Differential Transcriptomic Signatures of Small Airway Cell Cultures Derived from IPF and COVID-19-Induced Exacerbation of Interstitial Lung Disease
Abstract
:1. Introduction
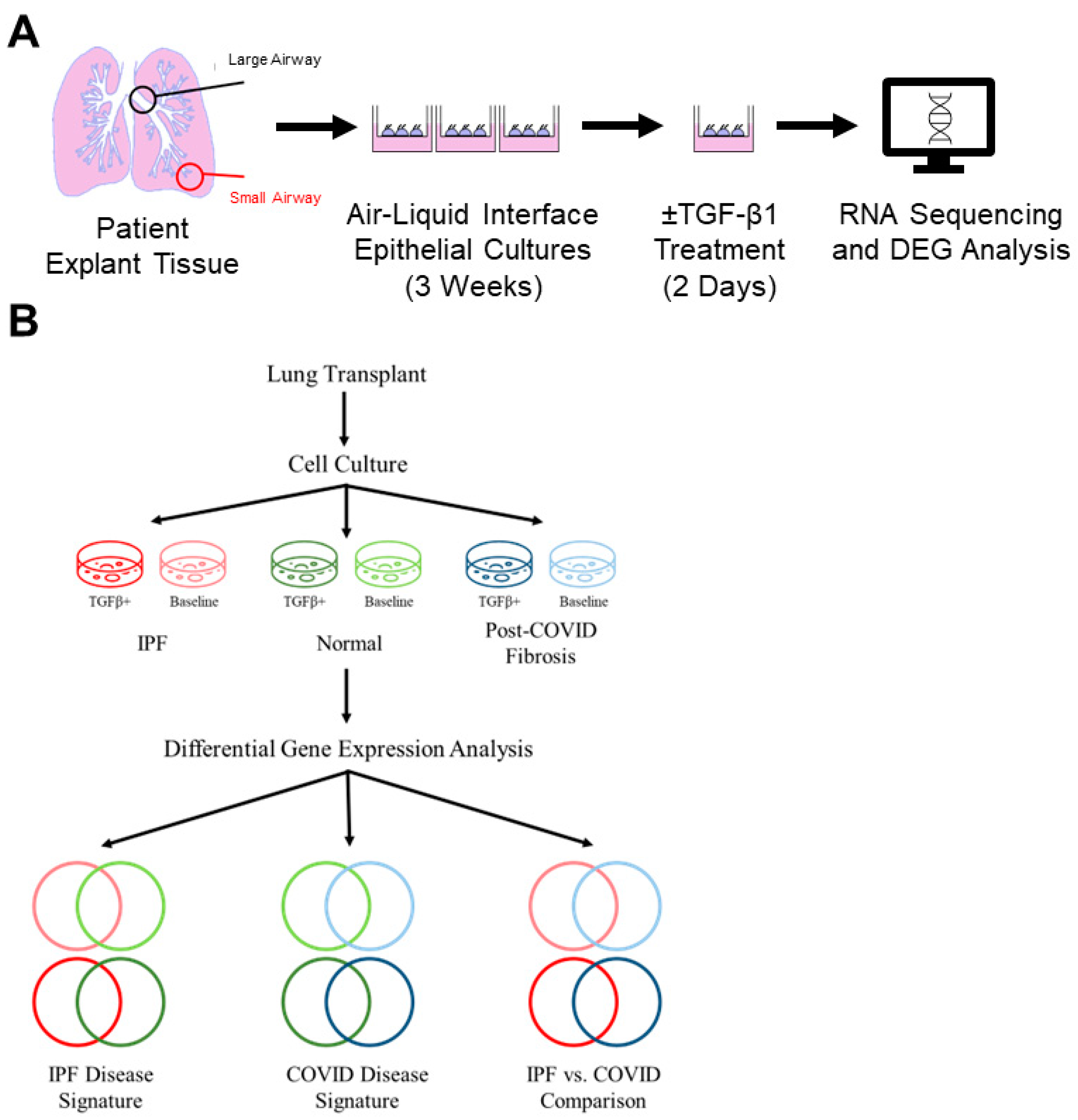
2. Materials and Methods
2.1. Human Lung Explant Samples
2.2. Isolation and Propagation of Distal Small Airways
2.3. RNA Isolation and Sequencing
2.4. Differential Expression Analysis
2.5. cDNA Synthesis and Real-Time PCR
2.6. Protein Extraction and Western Blotting
2.7. Immunohistochemistry
2.7.1. Immunostaining and Quantification of Patient-Derived Cell Cultures for Epithelial Cell Markers
2.7.2. Immunofluorescent Detection of pSMAD1/5/8 in Patient Lung Tissue
3. Results
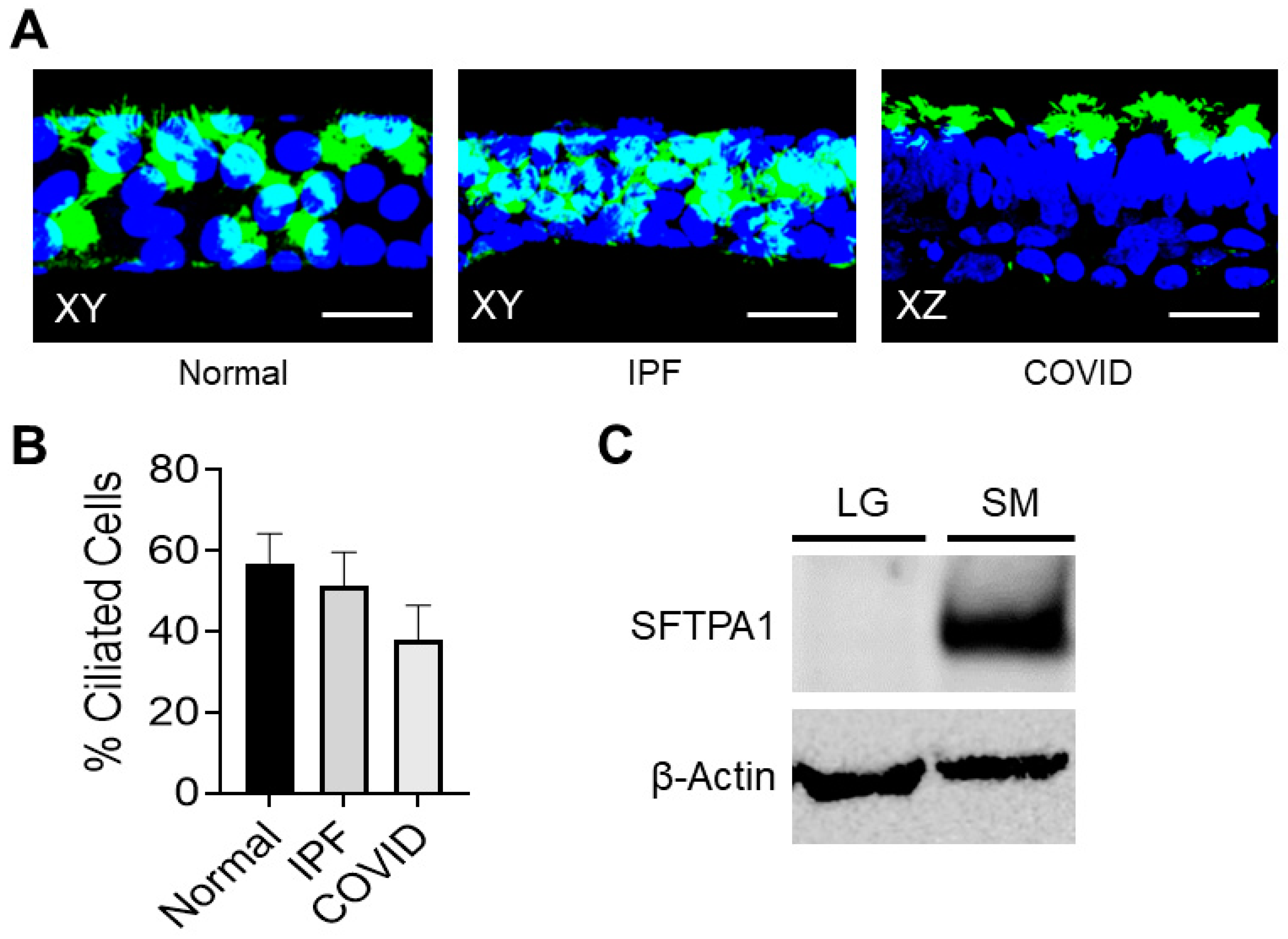
3.1. Patient-Derived Small Airway Cell Cultures Maintain Cell Markers and Characteristics Found in Fibrotic Lung Tissue
Patient-Derived Small Airway Cell Cultures Show Evidence of Ciliated Cells
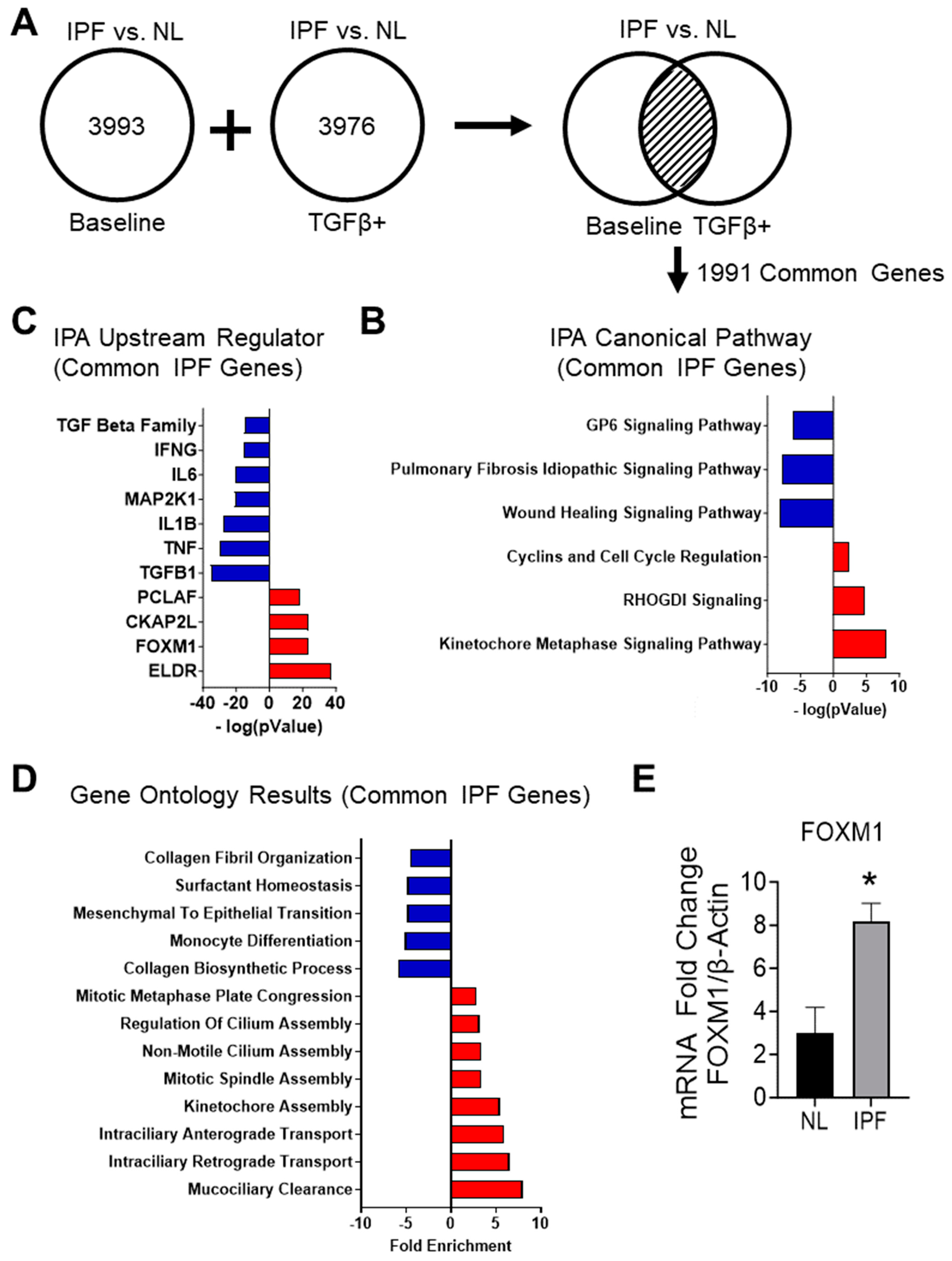
3.2. Determination of IPF Transcriptomic Signature
3.2.1. RNA-Sequencing of IPF and Non-IPF Control Small Airway Cells Demonstrate Unique Transcriptomic Signatures
3.2.2. Analyzing the Canonical Pathways in Small Airway Cultures of IPF Compared to Non-IPF Controls Reveals a Suppression of Pathways Associated with IPF
3.2.3. Upstream Regulators Associated with Common IPF Genes Predict the Inhibition of the TGF-β1-Signaling Pathway and Host Immune Response
3.2.4. Gene Ontology Results for Common IPF Genes Show Activation of Ciliary Movement and Cell Cycle Regulation and a Downregulation of Genes Associated with ECM Components and Immune Response
3.2.5. Transcriptomic Signatures of Cultured IPF Patient Cells Reflect Gene Expression Changes Seen in Single-Cell RNA Sequencing Data
