Octopamine Rescues Endurance and Climbing Speed in Drosophila Clkout Mutants with Circadian Rhythm Disruption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Maintenance
2.2. Exercise
2.3. Octopamine Feeding
2.4. Endurance
2.5. Climbing Speed Evaluation
2.6. Flight Performance Test
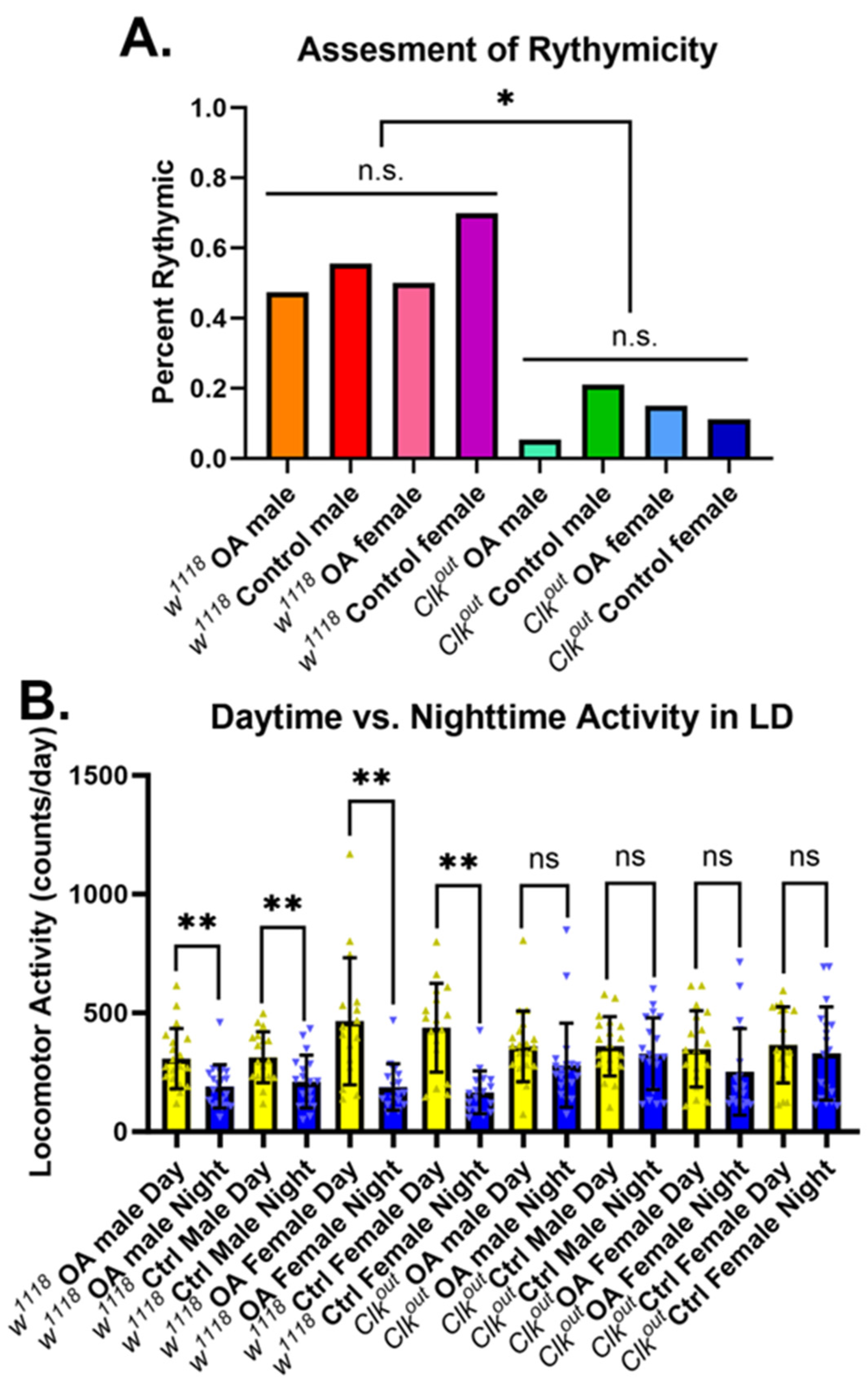
2.7. Activity Rhythm Evaluation
2.8. Quantitative Reverse Transcriptase PCR
3. Results
3.1. Clkout Mutants Have Severe and Robust Phenotypes in Climbing Speed, Endurance, and Flight Performance
3.2. Activation of Exercise Response Pathways by Octopamine Can Rescue Exercise Phenotypes of Clkout Mutants
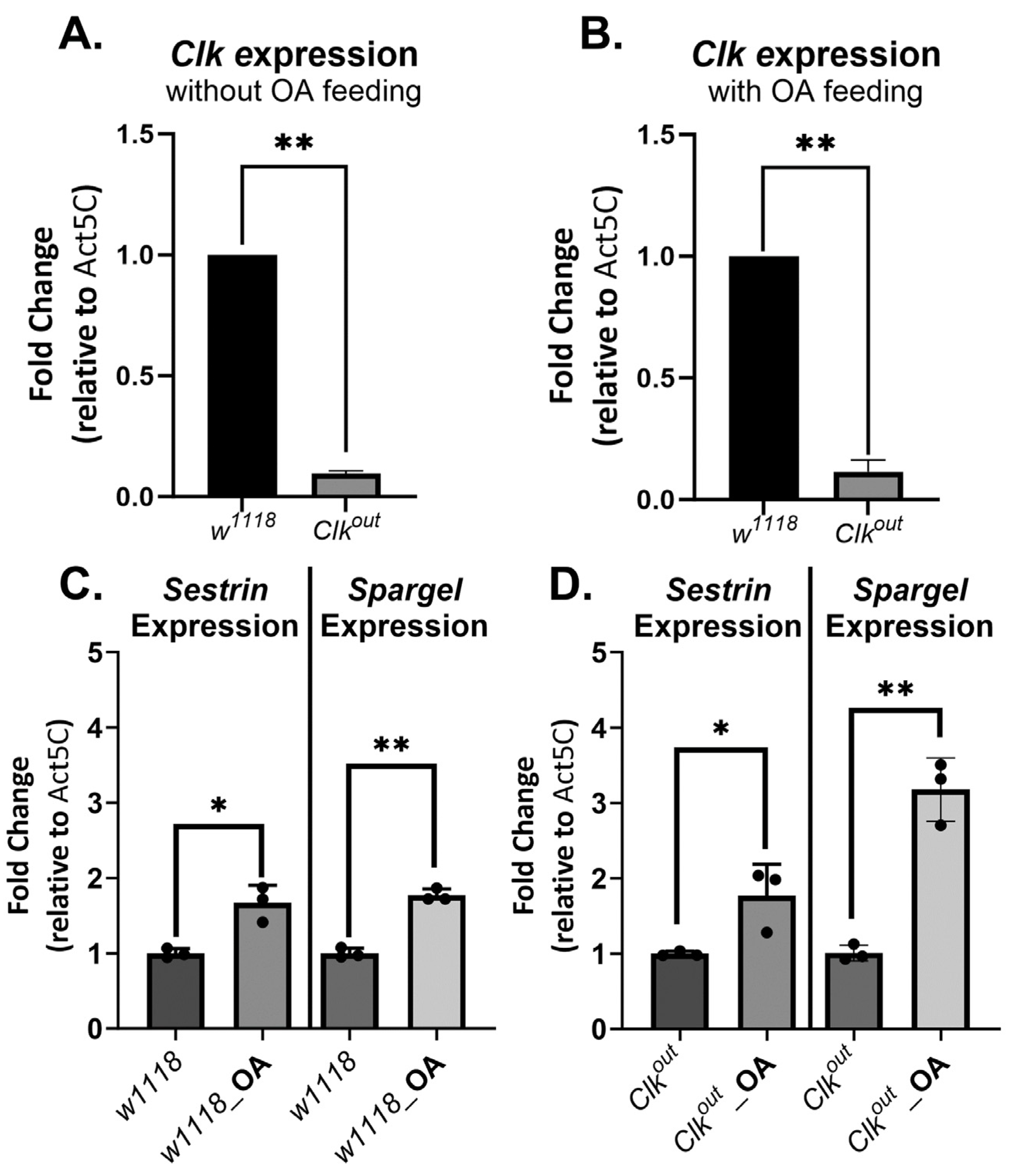
3.3. Octopamine Feeding Leads to Increased Expression of Two Exercise Response Transcripts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Videnovic, A.; Lazar, A.S.; Barker, R.A.; Overeem, S. ‘The clocks that time us’—Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2014, 10, 683–693. [Google Scholar] [CrossRef]
- Douma, L.G.; Gumz, M.L. Circadian clock-mediated regulation of blood pressure. Free Radic. Biol. Med. 2018, 119, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Krauchi, K. How is the circadian rhythm of core body temperature regulated? Clin. Auton. Res. 2002, 12, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Tomioka, K. Heterogeneity of the Peripheral Circadian Systems in Drosophila melanogaster: A Review. Front. Physiol. 2016, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Wolff, C.A.; Esser, K.A. Exercise timing and circadian rhythms. Curr. Opin. Physiol. 2019, 10, 64–69. [Google Scholar] [CrossRef]
- Peschel, N.; Helfrich-Förster, C. Setting the clock—By nature: Circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011, 585, 1435–1442. [Google Scholar] [CrossRef]
