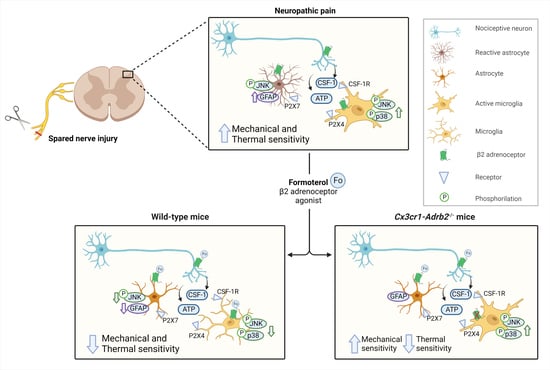
Activation of β2-Adrenergic Receptors in Microglia Alleviates Neuropathic Hypersensitivity in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Surgical Procedures and Nerve Injury
2.3. Pharmacological Drugs
2.4. Behavioral Tests
2.4.1. Experimental Design
- (i)
- The first one assessed the behavioral response of the mice on day three after the operation, one hour after receiving i.p. 50 μg/kg of Formoterol or saline.
- (ii)
- In the second paradigm, we tested mechanical and cold hypersensitivity six and 21 days after the operation, each day 1 h after injecting i.p. Formoterol or saline.
- (iii)
- In the third experimental scheme, we evaluated behavioral parameters only on day 21 after the surgery, one hour after receiving i.p. Formoterol or saline.
2.4.2. Mechanical Sensitivity
2.4.3. Cold Allodynia
2.4.4. Conditioned Place Preference Test
2.5. Immunohistochemistry, Imaging, and Cell Counting
2.6. Microglia Isolation
2.7. Cell Culture
2.8. Dot Blot
2.9. RNA Extraction and qPCR
2.10. Statistical Analysis
2.11. Data Availability
3. Results
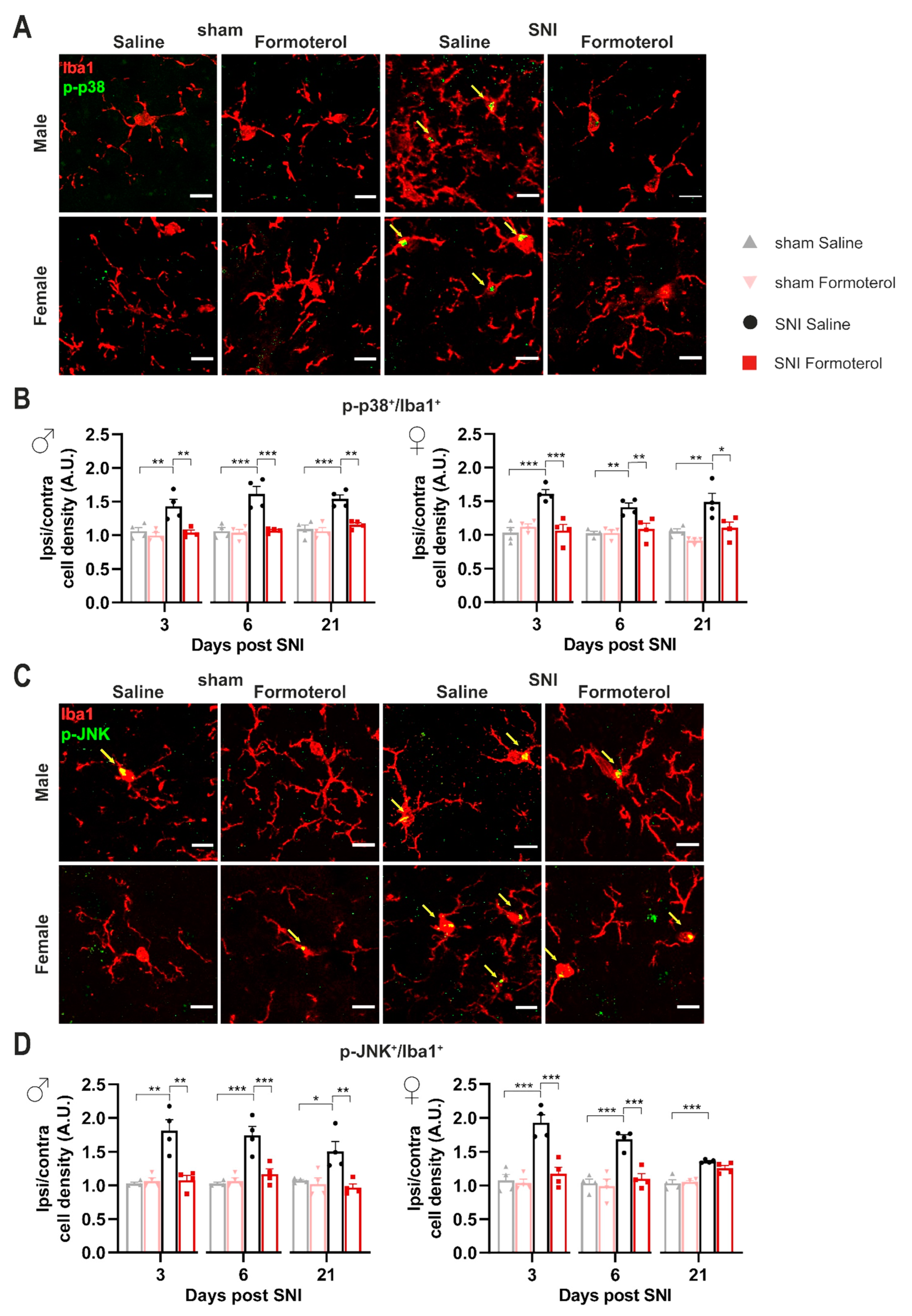
3.1. β2-ARs Are Upregulated in Spinal Microglia Early after Nerve Injury and Their Activation Attenuates Inflammatory Mediators in Microglia
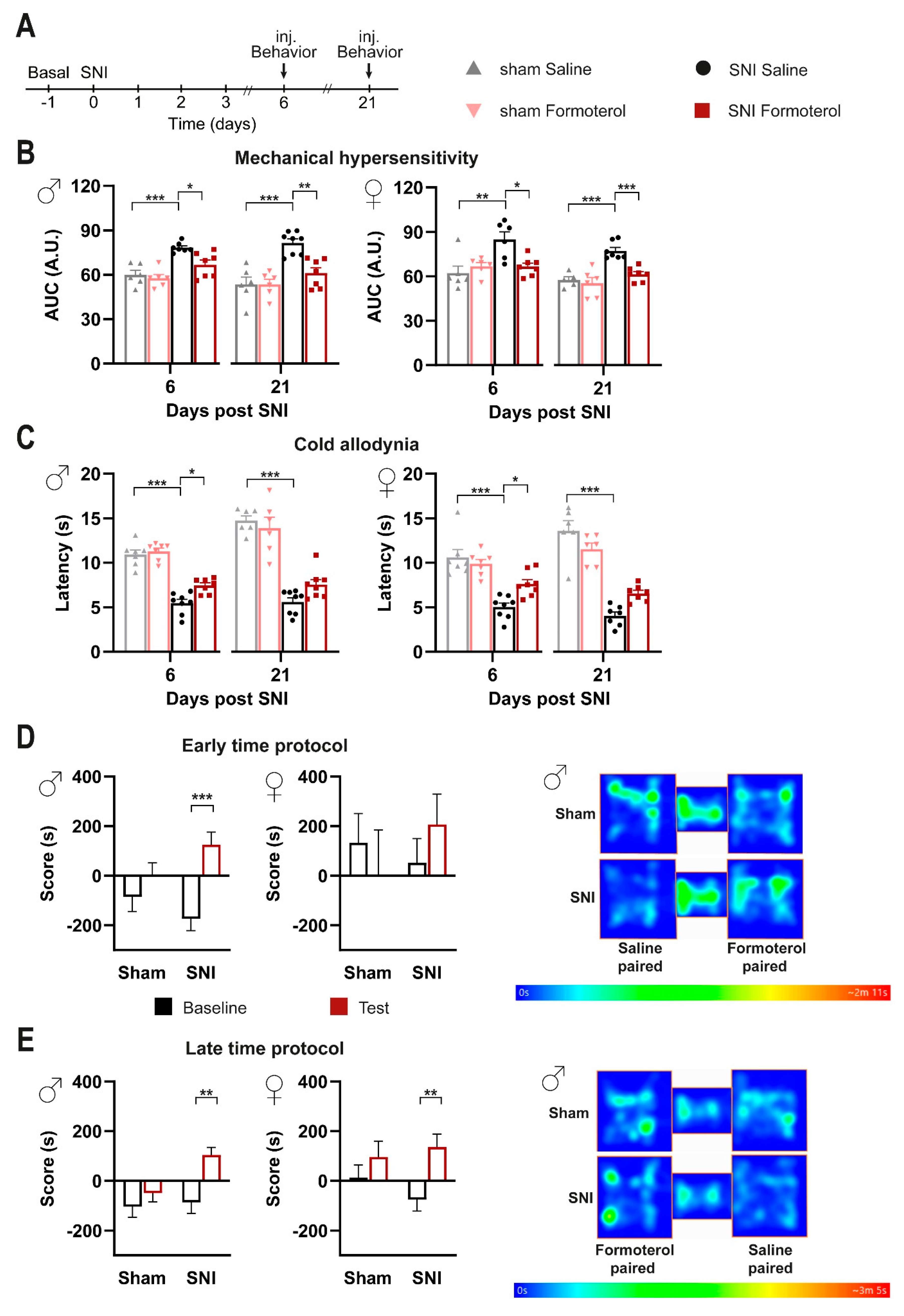
3.2. Impact of In Vivo Administration of β2-AR Agonist on Mechanical and Cold Hypersensitivity in Mice over Early Stages Post-Nerve Injury
3.3. Formoterol Reverses Hypersensitivity Established over Several Days to Weeks and Alleviates Spontaneous Pain in Mice Post-SNI
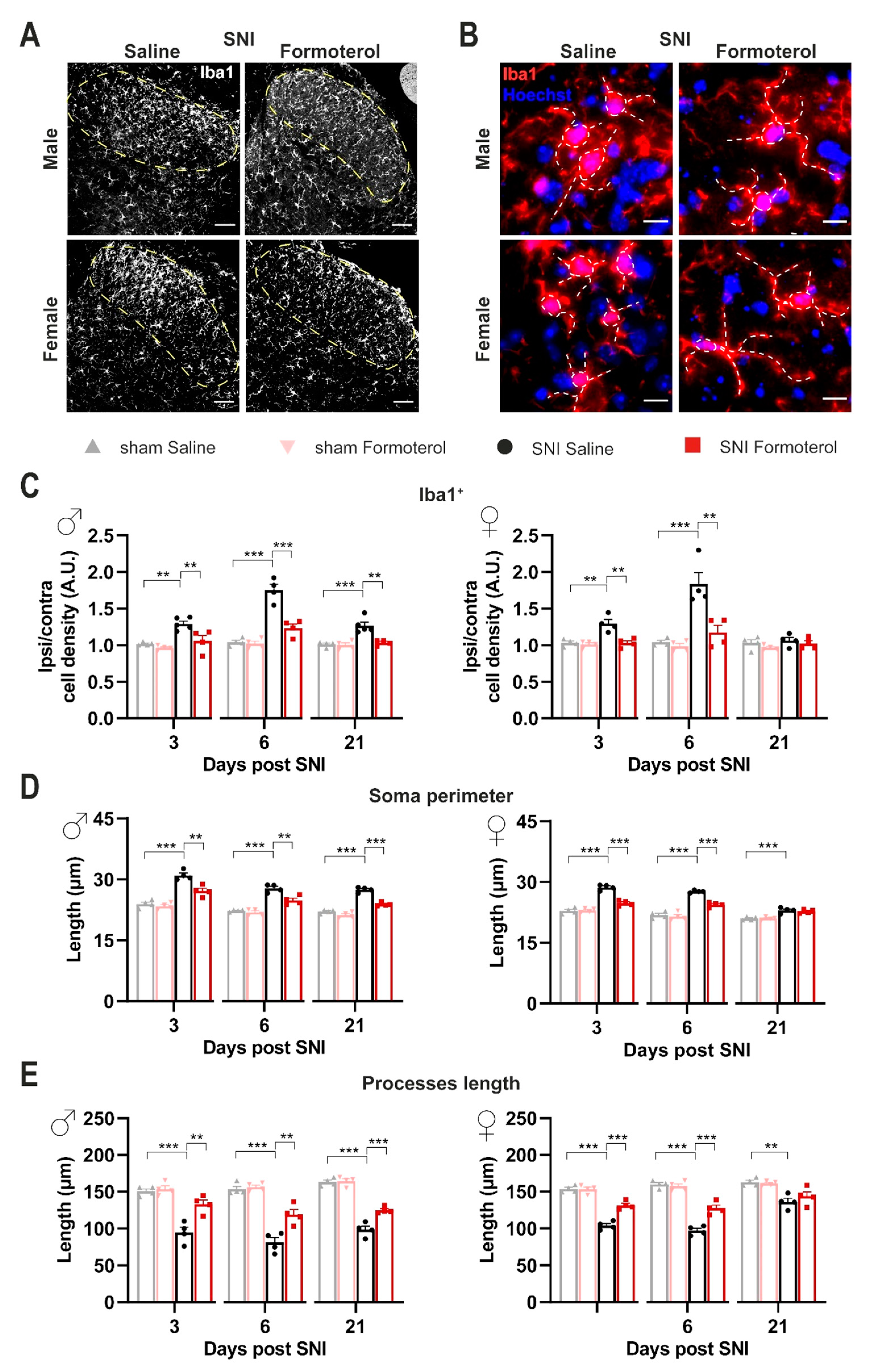
3.4. In Vivo Administration of Formoterol Dampens Structural Remodeling and Activation of Microglia in Neuropathic Mice
3.5. Formoterol Diminishes Astrocytic Activation at Late Stages after Nerve Injury in Female Mice
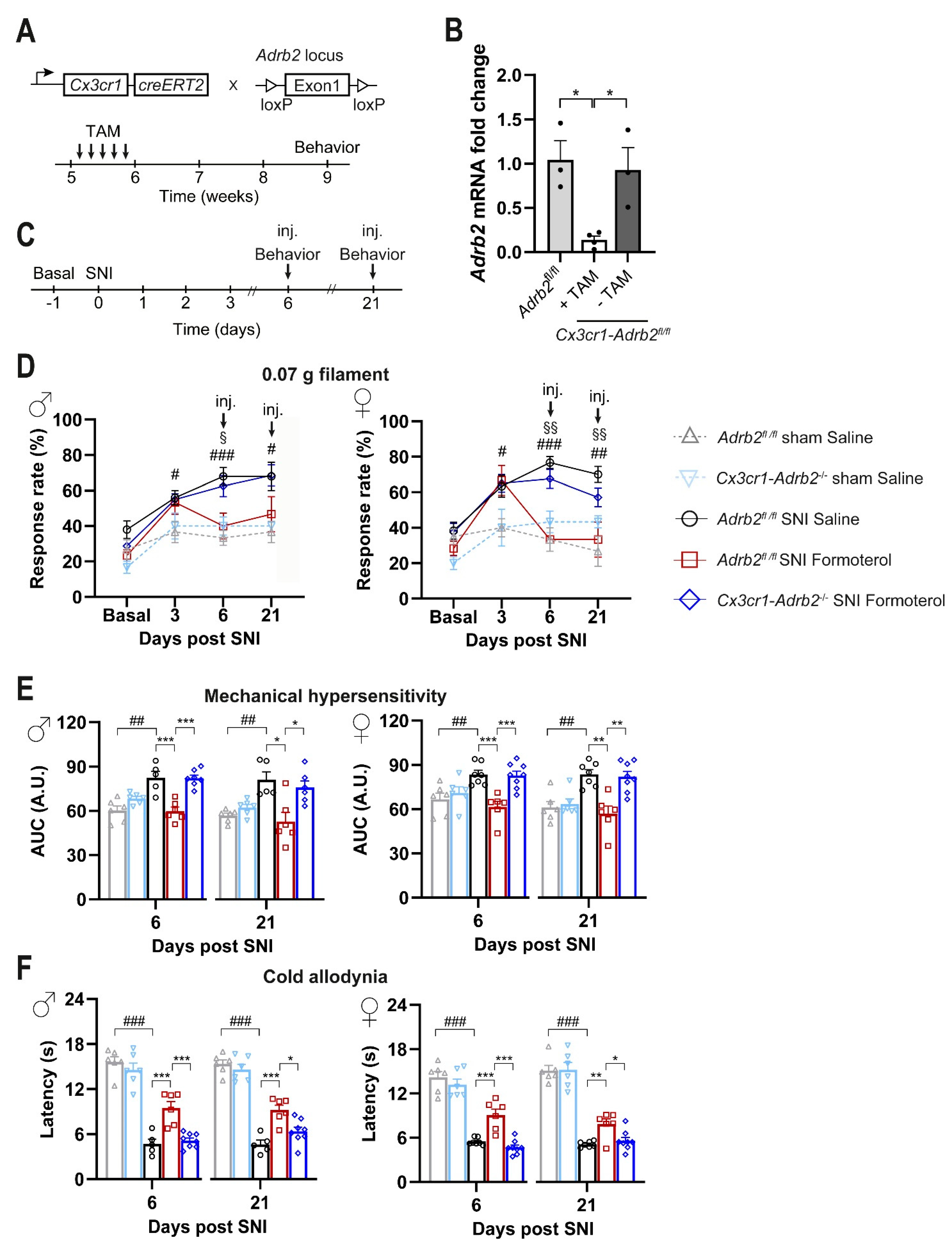
3.6. Contribution of Microglial ß2-ARs to Anti-Nociceptive Effects of Formoterol in Mice with Neuropathic Pain
