HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer
Abstract
1. Introduction
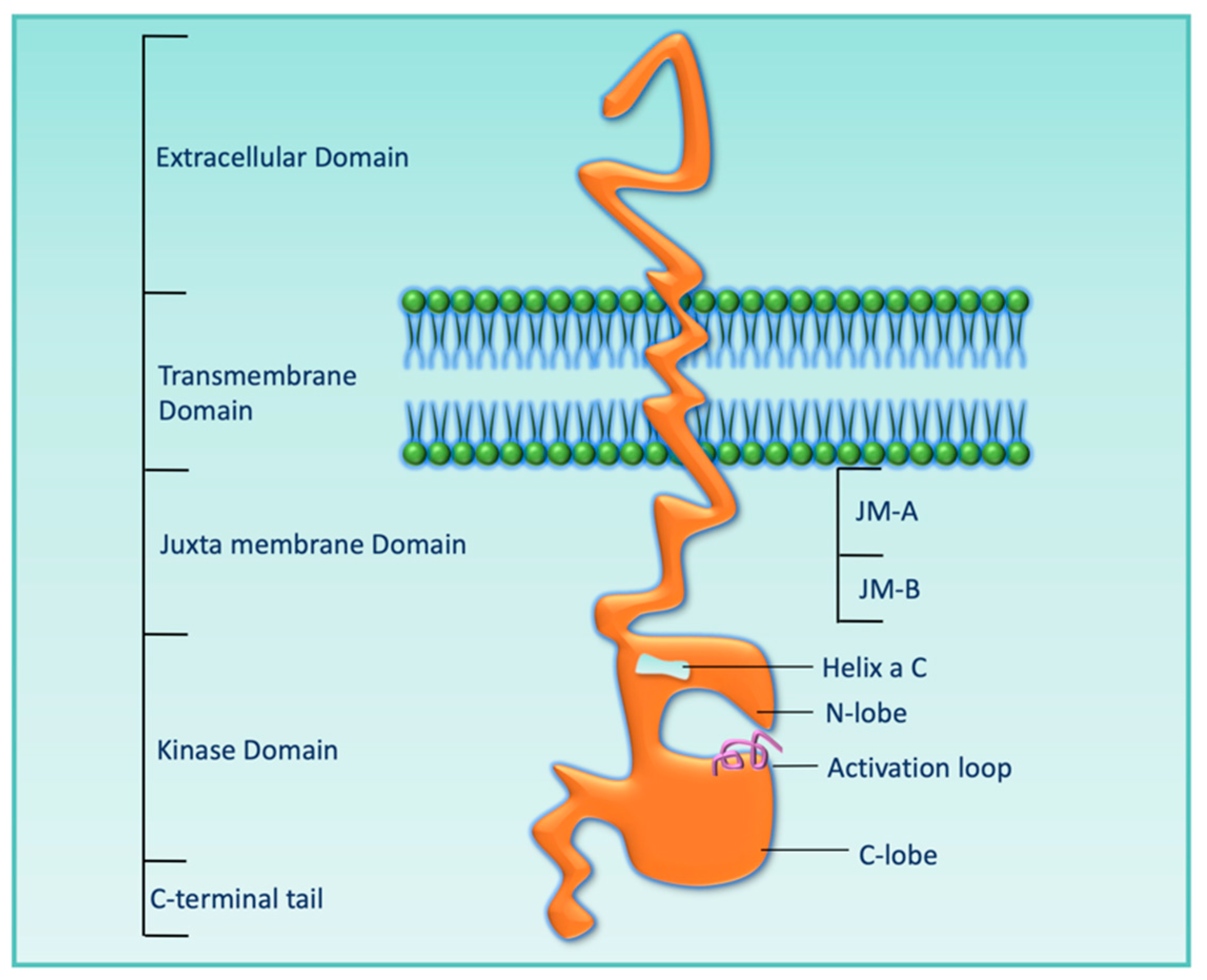
2. HER3 Structure and Mechanism of Activation
3. Expressions of HER3 in Different Types of Cancers
| Cancer Type | % of HER3 Overexpression | Antibody Used in IHC | Cutoff for Overexpression | Reference |
|---|---|---|---|---|
| Pancreatic | 41.3% | Nanotools, Teningen, Germany | Moderate staining is observed in >10% of tumor cells (score 2+), and strong staining is observed in >10% of tumor cells (score 3) | [56] |
| Breast | 43.0% | Clone 2F12, Labvision, Cheshire, UK | Positive: Optimal cutoffs for HER2:HER3 dimers were assessed by performing a minimum P value estimation using approximate 5% cutoffs across the entire dataset using relapse-free survival as an endpoint | [57] |
| 17.5% | IgG1, Neomarkers, UK | 4-point scale, where 0 = no staining, 1 = light staining, 2 = moderate staining, and 3 = strong staining | [58] | |
| Colorectal | 17.0% | MAb-MS-725-P, Neomarkers, Fremont, CA | Membranous staining: >1% of tumor cells stained. Cytoplasmic staining: 2+: moderate immunostaining in >10% of tumor cells and 3+: strong immunostaining in >10% of tumor cells | [59] |
| 69.7% | Lab Vision, Fremont, CA Cytoplasmic and membrane Cytoplasmic | Cytoplasmic staining: 0: no staining or weak staining in <10% of tumor cells. membranous staining: 0: no staining in <10% of tumor cells; 1: weak staining in >10% tumor cells; 2+: moderate staining in > 10% tumor cells; 3: strong staining in >10% tumor cells | [59] | |
| 20.9% | Clone C-17, 1:50; Santa Cruz, CA | Depending on the intensity of staining, HER3 expression was classified as weak, intermediate, or strong | [60] | |
| Gastric | 59.0% | Mouse monoclonal antibody, Neomarkers | 2+ = moderate staining, and 3+ = strong staining | [61] |
| 34.0% | RB-9211 rabbit polyclonal, dilution 1:100, N terminal; Neomarkers, Fremont, CA | 0 = <10% of positively stained cells; 1 = 10–25%; 2 = 26–50%; 3 = 51–75%; 4 = >75% | [52] | |
| Melanoma | approximately 65.0% | Clone C-17, 1:50 dilution; SantaCruz, CA | Positive: high GIS > 6 (GIS: German immunohistochemical scoring) | [60] |
| Ovary | 53.4% | C-17, rabbit polyclonal antibody; dilution: 1:25; Santa Cruz, CA | Positive: scores >8 | [62] |
| Head and Neck | 8.8% | RTJ.2, mouse monoclonal antibody; Santa Cruz, CA | Scores of 0, +1, +2, and +3 for increasing intensity | [55] |
| Cervix | 55.5% | MS-725-P, mouse antibody; Neomarkers | Intensity scale 0–4 based on pixel density | [63] |
4. Role of HER3 in the Genesis and Progression of Different Types of Cancer
5. Role of HER3 Ligands in Tumor Growth and Resistance to Different Anti-Cancer Therapies
6. Role of HER3 in Resistance to Different Anti-Cancer Therapies
6.1. HER3 as a Mediator of Resistance to Targeted Therapies
6.2. HER3 as a Mediator of Resistance to Hormonal Therapy, Chemotherapy, and Radiotherapy
7. Targeting HER3 and Its Challenges
8. Development of HER3-Directed Therapy and Its Clinical Stages
8.1. HER3-Targeting Monoclonal Antibodies and Clinical Trials
| Monoclonal Antibodies | Study Population | Clinical Trial Phase | Adverse Events | Status Finding | Reference |
|---|---|---|---|---|---|
| Lumretuzumab | Advanced or metastatic NSCLC | NCT02204345 phase I + II | Gastrointestinal, hematological, and nervous system toxicities, but generally mild and manageable | Terminated Efficacy of lumretuzumab + carboplatin + paclitaxel is similar to chemotherapy alone | [140] |
| Metastatic BC expressing HER3 and HER2 | NCT01918254 phase I | Diarrhea and hypokalemia | Completed Lumretuzumab + pertuzumab + paclitaxel was correlated with a serious incidence of diarrhea | [141] | |
| Metastatic and/or locally advanced malignant HER3 + solid tumors of epithelial cell origin | NCT01482377 phase I | Gastrointestinal and skin toxicities | Completed Moderate clinical activity with toxicity manageable | [142,143] | |
| ISU104 | Advanced solid tumors | NCT03552406 phase I Dose escalation study (PART I) Dose-expansion study (PART II) | PART I: oral mucositis, pruritus, diarrhea, and fatigue PART II: anorexia, mucositis oral and diarrhea in monotherapy and diarrhea and acneiform rash in combination with cetuximab | Status unknown ISU104 was well tolerated up to 20 mg/kg/day without DLT and showed a disease control rate of 60% ISU104 monotherapy or with cetuximab was safe with promising clinical outcomes in recurrent or metastatic HNSCC treated with the combination | [144] |
| CDX-3379 | Advanced cancer | NCT02014909 phase I | Diarrhea, fatigue, nausea, and rash | Completed CDX-3379 can be combined safely with cetuximab, erlotinib, vemurafenib, or trastuzumab at 15 to 20 mg/kg | [145] |
| HNSCC | NCT02473731, phase I | Diarrhea, fatigue, and acneiform dermatitis, but mild or moderate | Completed CDX-3379 was well tolerated and associated with tumor regression | [146] | |
| Advanced stage NRAS mutant and BRAF/NRAS wild-type melanoma | NCT03580382 phase I + II | Terminated | |||
| Advanced HNSCC | NCT03254927 phase II | Terminated CDX-3379 in combination with cetuximab is well tolerated with signs of antitumor activity | [147] | ||
| Thyroid cancer | NCT02456701 phase I | Completed Vemurafenib + CDX-3379 is safe and enhances efficacy for RAI uptake | [148] | ||
| AV-203 | Metastatic or advanced solid tumors | NCT01603979 phase I | Completed AV-203 was well tolerated. RP2D is 20 mg/kg IV every 2 weeks. The PR in a patient with squamous NSCLC guarantees future testing of AV-203 in this indication | [149] | |
| GSK2849330 | Advanced HER3 + solid tumors | NCT01966445 phase I | Drug tolerated with no major issues | Completed GSK2849330 has a durable response in an exceptional responder with an advanced CD74–NRG1-rearranged IMA | [150] |
| Advanced HER3 + solid tumors | NCT02345174 phase I | Decreased appetite and diarrhea | Completed Despite the restricted number of patients, an exploratory ID50 of 2 mg/kg and ID90 of 18 mg/kg have been reported | [151] | |
| Seribantumab | Advanced NSCLC | NCT00994123 phase I + II | Diarrhea, rash, decreased appetite, fatigue, and nausea | Completed Phase I: no maximum tolerated dose was determined and the AE profile was similar between comparative treatment Phase II: there was no significant difference in PFS between monotherapy and combination therapy. However, retrospective analyses suggest that detectable NRG mRNA levels identified patients who may benefit from MM-121 | [152] |
| NSCLC expressing NRG | NCT02387216 phase II | Diarrhea, fatigue, and neutropenia in the combination treatment | Terminated Seribantumab does not improve PFS when added to docetaxel | [153] | |
| CRC, HNSCC, NSCLC, TNBC, and other tumors with EGFR dependence | NCT01451632 phase I | Part 1: fatigue, dermatitis acneiform, hypomagnesemia, diarrhea, decreased appetite, and hypokalemia Part 2: diarrhea, hypokalemia, nausea, fatigue, hypomagnesemia, decreased appetite, dermatitis acneiform, mucosal inflammation, dehydration, and weight decrease | Completed Unlike doublet treatment, seribantumab + cetuximab + irinotecan was difficult to tolerate. However, MM121 + cetuximab with and without irinotecan had no activity in the vast majority of patients with prior exposure to EGFR-directed therapy | [154] | |
| Advanced gynecologic and breast cancers | NCT01209195 phase I | Completed | |||
| ER +, HER2- BC, and TNBC | NCT01421472 phase II | Completed | |||
| Platinum-resistant or refractory recurrent/advanced ovarian cancers | NCT01447706, phase II | Diarrhea, vomiting, stomatitis, and mucosal inflammation | Completed | [155] | |
| Locally advanced or metastatic ER + and/or PR + and HER2- BC | NCT01151046 phase II | Diarrhea, nausea, fatigue, and arthralgia | Completed The addition of MM-121 to exemestane did not significantly prolong PFS in the unselected population | [156] | |
| CRC, NSCLC, and HNSCC | NCT02538627 phase I | Terminated | |||
| Advanced solid tumors | NCT00734305 phase I | Completed | |||
| Advanced solid | NCT01447225 | Diarrhea, nausea, and fatigue, | Completed MM-121 can be administrated with | [157] | |
| Tumors | phase I | Anemia, vomiting, hypokalemia, decreased appetite, thrombocytopenia, peripheral edema, neutropenia, and constipation | Gemcitabine, pemetrexed, cabazitaxel, and carboplatin | ||
