Proinflammatory IFNγ Is Produced by but Not Required for the Generation of Eomes+ Thymic Innate CD8 T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antibodies
2.3. Cell Isolation
2.4. Flow Cytometry
2.5. Intracellular Cytokine Expression Assays
2.6. Nuclear Transcription Factor Staining
2.7. In-Vivo Treatment of IL-2RβTg Mice with IL-4 and Anti-IL-4 Antibody Complex
2.8. Co-Staining for Intracellular pSTAT1/Eomes
2.9. Statistics
3. Results
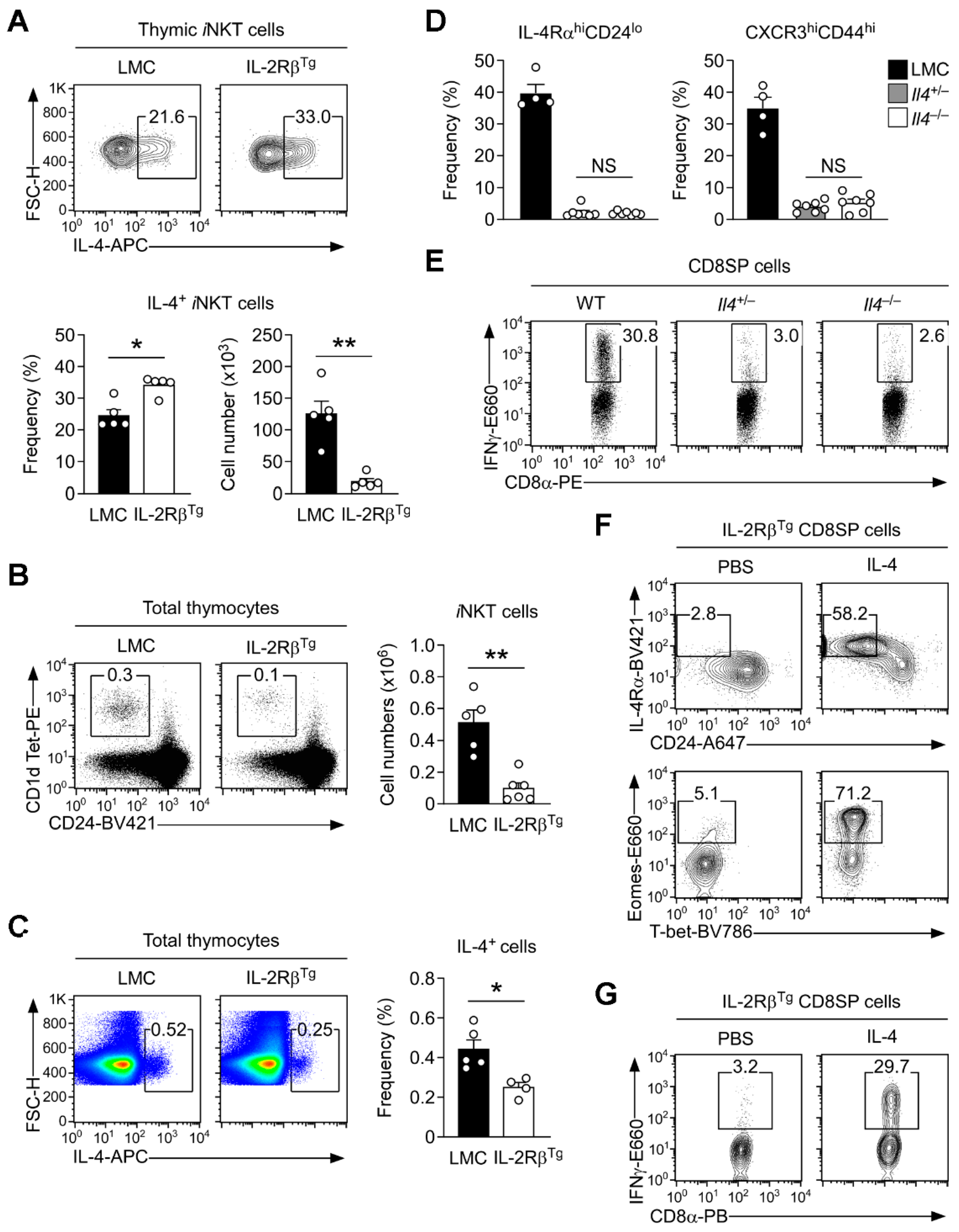
3.1. Distinct Cytokine Requirements for IL-4-Producing iNKT and IFNγ-Expression Innate CD8 T Cells
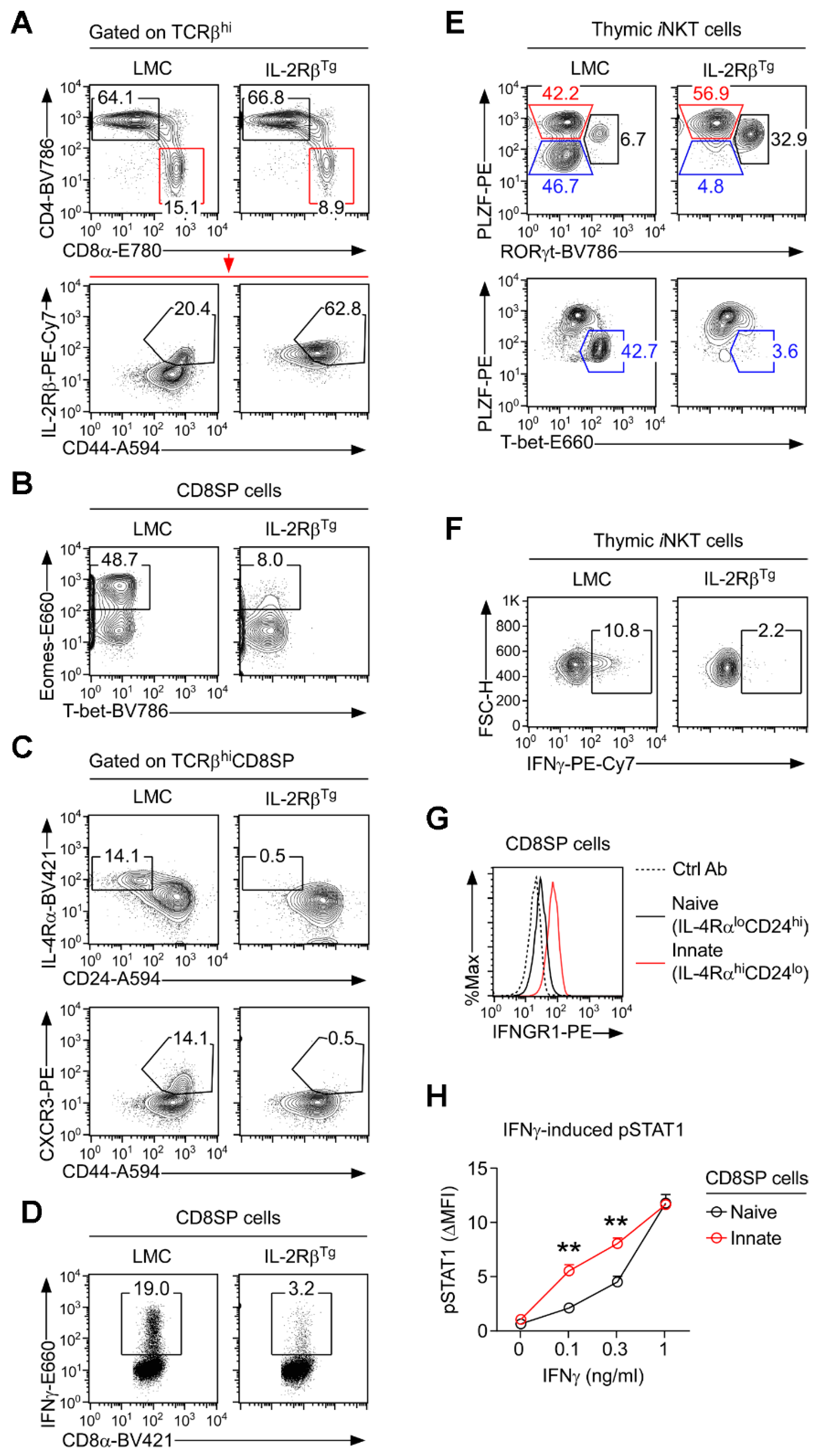
3.2. Forced Expression of IL-2Rβ Suppresses the Generation of Innate CD8 T Cells
3.3. Lack of Innate CD8 T Cells in IL-2RβTg Mice Is Associated with the Lack of IFNγ Expression
3.4. IFNγ Is Dispensable for the Generation of Innate CD8 T Cells in the Thymus
3.5. Innate CD8 T Cell Development Is Controlled by the Abundance of Intrathymic IL-4
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jameson, S.C. The Naming of Memory T-Cell Subsets. Cold Spring Harb. Perspect. Biol. 2021, 13, a037788. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, M.A.; Odumade, O.A.; Jameson, S.C.; Hogquist, K.A. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 2010, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Won, H.Y.; DiPalma, D.T.; Hong, C.; Park, J.H. Protein abundance of the cytokine receptor γc controls the thymic generation of innate-like T cells. Cell. Mol. Life Sci. 2021, 79, 17. [Google Scholar] [CrossRef] [PubMed]
- Jacomet, F.; Cayssials, E.; Basbous, S.; Levescot, A.; Piccirilli, N.; Desmier, D.; Robin, A.; Barra, A.; Giraud, C.; Guilhot, F.; et al. Evidence for eomesodermin-expressing innate-like CD8(+) KIR/NKG2A(+) T cells in human adults and cord blood samples. Eur. J. Immunol. 2015, 45, 1926–1933. [Google Scholar] [CrossRef]
- Lee, A.; Park, S.P.; Park, C.H.; Kang, B.H.; Park, S.H.; Ha, S.J.; Jung, K.C. IL-4 Induced Innate CD8+ T Cells Control Persistent Viral Infection. PLoS Pathog. 2015, 11, e1005193. [Google Scholar] [CrossRef]