3.2.6. RT-PCR Shows an Increase in FOXM1 in the IPF Cell Cultures
3.3. Determination of Post-COVID Fibrosis Transcriptomic Signature
3.3.1. Gene Expression Differences Are Observed between Post-COVID Fibrosis Cells and Non-IPF Control Small Airway Cells Cultured from Human Patients
3.3.2. Ingenuity Pathway Analysis of Post-COVID Fibrosis vs. Non-IPF Control Small Airway Cultures Demonstrates a Trend toward Host Immune Response and Interferon Signaling
3.3.3. Upstream Regulator Results for Post-COVID Fibrosis Common Genes Show Predicted Activation of the Immune Response but Not Cytokine-Related Molecules
3.3.4. Post-COVID Fibrosis Gene Ontology Results Show Activation of Host Immune Response and Decrease in Cilium Movement and DNA Repair
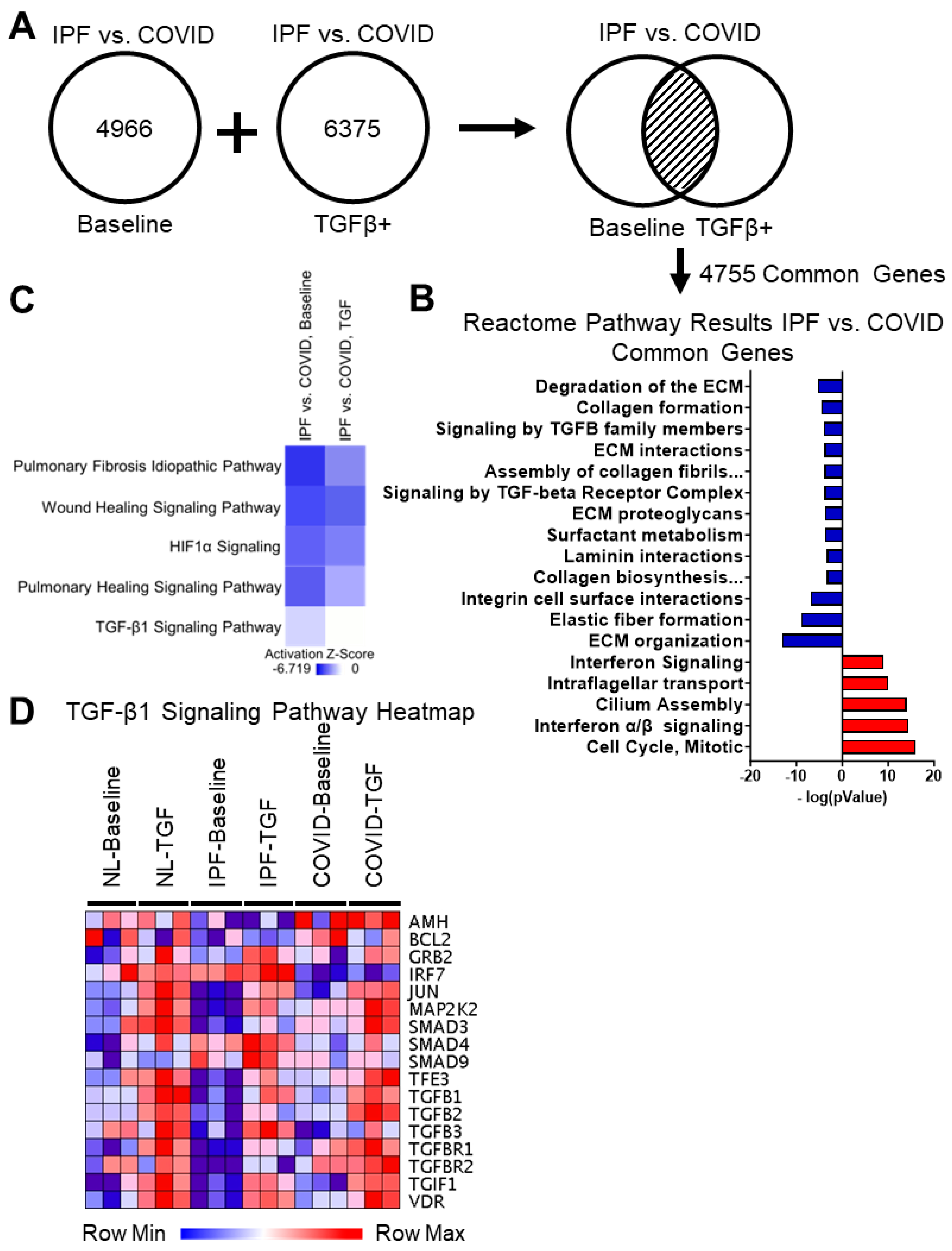
3.4. Identification of Crucial Differences between IPF and Post-COVID Fibrosis Small Airway Cell Cultures
3.4.1. Differential Gene Expression Analysis of IPF vs. Post-COVID Fibrosis Shows Common Genes between Baseline Cultures and TGF-β1 Culture Conditions
3.4.2. Reactome Pathway Results for the IPF vs. Post-COVID Fibrosis Common Genes Show Upregulation in Interferon Signaling, Microtubule Organization, and Cell Cycle in the IPF Cell Cultures Compared to the Post-COVID Fibrosis
3.4.3. Reactome Pathway Results for the IPF vs. Post-COVID Fibrosis Common Genes Show Downregulation in Extracellular Matrix Assembly, Cell-Surface Interactions, and Integrin Signaling in the IPF Cell Cultures
3.4.4. Comparing Gene Expression in Small Airway Cell Cultures from IPF and Post-COVID Fibrosis Reveals Distinctions in Baseline TGF-β1 Signaling