| Postmenopausal women with metastatic BC | NCT03241810 phase II | Terminated | |||
| Locally advanced or metastatic solid tumors | NCT01436565 phase I | Completed | |||
| NRG1 gene fusion-positive advanced solid tumors | NCT04383210 phase II | Active, not recruiting | |||
| An NRG1 fusion-positive metastatic pancreatic cancer patient | NCT04790695 phase II | Completed | |||
| Patritumab | Advanced, refractory solid tumors | NCT01957280 phase I | The most frequently reported treatment-related AEs were gastrointestinal | Completed Well tolerated with no anti-patritumab neutralizing antibodies formation and with normal bioavailability | [158] |
| EGFR wild-type subjects with locally advanced or metastatic NSCLC who have progressed on at least one prior systemic therapy | NCT02134015 phase III | In placebo + erlotinib, the most frequent AEs were rash, diarrhea, and fatigue, in patritumab + erlotinib were diarrhea, rash, and decreased appetite | Terminated Patritumab + erlotinib apparently do not have better results than placebo + erlotinib | ||
| Recurrent or metastatic HNSCC | NCT02633800 phase II | Rash, anemia, neutropenia, hypomagnesemia, and nausea | Terminated Patritumab + cetuximab + platinum was safe but not more efficacious than cetuximab + platinum | [159] | |
| EGFR treatment naïve subjects with advanced NSCLC who have progressed on at least one prior chemotherapy | NCT01211483 phase I + II | AE grade > 3 included diarrhea and rash | Completed Patritumab improved PFS in the NRG high, but not in the ITT population | ||
| Recurrent or metastatic HNSCC | NCT02350712 phase I | Skin and subcutaneous tissue disorders | Completed Patritumab (18 mg/kg loading dose, 9 mg/kg maintenance dose) with cetuximab, and platinum therapy was tolerated and active in HNSCC | [160] | |
| Advanced solid tumors | NCT01479023, phase I | Diarrhea, dizziness, fatigue, headache, hypertension, and weight loss | Terminated [64Cu]DOTA-patritumab and unlabeled patritumab are safe and well tolerated | [161] | |
| Newly diagnosed HER2 + metastatic BC | NCT01512199 phase I + II | Terminated | |||
| Advanced solid tumors | NCT00730470 phase I | Fatigue, diarrhea, nausea, decreased appetite, and dysgeusia | Completed Patritumab treatment was well tolerated and some evidence of disease stabilization was observed | [132] | |
| Elgemtumab (LJM716) | Platinum-pretreated recurrent/metastatic HNSCC | NCT02143622 phase I + II | Withdrawn | ||
| Advanced HER2 + BC or gastric cancer | NCT01602406 phase I | Diarrhea, nausea, fatigue, and chills | Completed As of 4 October 2013, LJM716 demonstrated clinical activity in combination with trastuzumab in trastuzumab-resistant patients with an acceptable safety profile | [162] | |
| Metastatic HER2 + BC | NCT02167854 phase I | Diarrhea, hyperglycemia, hypokalemia, mucositis, and transaminitis | Completed The combination treatment of LJM716, BYL719 (PI3K inhibitor) and trastuzumab has antitumor activity in these pretreated HER2 + metastatic BC with PIK3CA mutations | [163] | |
| Patients with previously treated ESCC | NCT01822613 phase I + II | Completed | |||
| HER2 + BC, HER2 + gastric cancer, HNSCC and ESCC | NCT01598077 phase I | Diarrhea, decreased appetite, pyrexia, fatigue, nausea, infusion-related reactions, vomiting, constipation and dyspnea and anemia and hypomagnesemia | Completed LJM716 was well tolerated, with a manageable safety profile | [164] | |
| Japanese patients with advanced solid tumors | NCT01911936 phase I | Diarrhea, stomatitis, fatigue, pyrexia and paronychia | Completed LJM716 was well tolerated and a degree of tumor shrinkage was reported | [165] | |
| REGN1400 | Patients with advanced NSCLC, CRC, or HNSCC who progressed on prior erlotinib or cetuximab | NCT01727869 phase I | Rash, diarrhea, nausea, and hypomagnesemia | Completed REGN1400 as monotherapy or combined with erlotinib or cetuximab was generally tolerated | [166] |
| Sym013 | Patients with advanced epithelial malignancies | NCT02906670 phase I + II | Terminated |
8.1.1. HER3-Directed Monoclonal Antibodies in Breast Cancer
8.1.2. HER3-Directed Monoclonal Antibodies in NSCLC
8.1.3. HER3-Directed Monoclonal Antibodies in Other Cancers
8.2. HER3-Targeting Bispecific Antibodies and Clinical Trials
| Bispecific Antibodies | Study Population | Clinical Trial Phase | Adverse Events | Status Finding | Reference |
|---|---|---|---|---|---|
| Zenocutuzumab (Zeno, MCLA-128) | Solid tumors harboring an NRG1 fusion | NCT02912949 phase I + II | Infusion-related reactions, diarrhea, rash, and fatigue | Recruiting As of January 2017, MCLA-128 reported a safety profile and antitumor activity in pretreated metastatic breast cancer patients progressing on HER2 therapies | [176] |
| A patient with advanced NRG1-fusion-positive solid tumor | NCT04100694 not given | Status and findings are not posted on www.clinicaltrials.gov (accessed on 20 October 2023) | |||
| Metastatic BC | NCT03321981 phase II | Neutropenia/neutrophil count decrease, diarrhea, asthenia/fatigue, and nausea | Active, not recruiting The combination of MCLA-128 + trastuzumab + vinorelbine is active in pretreated patients with HER2 + metastatic BC. The treatment is safe with manageable AEs | [177] | |
| SI-B001 | Locally advanced or metastatic epithelial tumors | NCT04603287 phase I | Recruiting | ||
| Recurrent and metastatic HNSCC | NCT05054439 phase II | Recruiting | |||
| Recurrent metastatic ESCC | NCT05022654 phase II | Recruiting | |||
| Recurrent and metastatic NSCLC | NCT05020769 phase II + III | Recruiting | |||
| EGFR/ALK wild-type recurrent or metastatic NSCLC | NCT05020457 phase II | Recruiting | |||
| Unresectable or metastatic digestive system malignancies (colorectal and gastric cancer) | NCT05039944 phase II | Terminated | |||
| MM-111 | Advanced, refractory HER2 amplified, NRG + BC | NCT01097460 phase I | Fatigue, diarrhea, and dyspnoea | Completed | |
| Advanced, refractory HER2 amplified, NRG + cancers | NCT00911898 phase I | Completed | |||
| Advanced HER2 + solid tumors | NCT01304784 phase I | Anemia, acute renal failure (assessed as cisplatin-related), chest pain, decreased appetite, diarrhea, febrile neutropenia, hyperuricemia, hypokalemia, hyponatremia, hypophosphatemia, mucosal inflammation, nausea, neutropenia, stomatitis, thrombocytopenia, and vomiting | Completed Treatment with MM-111 and standard-of-care HER2-directed regimens was viable | [178] | |
| HER2 + carcinomas of the distal esophagus, gastroesophageal junction, and stomach | NCT01774851 phase II | Diarrhea, anemia, decreased appetite, alopecia, fatigue, nausea, vomiting, asthenia, neutropenia, constipation, and cough | Terminated MM-111 did not improve PFS or OS when added to paclitaxel + trastuzumab | [179] | |
| Istiratumab (MM-141) | Advanced solid tumors | NCT01733004 phase I | Vomiting, nausea, fatigue, abdominal pain, increased AP, dyspnea, diarrhea, anemia, increased AST, and rash | Completed MM-141 was well tolerated as monotherapy and in combination with everolimus or paclitaxel + gemcitabine in patients with relapsed/refractory solid tumors | [180] |
| Metastatic pancreatic cancer | NCT02399137 phase II | Neutropenia, alopecia, diarrhea, fatigue, thrombocytopenia, anemia, and decreased appetite | Completed Istiratumab failed to improve the efficacy of chemotherapy | [175] | |
| CRC, NSCLC, and HNSCC | NCT02538627 phase I | Terminated | |||
| Duligotuzumab | Locally advanced or metastatic solid tumors with mutant KRAS | NCT01986166 phase I | Diarrhea, general disorders, dermatitis acneiform, rash, rash erythematous, rash maculo-papular, and nausea | Completed The combination of cobimetinib and duligotuzumab was correlated with increased toxicity and limited efficacy | [181] |
| Locally advanced or metastatic epithelial tumors | NCT01207323 phase I | Headache, rash, and diarrhea | Completed Duligotuzumab was well tolerated with evidence of tumor pharmacodynamic modulation and antitumor activity in HNSCC | [182] | |
| Recurrent/metastatic HNSCC | NCT01911598 phase I | Neutropenia, hypokalemia, dehydration, anemia, and diarrhea in arm A and neutropenia, anemia, febrile neutropenia, leukopenia, thrombocytopenia, and hypomagnesemia in arm B | Completed Duligotuzumab with cisplatin + 5-fluorouracil (arm A) or carboplatin + paclitaxel (arm B) demonstrated promising activity despite chemotherapy dose reductions and could be maintained with duligotuzumab alone | [183] | |