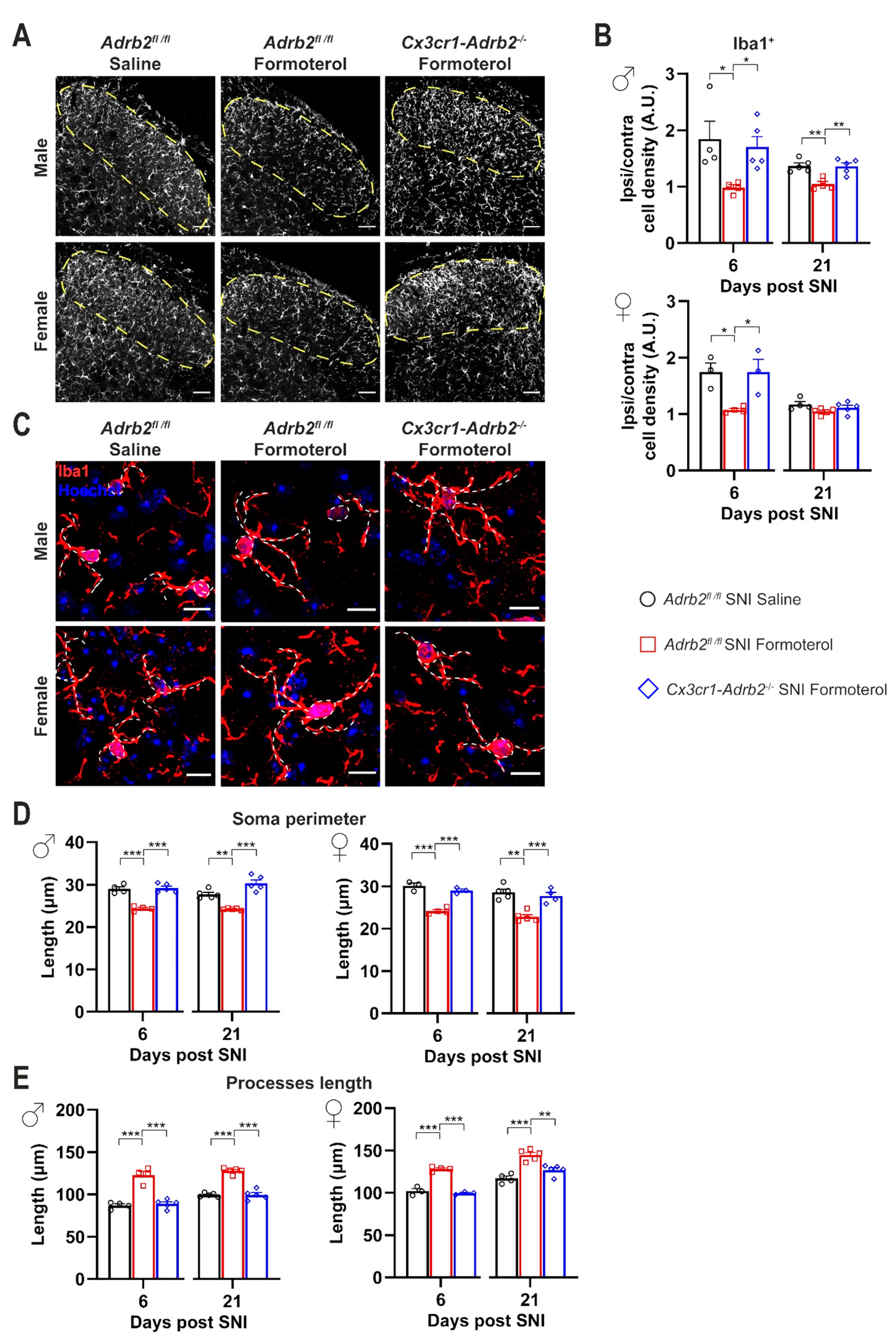
3.7. Contribution of Microglial β2-ARs to Inhibitory Effects of Formoterol on SNI-Induced Microgliosis and Astrogliosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caraci, F.; Merlo, S.; Drago, F.; Caruso, G.; Parenti, C.; Sortino, M.A. Rescue of Noradrenergic System as a Novel Pharmacological Strategy in the Treatment of Chronic Pain: Focus on Microglia Activation. Front. Pharmacol. 2019, 10, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahari, Z.; Meftahi, G.H. Spinal alpha2 -adrenoceptors and neuropathic pain modulation; therapeutic target. Br. J. Pharmacol. 2019, 176, 2366–2381. [Google Scholar] [CrossRef]
- Fricker, L.D.; Margolis, E.B.; Gomes, I.; Devi, L.A. Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions. Mol. Pharmacol. 2020, 98, 96–108. [Google Scholar] [CrossRef]
- Pan, H.L.; Wu, Z.Z.; Zhou, H.Y.; Chen, S.R.; Zhang, H.M.; Li, D.P. Modulation of Pain Transmission by G Protein-Coupled Receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Eisenach, J.C. Hyperexcitability of axotomized and neighboring unaxotomized sensory neurons is reduced days after perineural clonidine at the site of injury. J. Neurophysiol. 2005, 94, 3159–3167. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Qian, W.; Zhao, J.; Sun, L.; Qian, Y.; Xiao, H. Dexmedetomidine inhibits tumor necrosis factor-alpha and interleukin 6 in lipopolysaccharide-stimulated astrocytes by suppression of c-Jun N-terminal kinases. Inflammation 2014, 37, 942–949. [Google Scholar] [CrossRef]
- Ishii, Y.; Yamaizumi, A.; Kawakami, A.; Islam, A.; Choudhury, M.E.; Takahashi, H.; Yano, H.; Tanaka, J. Anti-inflammatory effects of noradrenaline on LPS-treated microglial cells: Suppression of NFκB nuclear translocation and subsequent STAT1 phosphorylation. Neurochem. Int. 2015, 90, 56–66. [Google Scholar] [CrossRef]