- Renkema, K.R.; Lee, J.Y.; Lee, Y.J.; Hamilton, S.E.; Hogquist, K.A.; Jameson, S.C. IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection. J. Exp. Med. 2016, 213, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Baez, N.S.; Cerban, F.; Savid-Frontera, C.; Hodge, D.L.; Tosello, J.; Acosta-Rodriguez, E.; Almada, L.; Gruppi, A.; Viano, M.E.; Young, H.A.; et al. Thymic expression of IL-4 and IL-15 after systemic inflammatory or infectious Th1 disease processes induce the acquisition of “innate” characteristics during CD8+ T cell development. PLoS Pathog. 2019, 15, e1007456. [Google Scholar] [CrossRef]
- Shi, X.; Guo, L.W.; Seedial, S.M.; Si, Y.; Wang, B.; Takayama, T.; Suwanabol, P.A.; Ghosh, S.; DiRenzo, D.; Liu, B.; et al. TGF-beta/Smad3 inhibit vascular smooth muscle cell apoptosis through an autocrine signaling mechanism involving VEGF-A. Cell Death Dis. 2014, 5, e1317. [Google Scholar] [CrossRef]
- Huang, W.; Huang, F.; Kannan, A.K.; Hu, J.; August, A. ITK tunes IL-4-induced development of innate memory CD8+ T cells in a gammadelta T and invariant NKT cell-independent manner. J. Leukoc. Biol. 2014, 96, 55–63. [Google Scholar] [CrossRef]
- Nayar, R.; Enos, M.; Prince, A.; Shin, H.; Hemmers, S.; Jiang, J.K.; Klein, U.; Thomas, C.J.; Berg, L.J. TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation. Proc. Natl. Acad. Sci. USA 2012, 109, E2794–E2802. [Google Scholar] [CrossRef]
- Lee, Y.J.; Holzapfel, K.L.; Zhu, J.; Jameson, S.C.; Hogquist, K.A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 2013, 14, 1146–1154. [Google Scholar] [CrossRef]
- Lai, D.; Zhu, J.; Wang, T.; Hu-Li, J.; Terabe, M.; Berzofsky, J.A.; Clayberger, C.; Krensky, A.M. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J. Exp. Med. 2011, 208, 1093–1103. [Google Scholar] [CrossRef]
- Waickman, A.T.; Park, J.Y.; Park, J.H. The common gamma-chain cytokine receptor: Tricks-and-treats for T cells. Cell. Mol. Life Sci. 2016, 73, 253–269. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNgamma: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Won, H.Y.; Kim, H.K.; Crossman, A.; Awasthi, P.; Gress, R.E.; Park, J.H. The Timing and Abundance of IL-2Rbeta (CD122) Expression Control Thymic iNKT Cell Generation and NKT1 Subset Differentiation. Front. Immunol. 2021, 12, 642856. [Google Scholar] [CrossRef]