- Mahesh, G.; Jeong, E.; Ng, F.S.; Liu, Y.; Gunawardhana, K.; Houl, J.H.; Yildirim, E.; Amunugama, R.; Jones, R.; Allen, D.L.; et al. Phosphorylation of the transcription activator CLOCK regulates progression through a approximately 24-h feedback loop to influence the circadian period in Drosophila. J. Biol. Chem. 2014, 289, 19681–19693. [Google Scholar] [CrossRef]
- Gutierrez-Monreal, M.A.; Harmsen, J.F.; Schrauwen, P.; Esser, K.A. Ticking for Metabolic Health: The Skeletal-Muscle Clocks. Obesity 2020, 28 (Suppl. S1), S46–S54. [Google Scholar] [CrossRef]
- Smagula, S.F.; Stone, K.L.; Fabio, A.; Cauley, J.A. Risk factors for sleep disturbances in older adults: Evidence from prospective studies. Sleep Med. Rev. 2016, 25, 21–30. [Google Scholar] [CrossRef]
- Kim, M.; Opsasnick, L.; Batio, S.; Benavente, J.Y.; Zheng, P.; Lovett, R.M.; Bailey, S.C.; Kwasny, M.J.; Ladner, D.P.; Chou, S.H.Y.; et al. Prevalence and risk factors of sleep disturbance in adults with underlying health conditions during the ongoing COVID-19 pandemic. Medicine 2022, 101, e30637. [Google Scholar] [CrossRef]
- Atkinson, G.; Fullick, S.; Grindey, C.; Maclaren, D. Exercise, energy balance and the shift worker. Sports Med. 2008, 38, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Kalesan, B. Sleep duration and mortality: A systematic review and meta-analysis. J. Sleep Res. 2009, 18, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Cogliati, C.; Fiorelli, E.M.; Nunziata, V.; Wu, M.A.; Prado, M.; Bevilacqua, M.; Trabattoni, D.; Porta, A.; Montano, N. One night on-call: Sleep deprivation affects cardiac autonomic control and inflammation in physicians. Eur. J. Intern. Med. 2013, 24, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Lasfargues, G.; Vol, S.; Caces, E.; Le