- Kremer, M.; Salvat, E.; Muller, A.; Yalcin, I.; Barrot, M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience 2016, 338, 183–206. [Google Scholar] [CrossRef]
- Old, E.A.; Clark, A.K.; Malcangio, M. The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb. Exp. Pharmacol. 2015, 227, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Taves, S.; Berta, T.; Chen, G.; Ji, R.R. Microglia and spinal cord synaptic plasticity in persistent pain. Neural. Plast. 2013, 2013, 753656. [Google Scholar] [CrossRef] [Green Version]
- Hald, A.; Nedergaard, S.; Hansen, R.R.; Ding, M.; Heegaard, A.M. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur. J. Pain 2009, 13, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Gwak, Y.S.; Kang, J.; Unabia, G.C.; Hulsebosch, C.E. Spatial and temporal activation of spinal glial cells: Role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp. Neurol. 2012, 234, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.; Kim, J.H.; Kim, J.H.; Jha, M.K.; Jung, J.Y.; Lee, M.G.; Choi, I.S.; Jang, I.S.; Lim, D.G.; Hwang, S.H.; et al. Reversible Induction of Pain Hypersensitivity following Optogenetic Stimulation of Spinal Astrocytes. Cell Rep. 2016, 17, 3049–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M.; et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Lubahn, C.L.; Lorton, D.; Schaller, J.A.; Sweeney, S.J.; Bellinger, D.L. Targeting alpha- and beta-Adrenergic Receptors Differentially Shifts Th1, Th2, and Inflammatory Cytokine Profiles in Immune Organs to Attenuate Adjuvant Arthritis. Front. Immunol. 2014, 5, 346. [Google Scholar] [CrossRef] [Green Version]
- Uzkeser, H.; Cadirci, E.; Halici, Z.; Odabasoglu, F.; Polat, B.; Yuksel, T.N.; Ozaltin, S.; Atalay, F. Anti-inflammatory and antinociceptive effects of salbutamol on acute and chronic models of inflammation in rats: Involvement of an antioxidant mechanism. Mediat. Inflamm. 2012, 2012, 438912. [Google Scholar] [CrossRef]