3.4.5. Differences in BMP Signaling Are Observed between Post-COVID Fibrosis and IPF Cell Cultures
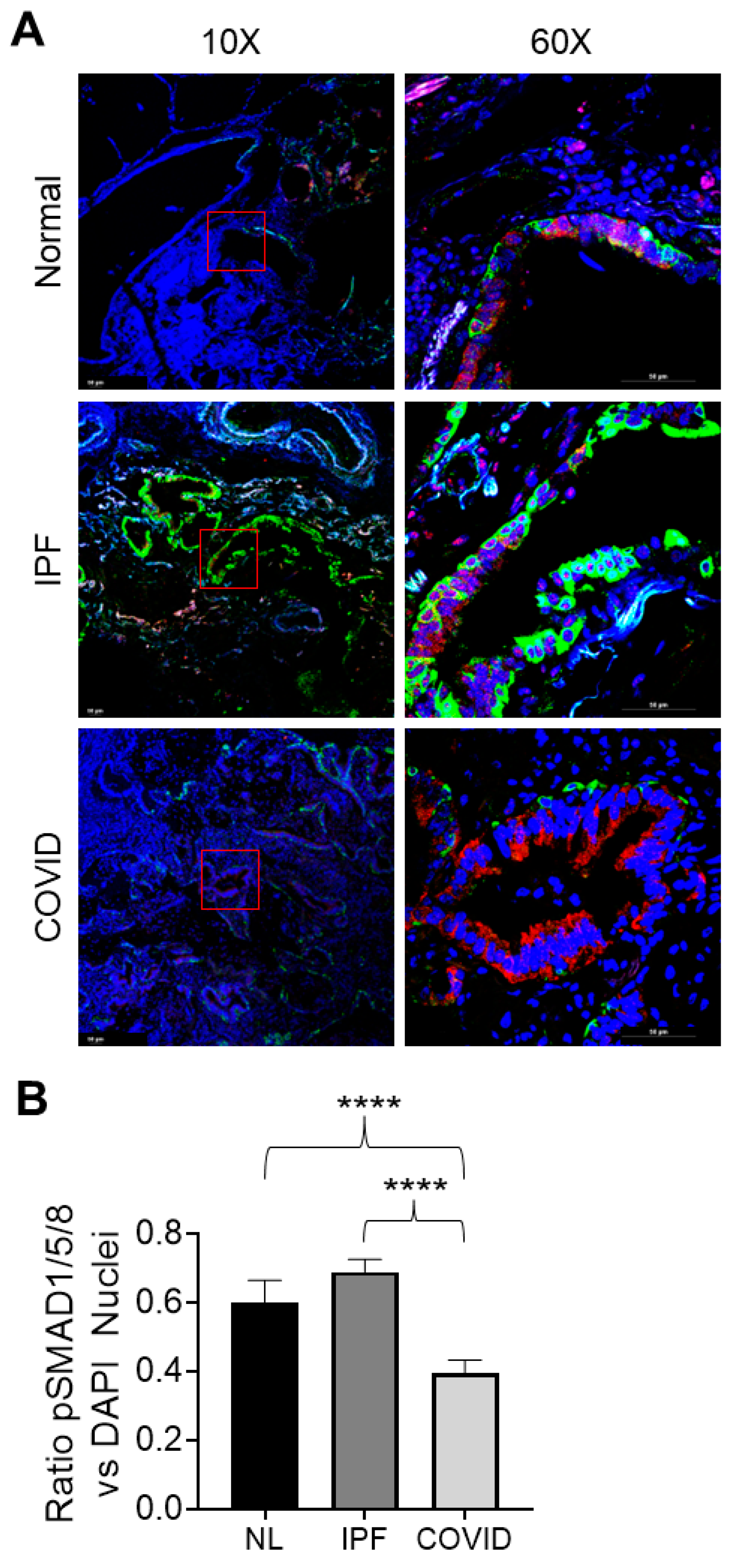
3.4.6. pSMAD1/5/8 Expression and Nuclear Localization in Normal, IPF, and Post-COVID Fibrosis Tissues
4. Discussion
4.1. Patient-Derived Small Airway Cell Cultures Maintain Tissue-Specific Markers and Reflect Gene Expression Patterns Observed in Fibrotic Lung Tissue
4.2. Transcriptomic Signatures of IPF Cell Cultures Include the Activation of Cell Cycle Regulators, the Upregulation of Ciliary Movement, and the Predicted Inhibition of the Pulmonary Fibrosis Idiopathic and TGF-β1 Signaling Pathways
4.3. The Disease Signature of Post-COVID Fibrosis Illustrates a Distinct Interplay of Activation and Inhibition within Components of the Immune System
4.4. Comparison of IPF vs. Post-COVID Fibrosis DEGs Highlights Mucociliary Dysfunction in Post-COVID Fibrosis Cell Cultures Compared to IPF
4.5. IPF Cell Cultures Demonstrate a Decrease in Extracellular Matrix-Associated Pathways and Collagen Fiber Biosynthesis Compared to Post-COVID Fibrosis Cell Cultures
4.6. IPF Small Airway Cell Cultures Demonstrate Lower Levels of Baseline TGF-β1 Signaling
4.7. Higher Levels of BMP Signaling in IPF Samples Decrease TGF-β1 Signaling and Contribute to the Predicted Inhibition of Key Fibrosis-Related Pathways
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ILD | Interstitial lung disease |
| IPF | Idiopathic pulmonary fibrosis |
| COPD | Chronic obstructive pulmonary disease |
| SARS-CoV-2, COVID-19 | Severe acute respiratory syndrome coronavirus 2 |
| TGF-β1 | Transforming growth factor beta |
References
