APA StylePensabene, E., Yurchenko, A., Kratochvíl, L., & Rovatsos, M. (2023). Madagascar Leaf-Tail Geckos (Uroplatus spp.) Share Independently Evolved Differentiated ZZ/ZW Sex Chromosomes. Cells, 12(2), 260. https://doi.org/10.3390/cells12020260