Clesiau, H.; Lecomte, P.; Tichet, J. Relations among night work, dietary habits, biological measure, and health status. Int. J. Behav. Med. 1996, 3, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Knutsson, A.; Lindahl, B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup. Environ. Med. 2001, 58, 747–752. [Google Scholar] [CrossRef]
- Mattis, J.; Sehgal, A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol. Metab. 2016, 27, 192–203. [Google Scholar] [CrossRef]
- Krishnan, N.; Rakshit, K.; Chow, E.S.; Wentzell, J.S.; Kretzschmar, D.; Giebultowicz, J.M. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol. Dis. 2012, 45, 1129–1135. [Google Scholar] [CrossRef]
- Means, J.C.; Venkatesan, A.; Gerdes, B.; Fan, J.Y.; Bjes, E.S.; Price, J.L. Drosophila spaghetti and doubletime link the circadian clock and light to caspases, apoptosis and tauopathy. PLoS Genet. 2015, 11, e1005171. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Kretzschmar, D.; Rakshit, K.; Chow, E.; Giebultowicz, J.M. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging 2009, 1, 937–948. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Spanos, M.; Li, G.; Lu, R.; Bei, Y.; Xiao, J. Exercise training maintains cardiovascular health: Signaling pathways involved and potential therapeutics. Signal Transduct. Target. Ther. 2022, 7, 306. [Google Scholar] [CrossRef]
- Streckmann, F.; Balke, M.; Cavaletti, G.; Toscanelli, A.; Bloch, W.; Decard, B.F.; Lehmann, H.C.; Faude, O. Exercise and Neuropathy: Systematic Review with Meta-Analysis. Sports Med. 2022, 52, 1043–1065. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Esser, K.A.; Kraus, W.E.; Buford, T.W.; Redman, L.M. A Role for Exercise to Counter Skeletal Muscle Clock Disruption. Exerc. Sport Sci. Rev. 2021, 49, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Min, S.K.; Kwon, Y.C.; Park, S.K.; Park, H. Effects of intermittent exercise on biomarkers of cardiovascular risk in night shift workers. Atherosclerosis 2015, 242, 186–190. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.I.; Honma, S. Roles of Neuropeptides, VIP and AVP, in the Mammalian Central Circadian Clock. Front. Neurosci. 2021, 15, 650154. [Google Scholar] [CrossRef]
- Schroeder, A.M.; Truong, D.; Loh, D.H.; Jordan, M.C.; Roos, K.P.; Colwell, C.S. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J. Physiol. 2012, 590, 6213–6226. [Google Scholar] [CrossRef]
- Adamovich, Y.; Dandavate, V.; Ezagouri, S.; Manella, G.; Zwighaft, Z.; Sobel, J.; Kuperman, Y.; Golik, M.; Auerbach, A.; Itkin, M.; et al. Clock proteins and training modify exercise capacity in a daytime-dependent manner. Proc. Natl. Acad. Sci. USA 2021, 118, e2101115118. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yan, L.; Zhu, R.; Wei, H.; Wang, J.; Zheng, L.; Zhang, Y. Skeletal-Muscle-Specific Overexpression of Chrono Leads to Disruption of Glucose Metabolism and Exercise Capacity. Life 2022, 12, 1233. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Machado, F.S.; Foscolo, D.R.; Poletini, M.O.; Coimbra, C.C. Influence of Time-of-Day on Maximal Exercise Capacity Is Related to Daily Thermal Balance but Not to Induced Neuronal Activity in Rats. Front. Physiol. 2016, 7, 464. [Google Scholar] [CrossRef]
- Dalbram, E.; Basse, A.L.; Zierath, J.R.; Treebak, J.T. Voluntary wheel running in the late dark phase ameliorates diet-induced obesity in mice without altering insulin action. J. Appl. Physiol. (1985) 2019, 126, 993–1005. [Google Scholar] [CrossRef]