| KRAS wild-type metastatic CRC | NCT01652482 phase II | Rash, diarrhea, fatigue, and nausea. There were fewer rash events of any grade in the duligotuzumab arm but more diarrhea | Completed The combination of FOLFIRI with duligotuzumab did not improve clinical outcomes compared with the cetuximab combination | [174] | |
| Recurrent/metastatic HNSCC | NCT01577173 phase II | Rash, infections, diarrhea, fatigue, and nausea | Completed Duligotuzumab demonstrated similar activity to cetuximab, but not superior | [173] |
8.3. HER3-Targeting Antibody–Drug Conjugates and Clinical Trials
8.3.1. Development of Antibody–Drug Conjugates in Breast Cancer
8.3.2. Development of Antibody–Drug Conjugates in NSCLC
9. Emerging Treatment Strategies
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADC | Antibody–drug conjugate |
| AE(s) | Adverse event(s) |
| bAbs | Bispecific antibodies |
| BC | Breast cancer |
| ECD | Extracellular domain |
| EGFR | Epidermal growth factor receptor |
| ER + | Estrogen receptor positive |
| ESCC | Esophageal squamous cell carcinoma |
| HER2 + | HER2-amplified |
| HNSCC | Head and neck squamous cell carcinoma |
| HRG | Heregulin |
| IGF1R | Insulin-like growth factor 1 receptor |
| IMA | Invasive mucinous adenocarcinomas |
| MoAbs | Monoclonals antibodies |
| NRG(s) | Neuregulin(s) |
| NSCLC | Non-small cell lung cancers |
| OS | Overall survival |
| PD-1 | Programmed cell death-1 |
| PFS | Progression-free survival |
| PR | Partial response |
| RAI | Radioactive iodine |
| RP2D | Recommended phase 2 dose |
| RTK | Receptor tyrosine kinases |
| scDb | Bispecific single-chain diabody |
| T-DM1 | Trastuzumab-emtansine |
| TKIs | Tyrosine kinase inhibitors |
| TNBC | Triple-negative breast cancer |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer, J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for grOsada, T.; Morse, M.A.; Hobeika, A.; Diniz, M.A.; Gwin, W.R.; Hartman, Z.; Wei, J.; Guo, H.; Yang, X.Y.; Liu, C.X.; et al. Vaccination targeting human HER3 alters the phenotype of infiltrating T cells and responses to immune checkpoint inhibition. Oncoimmunology 2017, 6, e1315495. [Google Scholar] [CrossRef]
- Kumai, T.; Ohkuri, T.; Nagato, T.; Matsuda, Y.; Oikawa, K.; Aoki, N.; Kimura, S.; Celis, E.; Harabuchi, Y.; Kobayashi, H. Targeting HER-3 to elicit antitumor helper T cells against head and neck squamous cell carcinoma. Sci. Rep. 2015, 5, 16280. [Google Scholar] [CrossRef] [PubMed]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for growth factors in cancer progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Wu, Y.M.; Lin, S.F. The protein tyrosine kinase family of the human genome. Oncogene 2000, 19, 5548–5557. [Google Scholar] [CrossRef]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J.; Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 2003, 21, 2787–2799. [Google Scholar] [CrossRef]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef]
- Haikala, H.M.; Jänne, P.A. Thirty Years of HER3: From Basic Biology to Therapeutic Interventions. Clin. Cancer Res. 2021, 27, 3528–3539. [Google Scholar] [CrossRef]
- Jura, N.; Shan, Y.; Cao, X.; Shaw, D.E.; Kuriyan, J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc. Natl. Acad. Sci. USA 2009, 106, 21608–21613. [Google Scholar] [CrossRef]
- Berger, M.B.; Mendrola, J.M.; Lemmon, M.A. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 2004, 569, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Kunii, K.; Davis, L.; Gorenstein, J.; Hatch, H.; Yashiro, M.; Di Bacco, A.; Elbi, C.; Lutterbach, B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008, 68, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Soltoff, S.P.; Carraway, K.L., 3rd; Prigent, S.A.; Gullick, W.G.; Cantley, L.C. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol. Cell Biol. 1994, 14, 3550–3558. [Google Scholar] [CrossRef]
- Prigent, S.A.; Gullick, W.J. Identification of c-erbB-3 binding sites for phosphatidylinositol 3’-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994, 13, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, L.; Wang, S.; McManaman, J.L.; Thor, A.D.; Yang, X.; Esteva, F.J.; Liu, B. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-i receptor in breast cancer cells resistant to herceptin. Cancer Res. 2010, 70, 1204–1214. [Google Scholar] [CrossRef]
- Liu, J.; Kern, J.A. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am. J. Respir. Cell Mol. Biol. 2002, 27, 306–313. [Google Scholar] [CrossRef]
- Sergina, N.V.; Rausch, M.; Wang, D.; Blair, J.; Hann, B.; Shokat, K.M.; Moasser, M.M. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007, 445, 437–441. [Google Scholar] [CrossRef]
- Campbell, M.R.; Ruiz-Saenz, A.; Zhang, Y.; Peterson, E.; Steri, V.; Oeffinger, J.; Sampang, M.; Jura, N.; Moasser, M.M. Extensive conformational and physical plasticity protects HER2-HER3 tumorigenic signaling. Cell Rep. 2022, 38, 110285. [Google Scholar] [CrossRef]
- Thakkar, D.; Sancenon, V.; Taguiam, M.M.; Guan, S.; Wu, Z.; Ng, E.; Paszkiewicz, K.H.; Ingram, P.J.; Boyd-Kirkup, J.D. 10D1F, an Anti-HER3 Antibody that Uniquely Blocks the Receptor Heterodimerization Interface, Potently Inhibits Tumor Growth Across a Broad Panel of Tumor Models. Mol. Cancer Ther. 2020, 19, 490–501. [Google Scholar] [CrossRef]
- Clayton, A.H.; Orchard, S.G.; Nice, E.C.; Posner, R.G.; Burgess, A.W. Predominance of activated EGFR higher-order oligomers on the cell surface. Growth Factors 2008, 26, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Hofman, E.G.; Bader, A.N.; Voortman, J.; van den Heuvel, D.J.; Sigismund, S.; Verkleij, A.J.; Gerritsen, H.C.; van Bergen en Henegouwen, P.M. Ligand-induced EGF receptor oligomerization is kinase-dependent and enhances internalization. J. Biol. Chem. 2010, 285, 39481–39489. [Google Scholar] [CrossRef]
- van Lengerich, B.; Agnew, C.; Puchner, E.M.; Huang, B.; Jura, N. EGF and NRG induce phosphorylation of HER3/ERBB3 by EGFR using distinct oligomeric mechanisms. Proc. Natl. Acad. Sci. USA 2017, 114, E2836–E2845. [Google Scholar] [CrossRef]
- Landgraf, R.; Eisenberg, D. Heregulin reverses the oligomerization of HER3. Biochemistry 2000, 39, 8503–8511. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bharill, S.; Karandur, D.; Peterson, S.M.; Marita, M.; Shi, X.; Kaliszewski, M.J.; Smith, A.W.; Isacoff, E.Y.; Kuriyan, J. Molecular basis for multimerization in the activation of the epidermal growth factor receptor. eLife 2016, 5. [Google Scholar] [CrossRef]
- Needham, S.R.; Roberts, S.K.; Arkhipov, A.; Mysore, V.P.; Tynan, C.J.; Zanetti-Domingues, L.C.; Kim, E.T.; Losasso, V.; Korovesis, D.; Hirsch, M.; et al. EGFR oligomerization organizes kinase-active dimers into competent signalling platforms. Nat. Commun. 2016, 7, 13307. [Google Scholar] [CrossRef]
- Saffarian, S.; Li, Y.; Elson, E.L.; Pike, L.J. Oligomerization of the EGF receptor investigated by live cell fluorescence intensity distribution analysis. Biophys. J. 2007, 93, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.H.; Issing, W.; Miki, T.; Popescu, N.C.; Aaronson, S.A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: Evidence for overexpression in a subset of human mammary tumors. Proc. Natl. Acad. Sci. USA 1989, 86, 9193–9197. [Google Scholar] [CrossRef] [PubMed]
- Plowman, G.D.; Whitney, G.S.; Neubauer, M.G.; Green, J.M.; McDonald, V.L.; Todaro, G.J.; Shoyab, M. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc. Natl. Acad. Sci. USA 1990, 87, 4905–4909. [Google Scholar] [CrossRef]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef]
- Hellyer, N.J.; Cheng, K.; Koland, J.G. ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem. J. 1998, 333 Pt 3, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.R.; Amin, D.; Moasser, M.M. HER3 comes of age: New insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin. Cancer Res. 2010, 16, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Darcy, K.M.; Zangani, D.; Wohlhueter, A.L.; Huang, R.Y.; Vaughan, M.M.; Russell, J.A.; Ip, M.M. Changes in ErbB2 (her-2/neu), ErbB3, and ErbB4 during growth, differentiation, and apoptosis of normal rat mammary epithelial cells. J. Histochem. Cytochem. 2000, 48, 63–80. [Google Scholar] [CrossRef]