- Dalton, D.K.; Pitts-Meek, S.; Keshav, S.; Figari, I.S.; Bradley, A.; Stewart, T.A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 1993, 259, 1739–1742. [Google Scholar] [CrossRef]
- Park, J.Y.; Won, H.Y.; DiPalma, D.T.; Kim, H.K.; Kim, T.H.; Li, C.; Sato, N.; Hong, C.; Abraham, N.; Gress, R.E.; et al. In vivo availability of the cytokine IL-7 constrains the survival and homeostasis of peripheral iNKT cells. Cell Rep. 2022, 38, 110219. [Google Scholar] [CrossRef]
- Prakhar, P.; Alvarez-DelValle, J.; Keller, H.; Crossman, A.; Tai, X.; Park, Y.K.; Park, J.H. The small intestine epithelium exempts Foxp3+ Tregs from their IL-2 requirement for homeostasis and effector function. JCI Insight 2021, 6, e149656. [Google Scholar] [CrossRef]
- Morris, S.C.; Heidorn, S.M.; Herbert, D.R.; Perkins, C.; Hildeman, D.A.; Khodoun, M.V.; Finkelman, F.D. Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J. Immunol. 2009, 182, 1429–1438. [Google Scholar] [CrossRef]
- Li, C.; Park, J.H. Assessing IL-2-Induced STAT5 Phosphorylation in Fixed, Permeabilized Foxp3(+) Treg Cells by Multiparameter Flow Cytometry. STAR Protoc. 2020, 1, 100195. [Google Scholar] [CrossRef]
- Yarilin, A.A.; Belyakov, I.M. Cytokines in the thymus: Production and biological effects. Curr. Med. Chem. 2004, 11, 447–464. [Google Scholar] [CrossRef]
- Do, J.S.; Fink, P.J.; Li, L.; Spolski, R.; Robinson, J.; Leonard, W.J.; Letterio, J.J.; Min, B. Cutting edge: Spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J. Immunol. 2010, 184, 1675–1679. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jameson, S.C.; Hogquist, K.A. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011, 32, 50–56. [Google Scholar] [CrossRef]
- Verykokakis, M.; Boos, M.D.; Bendelac, A.; Kee, B.L. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity 2010, 33, 203–215. [Google Scholar] [CrossRef]
- Oghumu, S.; Terrazas, C.A.; Varikuti, S.; Kimble, J.; Vadia, S.; Yu, L.; Seveau, S.; Satoskar, A.R. CXCR3 expression defines a novel subset of innate CD8+ T cells that enhance immunity against bacterial infection and cancer upon stimulation with IL-15. FASEB J. 2015, 29, 1019–1028. [Google Scholar] [CrossRef]