- Sedliak, M.; Zeman, M.; Buzgo, G.; Cvecka, J.; Hamar, D.; Laczo, E.; Okuliarova, M.; Vanderka, M.; Kampmiller, T.; Hakkinen, K.; et al. Morphological, molecular and hormonal adaptations to early morning versus afternoon resistance training. Chronobiol. Int. 2018, 35, 450–464. [Google Scholar] [CrossRef]
- Watanabe, L.P.; Riddle, N.C. New opportunities: Drosophila as a model system for exercise research. J. Appl. Physiol. (1985) 2019, 127, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Sujkowski, A.; Bazzell, B.; Carpenter, K.; Arking, R.; Wessells, R.J. Endurance exercise and selective breeding for longevity extend Drosophila healthspan by overlapping mechanisms. Aging 2015, 7, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Mahesh, G.; Houl, J.H.; Hardin, P.E. Circadian Activators Are Expressed Days before They Initiate Clock Function in Late Pacemaker Neurons from Drosophila. J. Neurosci. 2015, 35, 8662–8671. [Google Scholar] [CrossRef]
- Kim, M.; Sujkowski, A.; Namkoong, S.; Gu, B.; Cobb, T.; Kim, B.; Kowalsky, A.H.; Cho, C.S.; Semple, I.; Ro, S.H.; et al. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun. 2020, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Sujkowski, A.; Ramesh, D.; Brockmann, A.; Wessells, R. Octopamine Drives Endurance Exercise Adaptations in Drosophila. Cell Rep. 2017, 21, 1809–1823. [Google Scholar] [CrossRef]
- Vargas-Ortiz, K.; Perez-Vazquez, V.; Macias-Cervantes, M.H. Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2717. [Google Scholar] [CrossRef]
- Sujkowski, A.; Gretzinger, A.; Soave, N.; Todi, S.V.; Wessells, R. Alpha- and beta-adrenergic octopamine receptors in muscle and heart are required for Drosophila exercise adaptations. PLoS Genet. 2020, 16, e1008778. [Google Scholar] [CrossRef]