- Stern, D.F. ErbBs in mammary development. Exp. Cell Res. 2003, 284, 89–98. [Google Scholar] [CrossRef]
- Levi, A.D.; Bunge, R.P.; Lofgren, J.A.; Meima, L.; Hefti, F.; Nikolics, K.; Sliwkowski, M.X. The influence of heregulins on human Schwann cell proliferation. J. Neurosci. 1995, 15, 1329–1340. [Google Scholar] [CrossRef]
- Maurer, C.A.; Friess, H.; Kretschmann, B.; Zimmermann, A.; Stauffer, A.; Baer, H.U.; Korc, M.; Büchler, M.W. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum. Pathol. 1998, 29, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Beji, A.; Horst, D.; Engel, J.; Kirchner, T.; Ullrich, A. Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer. Clin. Cancer Res. 2012, 18, 956–968. [Google Scholar] [CrossRef]
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Quinn, C.M.; Ostrowski, J.L.; Lane, S.A.; Loney, D.P.; Teasdale, J.; Benson, F.A. c-erbB-3 protein expression in human breast cancer: Comparison with other tumour variables and survival. Histopathology 1994, 25, 247–252. [Google Scholar] [CrossRef]
- Naidu, R.; Yadav, M.; Nair, S.; Kutty, M.K. Expression of c-erbB3 protein in primary breast carcinomas. Br. J. Cancer 1998, 78, 1385–1390. [Google Scholar] [CrossRef]
- Barnes, N.L.; Khavari, S.; Boland, G.P.; Cramer, A.; Knox, W.F.; Bundred, N.J. Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin. Cancer Res. 2005, 11, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.R.; Barnes, D.M.; Hollywood, D.P.; Hughes, C.M.; Smith, P.; Dublin, E.; Prigent, S.A.; Gullick, W.J.; Hurst, H.C. Expression of the ERBB3 gene product in breast cancer. Br. J. Cancer 1992, 66, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Travis, A.; Pinder, S.E.; Robertson, J.F.; Bell, J.A.; Wencyk, P.; Gullick, W.J.; Nicholson, R.I.; Poller, D.N.; Blamey, R.W.; Elston, C.W.; et al. C-erbB-3 in human breast carcinoma: Expression and relation to prognosis and established prognostic indicators. Br. J. Cancer 1996, 74, 229–233. [Google Scholar] [CrossRef]
- Ciardiello, F.; Kim, N.; Saeki, T.; Dono, R.; Persico, M.G.; Plowman, G.D.; Garrigues, J.; Radke, S.; Todaro, G.J.; Salomon, D.S. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc. Natl. Acad. Sci. USA 1991, 88, 7792–7796. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Wang, S.T.; Chow, N.H.; Yang, H.B. Investigation of the prognostic value of coexpressed erbB family members for the survival of colorectal cancer patients after curative surgery. Eur. J. Cancer 2002, 38, 1065–1071. [Google Scholar] [CrossRef]
- Kountourakis, P.; Pavlakis, K.; Psyrri, A.; Rontogianni, D.; Xiros, N.; Patsouris, E.; Pectasides, D.; Economopoulos, T. Prognostic significance of HER3 and HER4 protein expression in colorectal adenocarcinomas. BMC Cancer 2006, 6, 46. [Google Scholar] [CrossRef]
- Grivas, P.D.; Antonacopoulou, A.; Tzelepi, V.; Sotiropoulou-Bonikou, G.; Kefalopoulou, Z.; Papavassiliou, A.G.; Kalofonos, H. HER-3 in colorectal tumourigenesis: From mRNA levels through protein status to clinicopathologic relationships. Eur. J. Cancer 2007, 43, 2602–2611. [Google Scholar] [CrossRef]
- Shintani, S.; Funayama, T.; Yoshihama, Y.; Alcalde, R.E.; Matsumura, T. Prognostic significance of ERBB3 overexpression in oral squamous cell carcinoma. Cancer Lett. 1995, 95, 79–83. [Google Scholar] [CrossRef]
- Funayama, T.; Nakanishi, T.; Takahashi, K.; Taniguchi, S.; Takigawa, M.; Matsumura, T. Overexpression of c-erbB-3 in various stages of human squamous cell carcinomas. Oncology 1998, 55, 161–167. [Google Scholar] [CrossRef]
- Alaoui-Jamali, M.A.; Song, D.J.; Benlimame, N.; Yen, L.; Deng, X.; Hernandez-Perez, M.; Wang, T. Regulation of multiple tumor microenvironment markers by overexpression of single or paired combinations of ErbB receptors. Cancer Res. 2003, 63, 3764–3774. [Google Scholar] [PubMed]
- Begnami, M.D.; Fukuda, E.; Fregnani, J.H.; Nonogaki, S.; Montagnini, A.L.; da Costa, W.L., Jr.; Soares, F.A. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J. Clin. Oncol. 2011, 29, 3030–3036. [Google Scholar] [CrossRef]
- Koumakpayi, I.H.; Diallo, J.S.; Le Page, C.; Lessard, L.; Gleave, M.; Bégin, L.R.; Mes-Masson, A.M.; Saad, F. Expression and nuclear localization of ErbB3 in prostate cancer. Clin. Cancer Res. 2006, 12, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Trocmé, E.; Mougiakakos, D.; Johansson, C.C.; All-Eriksson, C.; Economou, M.A.; Larsson, O.; Seregard, S.; Kiessling, R.; Lin, Y. Nuclear HER3 is associated with favorable overall survival in uveal melanoma. Int. J. Cancer 2012, 130, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Takikita, M.; Xie, R.; Chung, J.Y.; Cho, H.; Ylaya, K.; Hong, S.M.; Moskaluk, C.A.; Hewitt, S.M. Membranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinoma. J. Transl. Med. 2011, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, T.; Nakata, B.; Amano, R.; Kimura, K.; Shimizu, S.; Ohira, G.; Yamada, N.; Ohira, M.; Hirakawa, K. HER3 overexpression as an independent indicator of poor prognosis for patients with curatively resected pancreatic cancer. Oncology 2011, 81, 192–198. [Google Scholar] [CrossRef]
- Spears, M.; Taylor, K.J.; Munro, A.F.; Cunningham, C.A.; Mallon, E.A.; Twelves, C.J.; Cameron, D.A.; Thomas, J.; Bartlett, J.M. In situ detection of HER2:HER2 and HER2:HER3 protein-protein interactions demonstrates prognostic significance in early breast cancer. Breast Cancer Res. Treat. 2012, 132, 463–470. [Google Scholar] [CrossRef]
- Witton, C.J.; Reeves, J.R.; Going, J.J.; Cooke, T.G.; Bartlett, J.M. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J. Pathol. 2003, 200, 290–297. [Google Scholar] [CrossRef]
- Baiocchi, G.; Lopes, A.; Coudry, R.A.; Rossi, B.M.; Soares, F.A.; Aguiar, S.; Guimarães, G.C.; Ferreira, F.O.; Nakagawa, W.T. ErbB family immunohistochemical expression in colorectal cancer patients with higher risk of recurrence after radical surgery. Int. J. Color. Dis. 2009, 24, 1059–1068. [Google Scholar] [CrossRef]
- Reschke, M.; Mihic-Probst, D.; van der Horst, E.H.; Knyazev, P.; Wild, P.J.; Hutterer, M.; Meyer, S.; Dummer, R.; Moch, H.; Ullrich, A. HER3 is a determinant for poor prognosis in melanoma. Clin. Cancer Res. 2008, 14, 5188–5197. [Google Scholar] [CrossRef]
- Hayashi, M.; Inokuchi, M.; Takagi, Y.; Yamada, H.; Kojima, K.; Kumagai, J.; Kawano, T.; Sugihara, K. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin. Cancer Res. 2008, 14, 7843–7849. [Google Scholar] [CrossRef]
- Tanner, B.; Hasenclever, D.; Stern, K.; Schormann, W.; Bezler, M.; Hermes, M.; Brulport, M.; Bauer, A.; Schiffer, I.B.; Gebhard, S.; et al. ErbB-3 predicts survival in ovarian cancer. J. Clin. Oncol. 2006, 24, 4317–4323. [Google Scholar] [CrossRef]
- Lee, C.M.; Shrieve, D.C.; Zempolich, K.A.; Lee, R.J.; Hammond, E.; Handrahan, D.L.; Gaffney, D.K. Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol. Oncol. 2005, 99, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Singh, M.; Behera, J.; Theilen, N.T.; George, A.K.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Hydrogen sulfide alleviates hyperhomocysteinemia-mediated skeletal muscle atrophy via mitigation of oxidative and endoplasmic reticulum stress injury. Am. J. Physiol. Cell Physiol. 2018, 315, C609–C622. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Singh, M.; George, A.K.; Behera, J.; Tyagi, N.; Tyagi, S.C. Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-β-synthase mutant mice via PPAR-γ/VEGF axis. Physiol. Rep. 2018, 6, e13858. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Singh, M.; George, A.K.; Tyagi, S.C. Restoration of skeletal muscle homeostasis by hydrogen sulfide during hyperhomocysteinemia-mediated oxidative/ER stress condition (1). Can. J. Physiol. Pharmacol. 2019, 97, 441–456. [Google Scholar] [CrossRef]
- Sithanandam, G.; Anderson, L.M. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008, 15, 413–448. [Google Scholar] [CrossRef]
- Fiddes, R.J.; Campbell, D.H.; Janes, P.W.; Sivertsen, S.P.; Sasaki, H.; Wallasch, C.; Daly, R.J. Analysis of Grb7 recruitment by heregulin-activated erbB receptors reveals a novel target selectivity for erbB3. J. Biol. Chem. 1998, 273, 7717–7724. [Google Scholar] [CrossRef] [PubMed]
- Schulze, W.X.; Deng, L.; Mann, M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol. Syst. Biol. 2005, 1, 2005.0008. [Google Scholar] [CrossRef]