- Stancil, I.T.; Michalski, J.E.; Schwartz, D.A. An Airway-Centric View of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2022, 206, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.S.; Tager, A.M.; Borok, Z.; Moore, B.B.; Schwartz, D.A.; Anstrom, K.J.; Bar-Joseph, Z.; Bitterman, P.; Blackburn, M.R.; Bradford, W.; et al. Future Directions in Idiopathic Pulmonary Fibrosis Research. An NHLBI Workshop Report. Am. J. Respir. Crit. Care Med. 2014, 189, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Stancil, I.T.; Michalski, J.E.; Davis-Hall, D.; Chu, H.W.; Park, J.A.; Magin, C.M.; Yang, I.V.; Smith, B.J.; Dobrinskikh, E.; Schwartz, D.A. Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat. Commun. 2021, 12, 4566. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; Tanabe, N.; McDonough, J.E.; Vasilescu, D.M.; Xu, F.; Wuyts, W.A.; Piloni, D.; De Sadeleer, L.; Willems, S.; Mai, C.; et al. Small airways pathology in idiopathic pulmonary fibrosis: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Abdelgied, M.; Uhl, K.; Chen, O.G.; Schultz, C.; Tripp, K.; Peraino, A.M.; Paithankar, S.; Chen, B.; Tamae Kakazu, M.; Castillo Bahena, A.; et al. Targeting ATP12A, a Non-Gastric Proton Pump Alpha Subunit, for Idiopathic Pulmonary Fibrosis Treatment. Am. J. Respir. Cell Mol. Biol. 2023, 68, 638–650. [Google Scholar] [CrossRef]
- Maher, T.M. Small airways in idiopathic pulmonary fibrosis: Quiet but not forgotten. Am. J. Respir. Crit. Care Med. 2021, 204, 1010–1011. [Google Scholar] [CrossRef]
- Ikezoe, K.; Hackett, T.-L.; Peterson, S.; Prins, D.; Hague, C.J.; Murphy, D.; LeDoux, S.; Chu, F.; Xu, F.; Cooper, J.D. Small airway reduction and fibrosis is an early pathologic feature of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 1048–1059. [Google Scholar] [CrossRef]