- Damschroder, D.; Cobb, T.; Sujkowski, A.; Wessells, R. Drosophila Endurance Training and Assessment of Its Effects on Systemic Adaptations. Bio Protoc. 2018, 8, e3037. [Google Scholar] [CrossRef]
- Sujkowski, A.; Richardson, K.; Prifti, M.V.; Wessells, R.J.; Todi, S.V. Endurance exercise ameliorates phenotypes in Drosophila models of spinocerebellar ataxias. eLife 2022, 11, e75389. [Google Scholar] [CrossRef]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Chiu, J.C.; Low, K.H.; Pike, D.H.; Yildirim, E.; Edery, I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J. Vis. Exp. 2010, 43, e2157. [Google Scholar] [CrossRef]
- Cichewicz, K.; Hirsh, J. ShinyR-DAM: A program analyzing Drosophila activity, sleep and circadian rhythms. Commun. Biol. 2018, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Konopka, R.J.; Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef]
- Piazza, N.; Gosangi, B.; Devilla, S.; Arking, R.; Wessells, R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE 2009, 4, e5886. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, D.; Zapata-Perez, R.; Richardson, K.; Vaz, F.M.; Houtkooper, R.H.; Wessells, R. Stimulating the sir2-spargel axis rescues exercise capacity and mitochondrial respiration in a Drosophila model of Barth syndrome. Dis. Model. Mech. 2022, 15, dmm049279. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, J.D. Transcriptional control of circadian metabolic rhythms in the liver. Diabetes Obes. Metab. 2015, 17 (Suppl. S1), 33–38. [Google Scholar] [CrossRef]
- Leone, T.C.; Lehman, J.J.; Finck, B.N.; Schaeffer, P.J.; Wende, A.R.; Boudina, S.; Courtois, M.; Wozniak, D.F.; Sambandam, N.; Bernal-Mizrachi, C.; et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005, 3, e101. [Google Scholar] [CrossRef]