- Vijapurkar, U.; Cheng, K.; Koland, J.G. Mutation of a Shc binding site tyrosine residue in ErbB3/HER3 blocks heregulin-dependent activation of mitogen-activated protein kinase. J. Biol. Chem. 1998, 273, 20996–21002. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.; Liu, N.; Wen, D.; Chang, D.; Thomason, A.; Yoshinaga, S.K. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J. Biol. Chem. 1996, 271, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.G.; Soung, Y.H.; Lee, J.W.; Lee, S.H.; Nam, S.W.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. ERBB3 kinase domain mutations are rare in lung, breast and colon carcinomas. Int. J. Cancer 2006, 119, 2986–2987. [Google Scholar] [CrossRef]
- Alimandi, M.; Romano, A.; Curia, M.C.; Muraro, R.; Fedi, P.; Aaronson, S.A.; Di Fiore, P.P.; Kraus, M.H. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995, 10, 1813–1821. [Google Scholar] [PubMed]
- Holbro, T.; Beerli, R.R.; Maurer, F.; Koziczak, M.; Barbas, C.F., 3rd; Hynes, N.E. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc. Natl. Acad. Sci. USA 2003, 100, 8933–8938. [Google Scholar] [CrossRef]
- Liles, J.S.; Arnoletti, J.P.; Tzeng, C.W.; Howard, J.H.; Kossenkov, A.V.; Kulesza, P.; Heslin, M.J.; Frolov, A. ErbB3 expression promotes tumorigenesis in pancreatic adenocarcinoma. Cancer Biol. Ther. 2010, 10, 555–563. [Google Scholar] [CrossRef]
- Wilson, T.R.; Lee, D.Y.; Berry, L.; Shames, D.S.; Settleman, J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell 2011, 20, 158–172. [Google Scholar] [CrossRef]
- Majumder, A.; Sandhu, M.; Banerji, D.; Steri, V.; Olshen, A.; Moasser, M.M. The role of HER2 and HER3 in HER2-amplified cancers beyond breast cancers. Sci. Rep. 2021, 11, 9091. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Rexer, B.N.; Wang, S.E.; Cook, R.S.; Engelman, J.A.; Arteaga, C.L. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene 2010, 29, 5193–5203. [Google Scholar] [CrossRef][Green Version]
- Ritter, C.A.; Perez-Torres, M.; Rinehart, C.; Guix, M.; Dugger, T.; Engelman, J.A.; Arteaga, C.L. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin. Cancer Res. 2007, 13, 4909–4919. [Google Scholar] [CrossRef] [PubMed]
- Révillion, F.; Lhotellier, V.; Hornez, L.; Bonneterre, J.; Peyrat, J.P. ErbB/HER ligands in human breast cancer, and relationships with their receptors, the bio-pathological features and prognosis. Ann. Oncol. 2008, 19, 73–80. [Google Scholar] [CrossRef]
- Wilson, T.R.; Fridlyand, J.; Yan, Y.; Penuel, E.; Burton, L.; Chan, E.; Peng, J.; Lin, E.; Wang, Y.; Sosman, J.; et al. Widespread potential for growth-factor-driven resistance to anti-cancer kinase inhibitors. Nature 2012, 487, 505–509. [Google Scholar] [CrossRef]
- Motoyama, A.B.; Hynes, N.E.; Lane, H.A. The efficacy of ErbB receptor-targeted anti-cancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002, 62, 3151–3158. [Google Scholar] [PubMed]
- Phillips, G.D.; Fields, C.T.; Li, G.; Dowbenko, D.; Schaefer, G.; Miller, K.; Andre, F.; Burris, H.A., 3rd; Albain, K.S.; Harbeck, N.; et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: Critical role for neuregulin blockade in antitumor response to combination therapy. Clin. Cancer Res. 2014, 20, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Vora, S.; Juric, D.; Morse, N.; Mino-Kenudson, M.; Muranen, T.; Tao, J.; Campos, A.B.; Rodon, J.; Ibrahim, Y.H.; et al. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci. Transl. Med. 2013, 5, 196ra199. [Google Scholar] [CrossRef]
- Lin, M.C.; Rojas, K.S.; Cerione, R.A.; Wilson, K.F. Identification of mTORC2 as a necessary component of HRG/ErbB2-dependent cellular transformation. Mol. Cancer Res. 2014, 12, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yashiro, M.; Takakura, N. Heregulin induces resistance to lapatinib-mediated growth inhibition of HER2-amplified cancer cells. Cancer Sci. 2013, 104, 1618–1625. [Google Scholar] [CrossRef]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Ono, M.; Kuwano, M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin. Cancer Res. 2006, 12, 7242–7251. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Leighl, N.B. Treatment paradigms for patients with metastatic non-small-cell lung cancer: First-, second-, and third-line. Curr. Oncol. 2012, 19, S52–S58. [Google Scholar] [CrossRef]
- Vincent, M.D.; Kuruvilla, M.S.; Leighl, N.B.; Kamel-Reid, S. Biomarkers that currently affect clinical practice: EGFR, ALK, MET, KRAS. Curr. Oncol. 2012, 19, S33–S44. [Google Scholar] [CrossRef]
- Murphy, C.G.; Morris, P.G. Recent advances in novel targeted therapies for HER2-positive breast cancer. Anti-Cancer Drugs 2012, 23, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Bang, Y.J.; Feng-Yi, F.; Xu, J.M.; Lee, K.W.; Jiao, S.C.; Chong, J.L.; López-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.V.; Basile, K.J.; Kugel, C.H., 3rd; Witkiewicz, A.K.; Le, K.; Amaravadi, R.K.; Karakousis, G.C.; Xu, X.; Xu, W.; Schuchter, L.M.; et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J. Clin. Investig. 2013, 123, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Petricoin, E.F., 3rd; Zhao, S.; Liu, L.; Osada, T.; Cheng, Q.; Wulfkuhle, J.D.; Gwin, W.R.; Yang, X.; Gallagher, R.I.; et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013, 15, R85. [Google Scholar] [CrossRef]
- Gwin, W.R.; Spector, N.L. Pertuzumab protects the achilles’ heel of trastuzumab–emtansine. Clin. Cancer Res. 2014, 20, 278–280. [Google Scholar] [CrossRef]
- Vlacich, G.; Coffey, R.J. Resistance to EGFR-targeted therapy: A family affair. Cancer Cell 2011, 20, 423–425. [Google Scholar] [CrossRef]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Capone, E.; Barcaroli, D.; D’Agostino, D.; Volpe, S.; Benfante, A.; van Hooff, S.; Iacobelli, V.; Rossi, C.; Iacobelli, S.; et al. ErbB-3 activation by NRG-1β sustains growth and promotes vemurafenib resistance in BRAF-V600E colon cancer stem cells (CSCs). Oncotarget 2015, 6, 16902–16911. [Google Scholar] [CrossRef]
- Watanabe, S.; Yonesaka, K.; Tanizaki, J.; Nonagase, Y.; Takegawa, N.; Haratani, K.; Kawakami, H.; Hayashi, H.; Takeda, M.; Tsurutani, J.; et al. Targeting of the HER2/HER3 signaling axis overcomes ligand-mediated resistance to trastuzumab in HER2-positive breast cancer. Cancer Med. 2019, 8, 1258–1268. [Google Scholar] [CrossRef]
- Liu, B.; Ordonez-Ercan, D.; Fan, Z.; Edgerton, S.M.; Yang, X.; Thor, A.D. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int. J. Cancer 2007, 120, 1874–1882. [Google Scholar] [CrossRef]
- Hutcheson, I.R.; Goddard, L.; Barrow, D.; McClelland, R.A.; Francies, H.E.; Knowlden, J.M.; Nicholson, R.I.; Gee, J.M. Fulvestrant-induced expression of ErbB3 and ErbB4 receptors sensitizes oestrogen receptor-positive breast cancer cells to heregulin β1. Breast Cancer Res. 2011, 13, R29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, W.C.; Li, P.; Guo, H.; Poh, S.B.; Brady, S.W.; Xiong, Y.; Tseng, L.M.; Li, S.H.; Ding, Z.; et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat. Med. 2011, 17, 461–469. [Google Scholar] [CrossRef]
- Chandarlapaty, S.; Sakr, R.A.; Giri, D.; Patil, S.; Heguy, A.; Morrow, M.; Modi, S.; Norton, L.; Rosen, N.; Hudis, C.; et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin. Cancer Res. 2012, 18, 6784–6791. [Google Scholar] [CrossRef] [PubMed]
- Ogden, A.; Bhattarai, S.; Sahoo, B.; Mongan, N.P.; Alsaleem, M.; Green, A.R.; Aleskandarany, M.; Ellis, I.O.; Pattni, S.; Li, X.B.; et al. Combined HER3-EGFR score in triple-negative breast cancer provides prognostic and predictive significance superior to individual biomarkers. Sci. Rep. 2020, 10, 3009. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, X.; Lee, C.K.; Liu, B. Elevated expression of erbB3 confers paclitaxel resistance in erbB2-overexpressing breast cancer cells via upregulation of Survivin. Oncogene 2010, 29, 4225–4236. [Google Scholar] [CrossRef]
- Erjala, K.; Sundvall, M.; Junttila, T.T.; Zhang, N.; Savisalo, M.; Mali, P.; Kulmala, J.; Pulkkinen, J.; Grenman, R.; Elenius, K. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin. Cancer Res. 2006, 12, 4103–4111. [Google Scholar] [CrossRef]
- Wilson, F.H.; Johannessen, C.M.; Piccioni, F.; Tamayo, P.; Kim, J.W.; Van Allen, E.M.; Corsello, S.M.; Capelletti, M.; Calles, A.; Butaney, M.; et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015, 27, 397–408. [Google Scholar] [CrossRef]