- Bösmüller, H.; Matter, M.; Fend, F.; Tzankov, A. The pulmonary pathology of COVID-19. Virchows Arch. 2021, 478, 137–150. [Google Scholar] [CrossRef]
- Giacomelli, C.; Piccarducci, R.; Marchetti, L.; Romei, C.; Martini, C. Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: Lessons from post-COVID-19 patients. Biochem. Pharmacol. 2021, 193, 114812. [Google Scholar] [CrossRef]
- Polak, S.B.; Van Gool, I.C.; Cohen, D.; von der Thüsen, J.H.; van Paassen, J. A systematic review of pathological findings in COVID-19: A pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020, 33, 2128–2138. [Google Scholar] [CrossRef]
- Cho, J.L.; Villacreses, R.; Nagpal, P.; Guo, J.; Pezzulo, A.A.; Thurman, A.L.; Hamzeh, N.Y.; Blount, R.J.; Fortis, S.; Hoffman, E.A. Quantitative chest CT assessment of small airways disease in post-acute SARS-CoV-2 infection. Radiology 2022, 304, 185–192. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, N.; Liu, Y.; Yang, X.; He, Y.; Li, Q.; Shen, X.; Zhu, Y.; Yang, Y. The Interaction Between Pulmonary Fibrosis and COVID-19 and the Application of Related Anti-Fibrotic Drugs. Front. Pharmacol. 2022, 12, 805535. [Google Scholar] [CrossRef]
- Weng, D.; Chen, X.-Q.; Qiu, H.; Zhang, Y.; Li, Q.-H.; Zhao, M.-M.; Wu, Q.; Chen, T.; Hu, Y.; Wang, L.-S. The role of infection in acute exacerbation of idiopathic pulmonary fibrosis. Mediat. Inflamm. 2019, 2019, 5160694. [Google Scholar] [CrossRef]
- Yue, X.; Shan, B.; A Lasky, J. TGF-β: Titan of lung fibrogenesis. Curr. Enzym. Inhib. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Evans, R.M.; Lippman, S.M. Shining light on the COVID-19 pandemic: A vitamin D receptor checkpoint in defense of unregulated wound healing. Cell Metab. 2020, 32, 704–709. [Google Scholar] [CrossRef]