- Kochan, D.Z.; Ilnytskyy, Y.; Golubov, A.; Deibel, S.H.; McDonald, R.J.; Kovalchuk, O. Circadian-disruption-induced gene expression changes in rodent mammary tissues. Oncoscience 2016, 3, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liu, R.; de Jesus, D.; Kim, B.S.; Ma, K.; Moulik, M.; Yechoor, V. Circadian control of beta-cell function and stress responses. Diabetes Obes. Metab. 2015, 17 (Suppl. S1), 123–133. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, K.; Wambua, R.; Giebultowicz, T.M.; Giebultowicz, J.M. Effects of exercise on circadian rhythms and mobility in aging Drosophila melanogaster. Exp. Gerontol. 2013, 48, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Damulewicz, M.; Tyszka, A.; Pyza, E. Light exposure during development affects physiology of adults in Drosophila melanogaster. Front. Physiol. 2022, 13, 1008154. [Google Scholar] [CrossRef]
- Hunt, L.C.; Jiao, J.; Wang, Y.D.; Finkelstein, D.; Rao, D.; Curley, M.; Robles-Murguia, M.; Shirinifard, A.; Pagala, V.R.; Peng, J.; et al. Circadian gene variants and the skeletal muscle circadian clock contribute to the evolutionary divergence in longevity across Drosophila populations. Genome Res. 2019, 29, 1262–1276. [Google Scholar] [CrossRef]
- Ahsan, M.; Garneau, L.; Aguer, C. The bidirectional relationship between AMPK pathway activation and myokine secretion in skeletal muscle: How it affects energy metabolism. Front. Physiol. 2022, 13, 1040809. [Google Scholar] [CrossRef]