- Zhang, F.F.; Morioka, N.; Abe, H.; Fujii, S.; Miyauchi, K.; Nakamura, Y.; Hisaoka-Nakashima, K.; Nakata, Y. Stimulation of spinal dorsal horn β2-adrenergic receptor ameliorates neuropathic mechanical hypersensitivity through a reduction of phosphorylation of microglial p38 MAP kinase and astrocytic c-jun N-terminal kinase. Neurochem. Int. 2016, 101, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Morado-Urbina, C.E.; Gwak, Y.S.; Parker, R.A.; Kittel, C.A.; Munoz-Islas, E.; Miguel Jimenez-Andrade, J.; Romero-Sandoval, E.A.; Eisenach, J.C.; Peters, C.M. Systemic administration of a beta2-adrenergic receptor agonist reduces mechanical allodynia and suppresses the immune response to surgery in a rat model of persistent post-incisional hypersensitivity. Mol. Pain 2021, 17, 1744806921997206. [Google Scholar] [CrossRef] [PubMed]
- Albertini, G.; Etienne, F.; Roumier, A. Regulation of microglia by neuromodulators: Modulations in major and minor modes. Neurosci. Lett. 2020, 733, 135000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [Green Version]
- Gyoneva, S.; Traynelis, S.F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 2013, 288, 15291–15302. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Ozaki, E.; Zhang, B.; Yang, L.; Yokoyama, A.; Takeda, I.; Maeda, N.; Sakanaka, M.; Tanaka, J. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 2002, 43, 1026–1034. [Google Scholar] [CrossRef]
- Stowell, R.D.; Sipe, G.O.; Dawes, R.P.; Batchelor, H.N.; Lordy, K.A.; Whitelaw, B.S.; Stoessel, M.B.; Bidlack, J.M.; Brown, E.; Sur, M.; et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat. Neurosci. 2019, 22, 1782–1792. [Google Scholar] [CrossRef]
- Liu, Y.U.; Ying, Y.; Li, Y.; Eyo, U.B.; Chen, T.; Zheng, J.; Umpierre, A.D.; Zhu, J.; Bosco, D.B.; Dong, H.; et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 2019, 22, 1771–1781. [Google Scholar] [CrossRef]