- Li, X.; Molina-Molina, M.; Abdul-Hafez, A.; Ramirez, J.; Serrano-Mollar, A.; Xaubet, A.; Uhal, B.D. Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 291, L887–L895. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J. Regulation of transforming growth factor-β1–driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.; Liefeld, T.; Gould, J.; Lerner, J.; Tamayo, P.; Mesirov, J.P. GenePattern 2.0. Nat. Genet. 2006, 38, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Sauleda, J.; Núñez, B.; Sala, E.; Soriano, J. Idiopathic Pulmonary Fibrosis: Epidemiology, Natural History, Phenotypes. Med. Sci. 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Neumark, N.; Cosme, C., Jr.; Rose, K.-A.; Kaminski, N. The idiopathic pulmonary fibrosis cell atlas. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L887–L892. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, F.; Tan, Q.; Guo, M.; Ma, P.; Wang, X.; Zhang, S.; Xu, J.; Luo, P.; Jin, Y. The multifaceted roles of FOXM1 in pulmonary disease. Cell Commun. Signal. 2019, 17, 35. [Google Scholar] [CrossRef]
- Lee, J.; Oh, D.H.; Park, K.C.; Choi, J.E.; Kwon, J.B.; Lee, J.; Park, K.; Sul, H.J. Increased primary cilia in idiopathic pulmonary fibrosis. Mol. Cells 2018, 41, 224. [Google Scholar]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Sayit, A.T.; Elmali, M.; Deveci, A.; Gedikli, O. Relationship between acute phase reactants and prognosis in patients with or without COVID-19 pneumonia. Rev. Inst. Med. Trop. São Paulo 2021, 63, e51. [Google Scholar] [CrossRef]