- Cobb, T.; Hwang, I.; Soukar, M.; Namkoong, S.; Cho, U.S.; Safdar, M.; Kim, M.; Wessells, R.J.; Lee, J.H. Iditarod, a Drosophila Homolog of the Irisin Precursor Fndc5, Is Critical for Exercise Performance and Cardiac Autophagy. Proc. Natl. Acad. Sci. USA 2023, 120, e2220556120. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Cao, Y.; Wang, X.; Tang, C.; Zheng, L. Stable Expression of dmiR-283 in the Brain Promises Positive Effects in Endurance Exercise on Sleep-Wake Behavior in Aging Drosophila. Int. J. Mol. Sci. 2023, 24, 4180. [Google Scholar] [CrossRef]
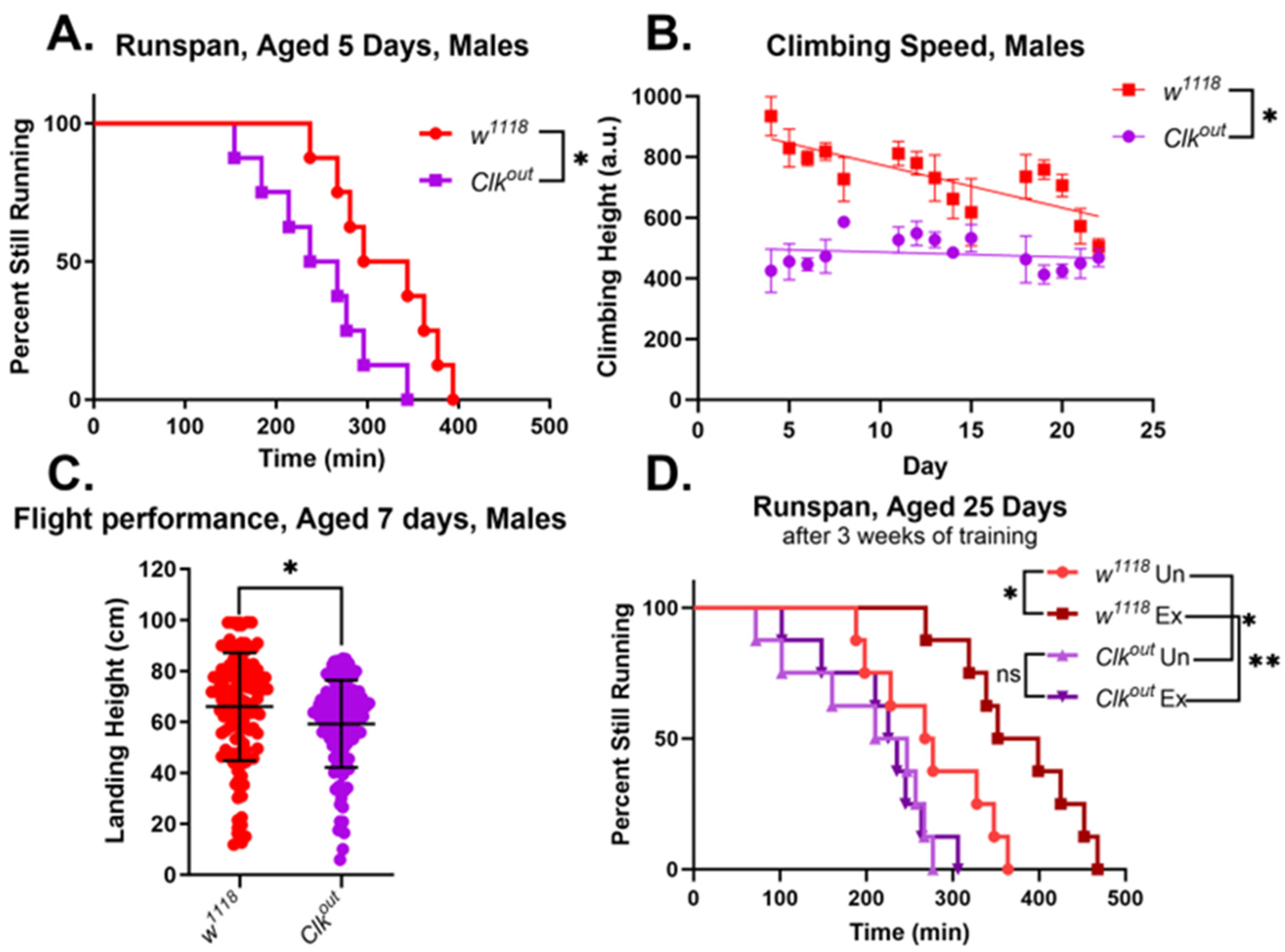

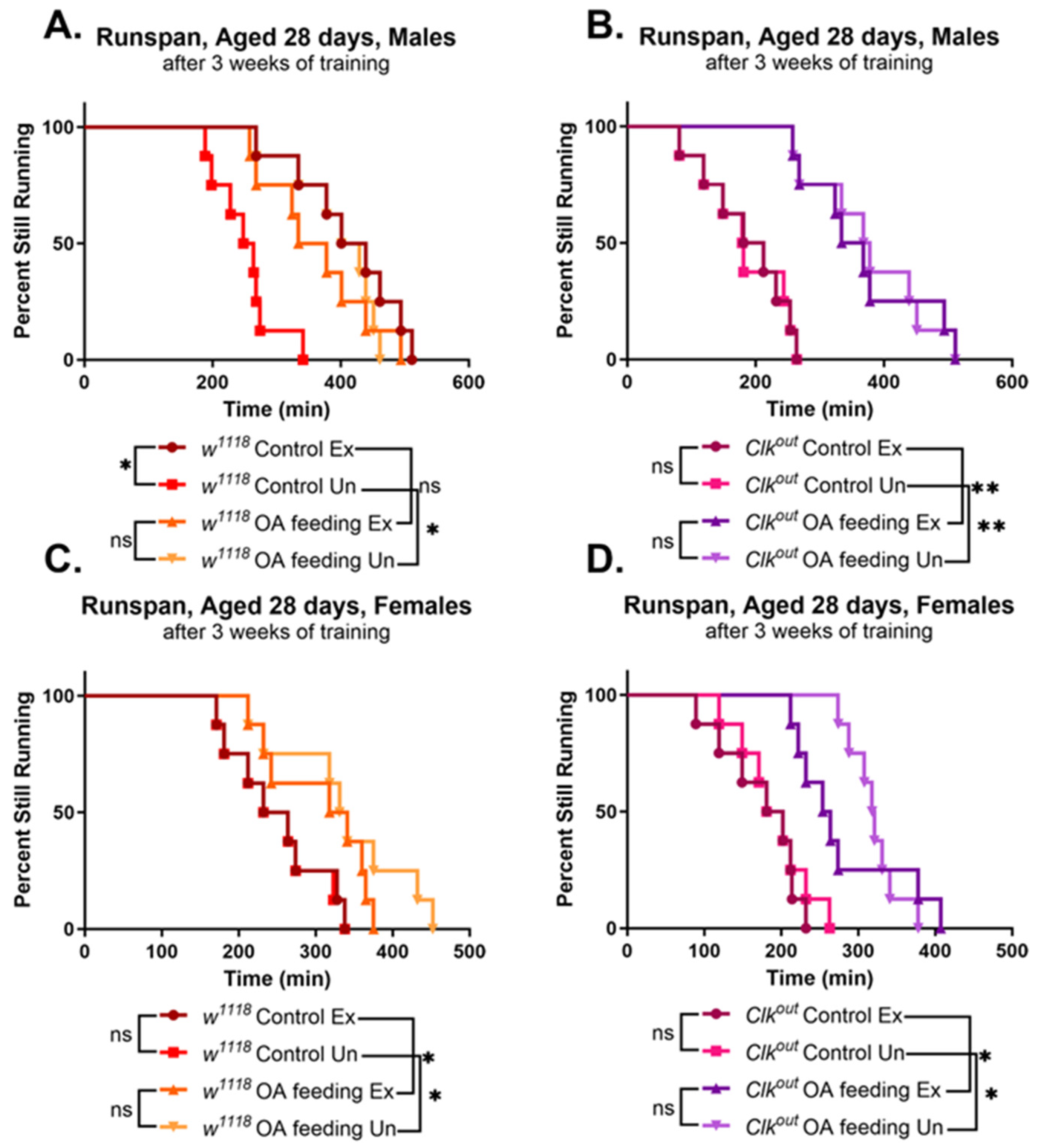
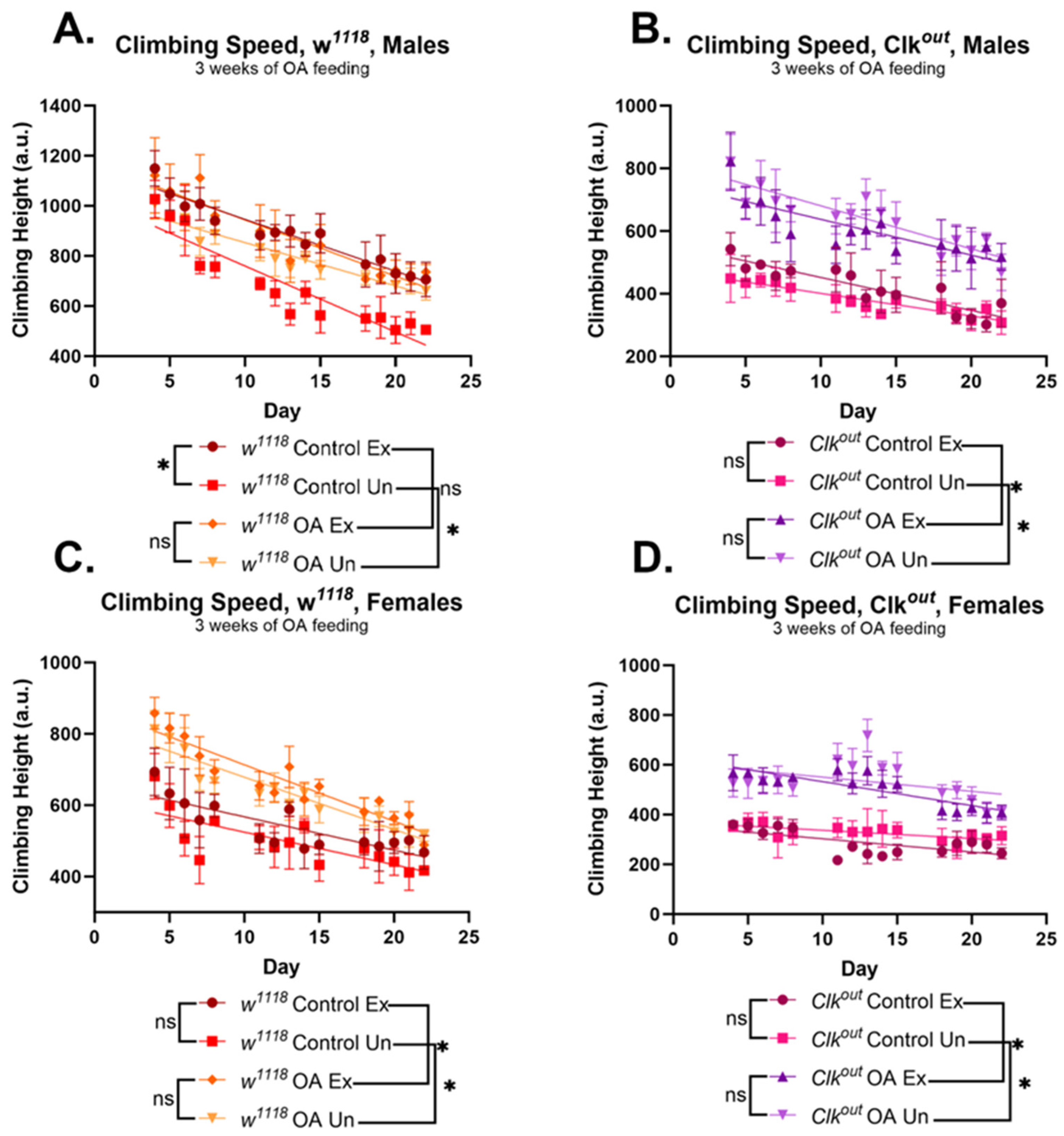
| Endurance | ||||
|---|---|---|---|---|
| w1118 Males | w1118 Females | Clkout Males | Clkout Females | |
| Exercise | + | - | - | - |
| OA Feeding | + | + | + | + |
| Exercise x OA | - | - | - | - |
| Climbing Speed | ||||
|---|---|---|---|---|
| w1118 Males | w1118 Females | Clkout Males | Clkout Females | |
| Exercise | + | - | - | - |
| OA Feeding | + | + | + | + |
| Exercise x OA | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safdar, M.; Wessells, R.J. Octopamine Rescues Endurance and Climbing Speed in Drosophila Clkout Mutants with Circadian Rhythm Disruption. Cells 2023, 12, 2515. https://doi.org/10.3390/cells12212515
Safdar M, Wessells RJ. Octopamine Rescues Endurance and Climbing Speed in Drosophila Clkout Mutants with Circadian Rhythm Disruption. Cells. 2023; 12(21):2515. https://doi.org/10.3390/cells12212515
Chicago/Turabian StyleSafdar, Maryam, and Robert J. Wessells. 2023. "Octopamine Rescues Endurance and Climbing Speed in Drosophila Clkout Mutants with Circadian Rhythm Disruption" Cells 12, no. 21: 2515. https://doi.org/10.3390/cells12212515
APA StyleSafdar, M., & Wessells, R. J. (2023). Octopamine Rescues Endurance and Climbing Speed in Drosophila Clkout Mutants with Circadian Rhythm Disruption. Cells, 12(21), 2515. https://doi.org/10.3390/cells12212515


.png)