- Hinoi, E.; Gao, N.; Jung, D.Y.; Yadav, V.; Yoshizawa, T.; Myers, M.G.; Chua, S.C.; Kim, J.K.; Kaestner, K.H.; Karsenty, G. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J. Cell Biol. 2008, 183, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Yalcin, I.; Tessier, L.H.; Petit-Demoulière, N.; Waltisperger, E.; Hein, L.; Freund-Mercier, M.J.; Barrot, M. Chronic treatment with agonists of beta(2)-adrenergic receptors in neuropathic pain. Exp. Neurol. 2010, 221, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Nees, T.A.; Wang, N.; Adamek, P.; Verkest, C.; La Porta, C.; Schaefer, I.; Virnich, J.; Balkaya, S.; Prato, V.; Morelli, C.; et al. The molecular mechanism and physiological role of silent nociceptor activation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Huang, E.Y.; Liu, T.C.; Tao, P.L. Co-administration of dextromethorphan with morphine attenuates morphine rewarding effect and related dopamine releases at the nucleus accumbens. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 368, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Schweizerhof, M.; Stösser, S.; Kurejova, M.; Njoo, C.; Gangadharan, V.; Agarwal, N.; Schmelz, M.; Bali, K.K.; Michalski, C.W.; Brugger, S.; et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat. Med. 2009, 15, 802–807. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773.e758. [Google Scholar] [CrossRef] [Green Version]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, T.; Vera-Portocarrero, L.; Gutierrez, T.; Vanderah, T.W.; Dussor, G.; Lai, J.; Fields, H.L.; Porreca, F. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 2009, 12, 1364–1366. [Google Scholar] [CrossRef] [Green Version]
- Pitzer, C.; Kuner, R.; Tappe-Theodor, A. EXPRESS: Voluntary and evoked behavioral correlates in neuropathic pain states under different housing conditions. Mol. Pain 2016, 12, 1744806916656635. [Google Scholar] [CrossRef]