- Renz, H.; Domenico, J.; Gelfand, E.W. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J. Immunol. 1991, 146, 3049–3055. [Google Scholar] [CrossRef]
- Leonard, W.J.; Lin, J.X.; O’Shea, J.J. The γ(c) Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef]
- Mathieu, C.; Beltra, J.C.; Charpentier, T.; Bourbonnais, S.; Di Santo, J.P.; Lamarre, A.; Decaluwe, H. IL-2 and IL-15 regulate CD8+ memory T-cell differentiation but are dispensable for protective recall responses. Eur. J. Immunol. 2015, 45, 3324–3338. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Ravkov, E.V.; Williams, M.A. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J. Immunol. 2010, 184, 6719–6730. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Escalante, N.K.; Jude, K.M.; Sotolongo Bellon, J.; Su, L.; Horton, T.M.; Tsutsumi, N.; Berardinelli, S.J.; Haltiwanger, R.S.; Piehler, J.; et al. Structure of the IFNγ receptor complex guides design of biased agonists. Nature 2019, 567, 56–60. [Google Scholar] [CrossRef]
- Kwesi-Maliepaard, E.M.; Jacobs, H.; van Leeuwen, F. Signals for antigen-independent differentiation of memory CD8(+) T cells. Cell. Mol. Life Sci. 2021, 78, 6395–6408. [Google Scholar] [CrossRef]
- Smith, N.L.; Patel, R.K.; Reynaldi, A.; Grenier, J.K.; Wang, J.; Watson, N.B.; Nzingha, K.; Yee Mon, K.J.; Peng, S.A.; Grimson, A.; et al. Developmental Origin Governs CD8(+) T Cell Fate Decisions during Infection. Cell 2018, 174, 117–130.e114. [Google Scholar] [CrossRef]
- Hussain, T.; Quinn, K.M. Similar but different: Virtual memory CD8 T cells as a memory-like cell population. Immunol. Cell Biol. 2019, 97, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.T.; Ernst, B.; Kieper, W.C.; LeRoy, E.; Sprent, J.; Surh, C.D. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002, 195, 1523–1532. [Google Scholar] [CrossRef]
- Istaces, N.; Splittgerber, M.; Lima Silva, V.; Nguyen, M.; Thomas, S.; Le, A.; Achouri, Y.; Calonne, E.; Defrance, M.; Fuks, F.; et al. EOMES interacts with RUNX3 and BRG1 to promote innate memory cell formation through epigenetic reprogramming. Nat. Commun. 2019, 10, 3306. [Google Scholar] [CrossRef]