- Narayan, M.; Wilken, J.A.; Harris, L.N.; Baron, A.T.; Kimbler, K.D.; Maihle, N.J. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009, 69, 2191–2194. [Google Scholar] [CrossRef]
- Tovey, S.; Dunne, B.; Witton, C.J.; Forsyth, A.; Cooke, T.G.; Bartlett, J.M. Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer? Clin. Cancer Res. 2005, 11, 4835–4842. [Google Scholar] [CrossRef]
- Tovey, S.M.; Witton, C.J.; Bartlett, J.M.; Stanton, P.D.; Reeves, J.R.; Cooke, T.G. Outcome and human epidermal growth factor receptor (HER) 1-4 status in invasive breast carcinomas with proliferation indices evaluated by bromodeoxyuridine labelling. Breast Cancer Res. 2004, 6, R246–R251. [Google Scholar] [CrossRef] [PubMed]
- Frogne, T.; Benjaminsen, R.V.; Sonne-Hansen, K.; Sorensen, B.S.; Nexo, E.; Laenkholm, A.V.; Rasmussen, L.M.; Riese, D.J., 2nd; de Cremoux, P.; Stenvang, J.; et al. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res. Treat. 2009, 114, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Karthaus, W.R.; Lee, Y.S.; Gao, V.R.; Wu, C.; Russo, J.W.; Liu, M.; Mota, J.M.; Abida, W.; Linton, E.; et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell 2020, 38, 279–296.e279. [Google Scholar] [CrossRef] [PubMed]
- Knuefermann, C.; Lu, Y.; Liu, B.; Jin, W.; Liang, K.; Wu, L.; Schmidt, M.; Mills, G.B.; Mendelsohn, J.; Fan, Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003, 22, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Bezler, M.; Hengstler, J.G.; Ullrich, A. Inhibition of doxorubicin-induced HER3-PI3K-AKT signalling enhances apoptosis of ovarian cancer cells. Mol. Oncol. 2012, 6, 516–529. [Google Scholar] [CrossRef]
- Yan, Y.; Hein, A.L.; Greer, P.M.; Wang, Z.; Kolb, R.H.; Batra, S.K.; Cowan, K.H. A novel function of HER2/Neu in the activation of G2/M checkpoint in response to γ-irradiation. Oncogene 2015, 34, 2215–2226. [Google Scholar] [CrossRef]
- He, G.; Di, X.; Yan, J.; Zhu, C.; Sun, X.; Zhang, S. Silencing human epidermal growth factor receptor-3 radiosensitizes human luminal A breast cancer cells. Cancer Sci. 2018, 109, 3774–3782. [Google Scholar] [CrossRef]
- Casalini, P.; Iorio, M.V.; Galmozzi, E.; Ménard, S. Role of HER receptors family in development and differentiation. J. Cell Physiol. 2004, 200, 343–350. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Bischoff, J. Heart valve development: Endothelial cell signaling and differentiation. Circ. Res. 2004, 95, 459–470. [Google Scholar] [CrossRef]
- Fouladkou, F.; Lu, C.; Jiang, C.; Zhou, L.; She, Y.; Walls, J.R.; Kawabe, H.; Brose, N.; Henkelman, R.M.; Huang, A.; et al. The ubiquitin ligase Nedd4-1 is required for heart development and is a suppressor of thrombospondin-1. J. Biol. Chem. 2010, 285, 6770–6780. [Google Scholar] [CrossRef]
- Erickson, S.L.; O’Shea, K.S.; Ghaboosi, N.; Loverro, L.; Frantz, G.; Bauer, M.; Lu, L.H.; Moore, M.W. ErbB3 is required for normal cerebellar and cardiac development: A comparison with ErbB2-and heregulin-deficient mice. Development 1997, 124, 4999–5011. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.T.; Arteaga, C.L. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: Mechanisms and clinical implications. Cancer Biol. Ther. 2011, 11, 793–800. [Google Scholar] [CrossRef]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef]
- Prenzel, N.; Fischer, O.M.; Streit, S.; Hart, S.; Ullrich, A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr. Relat. Cancer 2001, 8, 11–31. [Google Scholar] [CrossRef]
- Tzahar, E.; Waterman, H.; Chen, X.; Levkowitz, G.; Karunagaran, D.; Lavi, S.; Ratzkin, B.J.; Yarden, Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell Biol. 1996, 16, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ma, J.; Lyu, H.; Huang, J.; Kim, A.; Liu, B. Role of erbB3 receptors in cancer therapeutic resistance. Acta Biochim. Biophys. Sinica 2014, 46, 190–198. [Google Scholar] [CrossRef]
- Karachaliou, N.; Lazzari, C.; Verlicchi, A.; Sosa, A.E.; Rosell, R. HER3 as a Therapeutic Target in Cancer. BioDrugs 2017, 31, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Telesco, S.E.; Liu, Y.; Radhakrishnan, R.; Lemmon, M.A. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc. Natl. Acad. Sci. USA 2010, 107, 7692–7697. [Google Scholar] [CrossRef]
- Jacob, W.; James, I.; Hasmann, M.; Weisser, M. Clinical development of HER3-targeting monoclonal antibodies: Perils and progress. Cancer Treat. Rev. 2018, 68, 111–123. [Google Scholar] [CrossRef]
- Schoeberl, B.; Pace, E.A.; Fitzgerald, J.B.; Harms, B.D.; Xu, L.; Nie, L.; Linggi, B.; Kalra, A.; Paragas, V.; Bukhalid, R.; et al. Therapeutically targeting ErbB3: A key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci. Signal 2009, 2, ra31. [Google Scholar] [CrossRef]
- Garner, A.P.; Bialucha, C.U.; Sprague, E.R.; Garrett, J.T.; Sheng, Q.; Li, S.; Sineshchekova, O.; Saxena, P.; Sutton, C.R.; Chen, D.; et al. An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res. 2013, 73, 6024–6035. [Google Scholar] [CrossRef] [PubMed]
- LoRusso, P.; Jänne, P.A.; Oliveira, M.; Rizvi, N.; Malburg, L.; Keedy, V.; Yee, L.; Copigneaux, C.; Hettmann, T.; Wu, C.Y.; et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer Res. 2013, 19, 3078–3087. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, C.S.; Keedy, V.L.; Moyo, V.; MacBeath, G.; Shapiro, G.I. Phase 1 dose escalation study of seribantumab (MM-121), an anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Investig. New Drugs 2021, 39, 1604–1612. [Google Scholar] [CrossRef]
- Meulendijks, D.; Jacob, W.; Martinez-Garcia, M.; Taus, A.; Lolkema, M.P.; Voest, E.E.; Langenberg, M.H.; Fleitas Kanonnikoff, T.; Cervantes, A.; De Jonge, M.J.; et al. First-in-Human Phase I Study of Lumretuzumab, a Glycoengineered Humanized Anti-HER3 Monoclonal Antibody, in Patients with Metastatic or Advanced HER3-Positive Solid Tumors. Clin. Cancer Res. 2016, 22, 877–885. [Google Scholar] [CrossRef]
- Liles, J.S.; Arnoletti, J.P.; Kossenkov, A.V.; Mikhaylina, A.; Frost, A.R.; Kulesza, P.; Heslin, M.J.; Frolov, A. Targeting ErbB3-mediated stromal-epithelial interactions in pancreatic ductal adenocarcinoma. Br. J. Cancer 2011, 105, 523–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schoeberl, B.; Faber, A.C.; Li, D.; Liang, M.C.; Crosby, K.; Onsum, M.; Burenkova, O.; Pace, E.; Walton, Z.; Nie, L.; et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010, 70, 2485–2494. [Google Scholar] [CrossRef]
- Arnett, S.O.; Teillaud, J.L.; Wurch, T.; Reichert, J.M.; Dunlop, C.; Huber, M. IBC’s 21st Annual Antibody Engineering and 8th Annual Antibody Therapeutics International Conferences and 2010 Annual Meeting of the Antibody Society, 5–9 December 2010, San Diego, CA, USA. MAbs 2011, 3, 133–152. [Google Scholar] [CrossRef]
- Jathal, M.K.; Chen, L.; Mudryj, M.; Ghosh, P.M. Targeting ErbB3: The New RTK(id) on the Prostate Cancer Block. Immunol. Endocr. Metab. Agents Med. Chem. 2011, 11, 131–149. [Google Scholar] [CrossRef]
- Sithanandam, G.; Fornwald, L.W.; Fields, J.; Anderson, L.M. Inactivation of ErbB3 by siRNA promotes apoptosis and attenuates growth and invasiveness of human lung adenocarcinoma cell line A549. Oncogene 2005, 24, 1847–1859. [Google Scholar] [CrossRef]
- Cejalvo, J.M.; Jacob, W.; Fleitas Kanonnikoff, T.; Felip, E.; Navarro Mendivil, A.; Martinez Garcia, M.; Taus Garcia, A.; Leighl, N.; Lassen, U.; Mau-Soerensen, M.; et al. A phase Ib/II study of HER3-targeting lumretuzumab in combination with carboplatin and paclitaxel as first-line treatment in patients with advanced or metastatic squamous non-small cell lung cancer. ESMO Open 2019, 4, e000532. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Park-Simon, T.W.; Albanell, J.; Lassen, U.; Cortés, J.; Dieras, V.; May, M.; Schindler, C.; Marmé, F.; Cejalvo, J.M.; et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Investig. New Drugs 2018, 36, 848–859. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, J.Y.; Shin, D.H.; Lim, K.Y.; Lee, G.K.; Kim, J.Y.; Jacob, W.; Ceppi, M.; Weisser, M.; James, I. EGFR and HER3 signaling blockade in invasive mucinous lung adenocarcinoma harboring an NRG1 fusion. Lung Cancer 2018, 124, 71–75. [Google Scholar] [CrossRef]