- Li, L.; Chen, C. Contribution of acute-phase reaction proteins to the diagnosis and treatment of 2019 novel coronavirus disease (COVID-19). Epidemiol. Infect. 2020, 148, e164. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y. Editorial: Eicosanoids and cytokines: Resolution of inflammation. Front. Pharmacol. 2022, 13, 978331. [Google Scholar] [CrossRef]
- Dalskov, L.; Narita, R.; Andersen, L.L.; Jensen, N.; Assil, S.; Kristensen, K.H.; Mikkelsen, J.G.; Fujita, T.; Mogensen, T.H.; Paludan, S.R. Characterization of distinct molecular interactions responsible for IRF3 and IRF7 phosphorylation and subsequent dimerization. Nucleic Acids Res. 2020, 48, 11421–11433. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Santer, D.M.; O’Shea, D.; Tyrrell, D.L.; Houghton, M. The impact of the interferon-lambda family on the innate and adaptive immune response to viral infections. Emerg. Microbes Infect. 2014, 3, e51. [Google Scholar] [CrossRef]
- Robinot, R.; Hubert, M.; de Melo, G.D.; Lazarini, F.; Bruel, T.; Smith, N.; Levallois, S.; Larrous, F.; Fernandes, J.; Gellenoncourt, S. SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat. Commun. 2021, 12, 4354. [Google Scholar] [CrossRef]
- Huang, J.-J.; Wang, C.-W.; Liu, Y.; Zhang, Y.-Y.; Yang, N.-B.; Yu, Y.-C.; Jiang, Q.; Song, Q.-F.; Qian, G.-Q. Role of the extracellular matrix in COVID-19. World J. Clin. Cases 2023, 11, 73. [Google Scholar] [CrossRef]
- Dituri, F.; Cossu, C.; Mancarella, S.; Giannelli, G. The Interactivity between TGFβ and BMP Signaling in Organogenesis, Fibrosis, and Cancer. Cells 2019, 8, 1130. [Google Scholar] [CrossRef]