- Chen, Z.; Doyle, T.M.; Luongo, L.; Largent-Milnes, T.M.; Giancotti, L.A.; Kolar, G.; Squillace, S.; Boccella, S.; Walker, J.K.; Pendleton, A.; et al. Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc. Natl. Acad. Sci. USA 2019, 116, 10557–10562. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ceredig, R.A.; Pierre, F.; Doridot, S.; Alduntzin, U.; Hener, P.; Salvat, E.; Yalcin, I.; Gaveriaux-Ruff, C.; Barrot, M.; Massotte, D. Peripheral Delta Opioid Receptors Mediate Formoterol Anti-allodynic Effect in a Mouse Model of Neuropathic Pain. Front. Mol. Neurosci. 2019, 12, 324. [Google Scholar] [CrossRef]
- Kremer, M.; Megat, S.; Bohren, Y.; Wurtz, X.; Nexon, L.; Ceredig, R.A.; Doridot, S.; Massotte, D.; Salvat, E.; Yalcin, I.; et al. Delta opioid receptors are essential to the antiallodynic action of Beta2-mimetics in a model of neuropathic pain. Mol. Pain 2020, 16, 1744806920912931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, M.; Yalcin, I.; Goumon, Y.; Wurtz, X.; Nexon, L.; Daniel, D.; Megat, S.; Ceredig, R.A.; Ernst, C.; Turecki, G.; et al. A Dual Noradrenergic Mechanism for the Relief of Neuropathic Allodynia by the Antidepressant Drugs Duloxetine and Amitriptyline. J. Neurosci. 2018, 38, 9934–9954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damo, E. Glial cells as target for antidepressants in neuropathic pain. Neuroforum 2022, 28, 85–94. [Google Scholar] [CrossRef]
- Evans, A.K.; Ardestani, P.M.; Yi, B.; Park, H.H.; Lam, R.K.; Shamloo, M. Beta-adrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s Disease. Neurobiol. Dis. 2020, 146, 105089. [Google Scholar] [CrossRef] [PubMed]
- Izeboud, C.A.; Monshouwer, M.; van Miert, A.S.; Witkamp, R.F. The beta-adrenoceptor agonist clenbuterol is a potent inhibitor of the LPS-induced production of TNF-alpha and IL-6 in vitro and in vivo. Inflamm. Res. 1999, 48, 497–502. [Google Scholar] [CrossRef]
- Keranen, T.; Hommo, T.; Moilanen, E.; Korhonen, R. beta2-receptor agonists salbutamol and terbutaline attenuated cytokine production by suppressing ERK pathway through cAMP in macrophages. Cytokine 2017, 94, 1–7. [Google Scholar] [CrossRef]