- Bortoluzzi, S.; Dashtsoodol, N.; Engleitner, T.; Drees, C.; Helmrath, S.; Mir, J.; Toska, A.; Flossdorf, M.; Ollinger, R.; Solovey, M.; et al. Brief homogeneous TCR signals instruct common iNKT progenitors whose effector diversification is characterized by subsequent cytokine signaling. Immunity 2021, 54, 2497–2513.e2499. [Google Scholar] [CrossRef]
- Gordy, L.E.; Bezbradica, J.S.; Flyak, A.I.; Spencer, C.T.; Dunkle, A.; Sun, J.; Stanic, A.K.; Boothby, M.R.; He, Y.W.; Zhao, Z.; et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J. Immunol. 2011, 187, 6335–6345. [Google Scholar] [CrossRef]
- Havenar-Daughton, C.; Li, S.; Benlagha, K.; Marie, J.C. Development and function of murine RORγt+ iNKT cells are under TGF-beta signaling control. Blood 2012, 119, 3486–3494. [Google Scholar] [CrossRef]
- Watarai, H.; Sekine-Kondo, E.; Shigeura, T.; Motomura, Y.; Yasuda, T.; Satoh, R.; Yoshida, H.; Kubo, M.; Kawamoto, H.; Koseki, H.; et al. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012, 10, e1001255. [Google Scholar] [CrossRef]
- Hogquist, K.; Georgiev, H. Recent advances in iNKT cell development. F1000Res 2020, 9, F1000 Faculty Rev-127. [Google Scholar] [CrossRef]
- Stock, P.; Lombardi, V.; Kohlrautz, V.; Akbari, O. Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J. Immunol. 2009, 182, 5116–5122. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, H.Y.; Liman, N.; Li, C.; Park, J.-H. Proinflammatory IFNγ Is Produced by but Not Required for the Generation of Eomes+ Thymic Innate CD8 T Cells. Cells 2023, 12, 2433. https://doi.org/10.3390/cells12202433
Won HY, Liman N, Li C, Park J-H. Proinflammatory IFNγ Is Produced by but Not Required for the Generation of Eomes+ Thymic Innate CD8 T Cells. Cells. 2023; 12(20):2433. https://doi.org/10.3390/cells12202433
Chicago/Turabian StyleWon, Hee Yeun, Nurcin Liman, Can Li, and Jung-Hyun Park. 2023. "Proinflammatory IFNγ Is Produced by but Not Required for the Generation of Eomes+ Thymic Innate CD8 T Cells" Cells 12, no. 20: 2433. https://doi.org/10.3390/cells12202433
APA StyleWon, H. Y., Liman, N., Li, C., & Park, J.-H. (2023). Proinflammatory IFNγ Is Produced by but Not Required for the Generation of Eomes+ Thymic Innate CD8 T Cells. Cells, 12(20), 2433. https://doi.org/10.3390/cells12202433