- Ohkouchi, S.; Kanehira, M.; Saigusa, D.; Ono, M.; Tazawa, R.; Terunuma, H.; Hirano, T.; Numakura, T.; Notsuda, H.; Inoue, C. Metabolic and Epigenetic Regulation of SMAD7 by STC1 Ameliorates Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 67, 320–333. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhl, K.; Paithankar, S.; Leshchiner, D.; Jager, T.E.; Abdelgied, M.; Dixit, B.; Marashdeh, R.; Luo-Li, D.; Tripp, K.; Peraino, A.M.; et al. Differential Transcriptomic Signatures of Small Airway Cell Cultures Derived from IPF and COVID-19-Induced Exacerbation of Interstitial Lung Disease. Cells 2023, 12, 2501. https://doi.org/10.3390/cells12202501
Uhl K, Paithankar S, Leshchiner D, Jager TE, Abdelgied M, Dixit B, Marashdeh R, Luo-Li D, Tripp K, Peraino AM, et al. Differential Transcriptomic Signatures of Small Airway Cell Cultures Derived from IPF and COVID-19-Induced Exacerbation of Interstitial Lung Disease. Cells. 2023; 12(20):2501. https://doi.org/10.3390/cells12202501
Chicago/Turabian StyleUhl, Katie, Shreya Paithankar, Dmitry Leshchiner, Tara E. Jager, Mohamed Abdelgied, Bhavna Dixit, Raya Marashdeh, Dewen Luo-Li, Kaylie Tripp, Angela M. Peraino, and et al. 2023. "Differential Transcriptomic Signatures of Small Airway Cell Cultures Derived from IPF and COVID-19-Induced Exacerbation of Interstitial Lung Disease" Cells 12, no. 20: 2501. https://doi.org/10.3390/cells12202501
APA StyleUhl, K., Paithankar, S., Leshchiner, D., Jager, T. E., Abdelgied, M., Dixit, B., Marashdeh, R., Luo-Li, D., Tripp, K., Peraino, A. M., Tamae Kakazu, M., Lawson, C., Chesla, D. W., Luo-Li, N., Murphy, E. T., Prokop, J., Chen, B., Girgis, R. E., & Li, X. (2023). Differential Transcriptomic Signatures of Small Airway Cell Cultures Derived from IPF and COVID-19-Induced Exacerbation of Interstitial Lung Disease. Cells, 12(20), 2501. https://doi.org/10.3390/cells12202501