- Agac, D.; Estrada, L.D.; Maples, R.; Hooper, L.V.; Farrar, J.D. The beta2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav. Immun. 2018, 74, 176–185. [Google Scholar] [CrossRef]
- Keranen, T.; Hommo, T.; Hamalainen, M.; Moilanen, E.; Korhonen, R. Anti-Inflammatory Effects of beta2-Receptor Agonists Salbutamol and Terbutaline Are Mediated by MKP-1. PLoS ONE 2016, 11, e0148144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Arbabzada, N.; Flood, P.M. Mechanism underlying beta2-AR agonist-mediated phenotypic conversion of LPS-activated microglial cells. J. Neuroimmunol. 2019, 332, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Laureys, G.; Clinckers, R.; Gerlo, S.; Spooren, A.; Wilczak, N.; Kooijman, R.; Smolders, I.; Michotte, Y.; De Keyser, J. Astrocytic beta(2)-adrenergic receptors: From physiology to pathology. Prog. Neurobiol. 2010, 91, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Midavaine, E.; Cote, J.; Marchand, S.; Sarret, P. Glial and neuroimmune cell choreography in sexually dimorphic pain signaling. Neurosci. Biobehav. Rev. 2021, 125, 168–192. [Google Scholar] [CrossRef]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Taves, S.; Berta, T.; Liu, D.L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Batti, L.; Sundukova, M.; Murana, E.; Pimpinella, S.; De Castro Reis, F.; Pagani, F.; Wang, H.; Pellegrino, E.; Perlas, E.; Di Angelantonio, S.; et al. TMEM16F Regulates Spinal Microglial Function in Neuropathic Pain States. Cell Rep. 2016, 15, 2608–2615. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Gu, N.; Zhou, L.; B Eyo, U.; Murugan, M.; Gan, W.B.; Wu, L.J. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat. Commun. 2016, 7, 12029. [Google Scholar] [CrossRef]