- Meulendijks, D.; Jacob, W.; Voest, E.E.; Mau-Sorensen, M.; Martinez-Garcia, M.; Taus, A.; Fleitas, T.; Cervantes, A.; Lolkema, M.P.; Langenberg, M.H.G.; et al. Phase Ib Study of Lumretuzumab Plus Cetuximab or Erlotinib in Solid Tumor Patients and Evaluation of HER3 and Heregulin as Potential Biomarkers of Clinical Activity. Clin. Cancer Res. 2017, 23, 5406–5415. [Google Scholar] [CrossRef]
- Kim, S.; Keam, B.; Shin, S.; Chae, Y.; Seo, S.; Park, K.; Kim, T.; Park, L.; Hong, S.; Lim, E. 928P Phase I dose-expansion (part II) study of ISU104 (a novel anti-ErbB3 monoclonal antibody) alone and combination with cetuximab (CET), in patients (pts) with recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). Ann. Oncol. 2020, 31, S667–S668. [Google Scholar] [CrossRef]
- Falchook, G.S.; Bauer, T.M.; LoRusso, P.; McLaughlin, J.F.; LaVallee, T.; Peck, R.A.; Eder, J.P. Safety, pharmacokinetics (PK), pharmacodynamics (Pd), and antitumor activity in a phase 1b study evaluating anti-ErbB3 antibody KTN3379 in adults with advanced tumors alone and with targeted therapies. J. Clin. Oncol. 2016, 34, 2501. [Google Scholar] [CrossRef]
- Duvvuri, U.; George, J.; Kim, S.; Alvarado, D.; Neumeister, V.M.; Chenna, A.; Gedrich, R.; Hawthorne, T.; LaVallee, T.; Grandis, J.R.; et al. Molecular and Clinical Activity of CDX-3379, an Anti-ErbB3 Monoclonal Antibody, in Head and Neck Squamous Cell Carcinoma Patients. Clin. Cancer Res. 2019, 25, 5752–5758. [Google Scholar] [CrossRef]
- Bauman, J.E.; Saba, N.F.; Wise-Draper, T.M.; Adkins, D.; O’Brien, P.E.; Heath-Chiozzi, M.; Golden, P.; Drescher, J.; Alvarado, D.; Gedrich, R. CDX3379-04: Phase II evaluation of CDX-3379 in combination with cetuximab in patients with advanced head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2019, 37, 6025. [Google Scholar] [CrossRef]
- Tchekmedyian, V.; Dunn, L.; Sherman, E.; Baxi, S.S.; Grewal, R.K.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Sabra, M.M.; et al. Enhancing Radioiodine Incorporation in BRAF-Mutant, Radioiodine-Refractory Thyroid Cancers with Vemurafenib and the Anti-ErbB3 Monoclonal Antibody CDX-3379: Results of a Pilot Clinical Trial. Thyroid 2022, 32, 273–282. [Google Scholar] [CrossRef]
- Sarantopoulos, J.; Gordon, M.S.; Harvey, R.D.; Sankhala, K.K.; Malik, L.; Mahalingam, D.; Owonikoko, T.K.; Lewis, C.M.; Payumo, F.; Miller, J. First-in-human phase 1 dose-escalation study of AV-203, a monoclonal antibody against ERBB3, in patients with metastatic or advanced solid tumors. J. Clin. Oncol. 2014, 32, 11113. [Google Scholar] [CrossRef]
- Drilon, A.; Somwar, R.; Mangatt, B.P.; Edgren, H.; Desmeules, P.; Ruusulehto, A.; Smith, R.S.; Delasos, L.; Vojnic, M.; Plodkowski, A.J.; et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov. 2018, 8, 686–695. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; McGeoch, A.; Bergstrom, M.; McSherry, I.; Smith, D.A.; Cleveland, M.; Al-Azzam, W.; Chen, L.; Verheul, H.; Hoekstra, O.S.; et al. Immuno-PET Imaging to Assess Target Engagement: Experience from (89)Zr-Anti-HER3 mAb (GSK2849330) in Patients with Solid Tumors. J. Nucl. Med. 2019, 60, 902–909. [Google Scholar] [CrossRef]
- Sequist, L.V.; Gray, J.E.; Harb, W.A.; Lopez-Chavez, A.; Doebele, R.C.; Modiano, M.R.; Jackman, D.M.; Baggstrom, M.Q.; Atmaca, A.; Felip, E.; et al. Randomized Phase II Trial of Seribantumab in Combination with Erlotinib in Patients with EGFR Wild-Type Non-Small Cell Lung Cancer. Oncol. 2019, 24, 1095–1102. [Google Scholar] [CrossRef]
- Sequist, L.V.; Janne, P.A.; Huber, R.M.; Gray, J.E.; Felip, E.; Perol, M.; Hirsch, F.R.; Tan, D.S.-W.; Kuesters, G.; Zalutskaya, A. SHERLOC: A phase 2 study of MM-121 plus with docetaxel versus docetaxel alone in patients with heregulin (HRG) positive advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2019, 37, 9036. [Google Scholar] [CrossRef]
- Cleary, J.M.; McRee, A.J.; Shapiro, G.I.; Tolaney, S.M.; O’Neil, B.H.; Kearns, J.D.; Mathews, S.; Nering, R.; MacBeath, G.; Czibere, A.; et al. A phase 1 study combining the HER3 antibody seribantumab (MM-121) and cetuximab with and without irinotecan. Investig. New Drugs 2017, 35, 68–78. [Google Scholar] [CrossRef]
- Liu, J.F.; Ray-Coquard, I.; Selle, F.; Poveda, A.M.; Cibula, D.; Hirte, H.; Hilpert, F.; Raspagliesi, F.; Gladieff, L.; Harter, P.; et al. Randomized Phase II Trial of Seribantumab in Combination with Paclitaxel in Patients with Advanced Platinum-Resistant or -Refractory Ovarian Cancer. J. Clin. Oncol. 2016, 34, 4345–4353. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Doyle, C.; Paepke, S.; Azaro, A.; Martin, M.; Semiglazov, V.; Smirnova, I.; Krasnozhon, D.; Manikhas, A.; Harb, W.A. A randomized, double-blind phase II trial of exemestane plus MM-121 (a monoclonal antibody targeting ErbB3) or placebo in postmenopausal women with locally advanced or metastatic ER+/PR+, HER2-negative breast cancer. J. Clin. Oncol. 2014, 32, 15. [Google Scholar] [CrossRef]
- Arnedos, M.; Denlinger, C.S.; Harb, W.A.; Rixe, O.; Morris, J.C.; Dy, G.K.; Adjei, A.A.; Pearlberg, J.; Follows, S.; Czibere, A.G. A phase I study of MM-121 in combination with multiple anticancer therapies in patients with advanced solid tumors. J. Clin. Oncol. 2013, 31, 2609. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Moore, K.N.; Lush, R.; Desai, M.; Mahmood, S.; Beckman, R.A.; Mendell-Harary, J. Pharmacokinetics, safety, and tolerability of a new patritumab formulation in patients with advanced, refractory solid tumors. J. Clin. Oncol. 2015, 33, e14026. [Google Scholar] [CrossRef]
- Forster, M.D.; Dillon, M.T.; Kocsis, J.; Remenár, É.; Pajkos, G.; Rolland, F.; Greenberg, J.; Harrington, K.J. Patritumab or placebo, with cetuximab plus platinum therapy in recurrent or metastatic squamous cell carcinoma of the head and neck: A randomised phase II study. Eur. J. Cancer. 2019, 123, 36–47. [Google Scholar] [CrossRef]
- Dillon, M.T.; Grove, L.; Newbold, K.L.; Shaw, H.; Brown, N.F.; Mendell, J.; Chen, S.; Beckman, R.A.; Jennings, A.; Ricamara, M.; et al. Patritumab with Cetuximab plus Platinum-Containing Therapy in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: An Open-Label, Phase Ib Study. Clin. Cancer Res. 2019, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, A.C.; Liu, Y.; Dehdashti, F.; Laforest, R.; Picus, J.; Frye, J.; Trull, L.; Belanger, S.; Desai, M.; Mahmood, S.; et al. Phase 1 Evaluation of [(64)Cu]DOTA-Patritumab to Assess Dosimetry, Apparent Receptor Occupancy, and Safety in Subjects with Advanced Solid Tumors. Mol. Imaging Biol. 2016, 18, 446–453. [Google Scholar] [CrossRef]
- Im, S.-A.; Juric, D.; Baselga, J.; Kong, A.; Martin, P.; Lin, C.-C.; Dees, E.C.; Schellens, J.H.; De Braud, F.G.; Delgado, L. A phase 1 dose-escalation study of anti-HER3 monoclonal antibody LJM716 in combination with trastuzumab in patients with HER2-overexpressing metastatic breast or gastric cancer. J. Clin. Oncol. 2014, 32, 2519. [Google Scholar] [CrossRef]
- Shah, P.D.; Chandarlapaty, S.; Dickler, M.N.; Ulaner, G.; Zamora, S.J.; Sterlin, V.; Iasonos, A.; Coughlin, C.M.; Morozov, A.; Ero, J. Phase I study of LJM716, BYL719, and trastuzumab in patients (pts) with HER2-amplified (HER2+) metastatic breast cancer (MBC). J. Clin. Oncol. 2015, 33, 590. [Google Scholar] [CrossRef]
- Reynolds, K.L.; Bedard, P.L.; Lee, S.H.; Lin, C.C.; Tabernero, J.; Alsina, M.; Cohen, E.; Baselga, J.; Blumenschein, G., Jr.; Graham, D.M.; et al. A phase I open-label dose-escalation study of the anti-HER3 monoclonal antibody LJM716 in patients with advanced squamous cell carcinoma of the esophagus or head and neck and HER2-overexpressing breast or gastric cancer. BMC Cancer 2017, 17, 646. [Google Scholar] [CrossRef]
- Takahashi, S.; Kobayashi, T.; Tomomatsu, J.; Ito, Y.; Oda, H.; Kajitani, T.; Kakizume, T.; Tajima, T.; Takeuchi, H.; Maacke, H.; et al. LJM716 in Japanese patients with head and neck squamous cell carcinoma or HER2-overexpressing breast or gastric cancer. Cancer Chemother. Pharmacol. 2017, 79, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Adjei, A.A.; Rasco, D.W.; Liu, L.; Kao, R.J.; Brownstein, C.M.; DiCioccio, A.T.; Lowy, I.; Trail, P.; Wang, D. Phase 1 study of REGN1400 (anti-ErbB3) combined with erlotinib or cetuximab in patients (pts) with advanced non-small cell lung cancer (NSCLC), colorectal cancer (CRC), or head and neck cancer (SCCHN). J. Clin. Oncol. 2014, 32, 2516. [Google Scholar] [CrossRef]