- Gu, N.; Eyo, U.B.; Murugan, M.; Peng, J.; Matta, S.; Dong, H.; Wu, L.J. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain. Behav. Immun. 2016, 55, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staniland, A.A.; Clark, A.K.; Wodarski, R.; Sasso, O.; Maione, F.; D’Acquisto, F.; Malcangio, M. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J. Neurochem. 2010, 114, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Iglesias, P.; Pineda-Farias, J.B.; Cervantes-Duran, C.; Bravo-Hernandez, M.; Rocha-Gonzalez, H.I.; Murbartian, J.; Granados-Soto, V. Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: Possible involvement of glial cells. Mol. Pain 2014, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo-Castrillo, A.; Arevalo, M.A. Microglial and Astrocytic Function in Physiological and Pathological Conditions: Estrogenic Modulation. Int. J. Mol. Sci. 2020, 21, 3219. [Google Scholar] [CrossRef]




| Primer | Sequence 5′ → 3′ Forward | Sequence 5′ → 3′ Reverse |
|---|---|---|
| Syt1 V2 | CTCAACTGGCATTTGTTAGTCAA | AGACTGCGGATGTTGGTTGT |
| Aqp4 | TGGAGGATTGGGAGTCACC | TGAACACCAACTGGAAAGTGA |
| Mbp | ATTGGGTCGCCATGGGAAAC | CCAGCCTCTCCTCGGTGAAT |
| Cx3cr1 | CGTGAGACTGGGTGAGTGAC | GGACATGGTGAGGTCCTGAG |
| Primer | Sequence 5′ → 3′ Forward | Sequence 5′ → 3′ Reverse |
|---|---|---|
| Adrb2 | GCATGGAAGGCTTTGTGAAC | CTTGGGAGTCAACGCTAAGG |
| Gapdh | AGAAGGTGGTGAAGCAGGCATC | CGAAGGTGGAAGAGTGGGAGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damo, E.; Agarwal, A.; Simonetti, M. Activation of β2-Adrenergic Receptors in Microglia Alleviates Neuropathic Hypersensitivity in Mice. Cells 2023, 12, 284. https://doi.org/10.3390/cells12020284
Damo E, Agarwal A, Simonetti M. Activation of β2-Adrenergic Receptors in Microglia Alleviates Neuropathic Hypersensitivity in Mice. Cells. 2023; 12(2):284. https://doi.org/10.3390/cells12020284
Chicago/Turabian StyleDamo, Elisa, Amit Agarwal, and Manuela Simonetti. 2023. "Activation of β2-Adrenergic Receptors in Microglia Alleviates Neuropathic Hypersensitivity in Mice" Cells 12, no. 2: 284. https://doi.org/10.3390/cells12020284