- Mukai, H.; Saeki, T.; Aogi, K.; Naito, Y.; Matsubara, N.; Shigekawa, T.; Ueda, S.; Takashima, S.; Hara, F.; Yamashita, T.; et al. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Cancer Sci. 2016, 107, 1465–1470. [Google Scholar] [CrossRef]
- von Pawel, J.; Jotte, R.; Spigel, D.R.; O’Brien, M.E.; Socinski, M.A.; Mezger, J.; Steins, M.; Bosquée, L.; Bubis, J.; Nackaerts, K.; et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J. Clin. Oncol. 2014, 32, 4012–4019. [Google Scholar] [CrossRef]
- Paz-Arez, L.; Serwatowski, P.; Szczęsna, A.; Von Pawel, J.; Toschi, L.; Tibor, C.; Morabito, A.; Zhang, L.; Shuster, D.; Chen, S. P3. 02b-045 Patritumab plus erlotinib in EGFR wild-type advanced non–small cell lung cancer (NSCLC): Part a results of HER3-Lung Study: Topic: EGFR Clinical. J. Thorac. Oncol. 2017, 12, S1214–S1215. [Google Scholar] [CrossRef]
- Kim, S.-B.; Keam, B.; Shin, S.; Chae, Y.; Kim, T.; Kim, M.-S.; Kim, J.; Park, K.; Ahn, J.; Park, L. First in human, a phase I study of ISU104, a novel ErbB3 monoclonal antibody, in patients with advanced solid tumours. Ann. Oncol. 2019, 30, v168. [Google Scholar] [CrossRef]
- Schram, A.M.; O’Reilly, E.M.; Somwar, R.; Benayed, R.; Shameem, S.; Chauhan, T.; Torrisi, J.; Ford, J.; Maussang, D.; Wasserman, E. Abstract PR02: Clinical proof of concept for MCLA-128, a bispecific HER2/3 antibody therapy, in NRG1 fusion-positive cancers. Mol. Cancer Ther. 2019, 18, PR02. [Google Scholar] [CrossRef]
- Schram, A.M.; O’Reilly, E.; O’Kane, G. Efficacy and safety of zenocutuzumab in advanced pancreatic cancer and other solid tumors harboring NRG1 fusions. J. Clin. Oncol. 2021, 39, 3003. [Google Scholar] [CrossRef]
- Fayette, J.; Wirth, L.; Oprean, C.; Udrea, A.; Jimeno, A.; Rischin, D.; Nutting, C.; Harari, P.M.; Csoszi, T.; Cernea, D.; et al. Randomized Phase II Study of Duligotuzumab (MEHD7945A) vs. Cetuximab in Squamous Cell Carcinoma of the Head and Neck (MEHGAN Study). Front. Oncol. 2016, 6, 232. [Google Scholar] [CrossRef]
- Hill, A.G.; Findlay, M.P.; Burge, M.E.; Jackson, C.; Alfonso, P.G.; Samuel, L.; Ganju, V.; Karthaus, M.; Amatu, A.; Jeffery, M.; et al. Phase II Study of the Dual EGFR/HER3 Inhibitor Duligotuzumab (MEHD7945A) versus Cetuximab in Combination with FOLFIRI in Second-Line RAS Wild-Type Metastatic Colorectal Cancer. Clin. Cancer Res. 2018, 24, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Kundranda, M.; Gracian, A.C.; Zafar, S.F.; Meiri, E.; Bendell, J.; Algül, H.; Rivera, F.; Ahn, E.R.; Watkins, D.; Pelzer, U.; et al. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann. Oncol. 2020, 31, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Boni, V.; Schellens, J.H.; Moreno, V.; Bol, K.; Westendorp, M.; Sirulnik, L.A.; Tabernero, J.; Calvo, E. First-in-human phase 1/2 study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3: Final phase 1 data and preliminary activity in HER2+ metastatic breast cancer (MBC). J. Clin. Oncol. 2017, 35 (Suppl. S15). [Google Scholar] [CrossRef]
- Hamilton, E.P.; Petit, T.; Pistilli, B.; Goncalves, A.; Ferreira, A.A.; Dalenc, F.; Cardoso, F.; Mita, M.M.; Dezentjé, V.O.; Manso, L. Clinical activity of MCLA-128 (zenocutuzumab), trastuzumab, and vinorelbine in HER2 amplified metastatic breast cancer (MBC) patients (pts) who had progressed on anti-HER2 ADCs. J. Clin. Oncol. 2020, 38, 3093. [Google Scholar] [CrossRef]
- Richards, D.A.; Braiteh, F.S.; Garcia, A.; Denlinger, C.S.; Conkling, P.R.; Edenfield, W.J.; Anthony, S.P.; Hellerstedt, B.A.; Raju, R.N.; Becerra, C. A phase 1 study of MM-111, a bispecific HER2/HER3 antibody fusion protein, combined with multiple treatment regimens in patients with advanced HER2-positive solid tumors. J. Clin. Oncol. 2014, 32, 651. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Alsina Maqueda, M.; Watkins, D.J.; Sym, S.J.; Bendell, J.C.; Park, S.H.; Arkenau, H.-T.; Bekaii-Saab, T.S.; Kudla, A.J.; McDonagh, C.F. Randomized phase 2 study of paclitaxel (PTX), trastuzumab (T) with or without MM-111 in HER2 expressing gastroesophageal cancers (GEC). J. Clin. Oncol. 2016, 34, 4043. [Google Scholar] [CrossRef]
- Isakoff, S.; Bahleda, R.; Saleh, M.; Bordoni, R.; Shields, A.; Dauer, J.; Curley, M.; Baum, J.; McClure, T.; Louis, C. A phase 1 study of MM-141, a novel tetravalent monoclonal antibody targeting IGF-1R and ErbB3, in relapsed or refractory solid tumors. Eur. J. Cancer 2016, 69, S137–S138. [Google Scholar] [CrossRef]
- Lieu, C.H.; Hidalgo, M.; Berlin, J.D.; Ko, A.H.; Cervantes, A.; LoRusso, P.; Gerber, D.E.; Eder, J.P.; Eckhardt, S.G.; Kapp, A.V.; et al. A Phase Ib Dose-Escalation Study of the Safety, Tolerability, and Pharmacokinetics of Cobimetinib and Duligotuzumab in Patients with Previously Treated Locally Advanced or Metastatic Cancers with Mutant KRAS. Oncol. 2017, 22, 1024-e1089. [Google Scholar] [CrossRef]
- Juric, D.; Dienstmann, R.; Cervantes, A.; Hidalgo, M.; Messersmith, W.; Blumenschein, G.R., Jr.; Tabernero, J.; Roda, D.; Calles, A.; Jimeno, A.; et al. Safety and Pharmacokinetics/Pharmacodynamics of the First-in-Class Dual Action HER3/EGFR Antibody MEHD7945A in Locally Advanced or Metastatic Epithelial Tumors. Clin. Cancer Res. 2015, 21, 2462–2470. [Google Scholar] [CrossRef]
- Jimeno, A.; Machiels, J.P.; Wirth, L.; Specenier, P.; Seiwert, T.Y.; Mardjuadi, F.; Wang, X.; Kapp, A.V.; Royer-Joo, S.; Penuel, E.; et al. Phase Ib study of duligotuzumab (MEHD7945A) plus cisplatin/5-fluorouracil or carboplatin/paclitaxel for first-line treatment of recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer 2016, 122, 3803–3811. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Koyama, K.; Kamai, Y.; Hirotani, K.; Ogitani, Y.; Zembutsu, A.; Abe, M.; Kaneda, Y.; Maeda, N.; Shiose, Y.; et al. A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization. Clin. Cancer Res. 2019, 25, 7151–7161. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Yonesaka, K.; Takamura, S.; Maenishi, O.; Kato, R.; Takegawa, N.; Kawakami, H.; Tanaka, K.; Hayashi, H.; Takeda, M.; et al. U3-1402 sensitizes HER3-expressing tumors to PD-1 blockade by immune activation. J. Clin. Invest. 2020, 130, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Baik, C.; Su, W.C.; Johnson, M.L.; Hayashi, H.; Nishio, M.; Kim, D.W.; Koczywas, M.; Gold, K.A.; Steuer, C.E.; et al. Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor-Resistant, EGFR-Mutated Non-Small Cell Lung Cancer. Cancer Discov. 2022, 12, 74–89. [Google Scholar] [CrossRef]
- Yu, H.; Baik, C.; Gold, K.; Hayashi, H.; Johnson, M.; Koczywas, M.; Murakami, H.; Nishio, M.; Steuer, C.; Su, W. LBA62 Efficacy and safety of patritumab deruxtecan (U3-1402), a novel HER3 directed antibody drug conjugate, in patients (pts) with EGFR-mutated (EGFRm) NSCLC. Ann. Oncol. 2020, 31, S1189–S1190. [Google Scholar] [CrossRef]
- Janne, P.A.; Yu, H.A.; Johnson, M.L.; Steuer, C.E.; Vigliotti, M.; Iacobucci, C.; Chen, S.; Yu, C.; Sellami, D.B. Safety and preliminary antitumor activity of U3-1402: A HER3-targeted antibody drug conjugate in EGFR TKI-resistant, EGFRm NSCLC. J. Clin. Oncol. 2019, 37, 9010. [Google Scholar] [CrossRef]
- Masuda, N.; Yonemori, K.; Takahashi, S.; Kogawa, T.; Nakayama, T.; Iwase, H.; Takahashi, M.; Toyama, T.; Saeki, T.; Saji, S. Abstract PD1-03: Single agent activity of U3-1402, a HER3-targeting antibody-drug conjugate, in HER3-overexpressing metastatic breast cancer: Updated results of a phase 1/2 trial. Cancer Res. 2019, 79 (Suppl. S4), PD1-03-PD01-03. [Google Scholar] [CrossRef]
- Kogawa, T.; Yonemori, K.; Masuda, N.; Takahashi, S.; Takahashi, M.; Iwase, H.; Nakayama, T.; Saeki, T.; Toyama, T.; Takano, T. Single agent activity of U3-1402, a HER3-targeting antibody-drug conjugate, in breast cancer patients: Phase 1 dose escalation study. J. Clin. Oncol. 2018, 36, 2512. [Google Scholar] [CrossRef]
- Krop, I.E.; Masuda, N.; Mukohara, T.; Takahashi, S.; Nakayama, T.; Inoue, K.; Iwata, H.; Toyama, T.; Yamamoto, Y.; Hansra, D.M. Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). J. Clin. Oncol. 2022, 40, 16. [Google Scholar] [CrossRef]
- Nuciforo, P.; Pascual, T.; Cortés, J.; Llombart-Cussac, A.; Fasani, R.; Paré, L.; Oliveira, M.; Galvan, P.; Martínez, N.; Bermejo, B. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann. Oncol. 2018, 29, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Falato, C.; Brunet, L.P.; Saez, O.M.; Andujar, J.C.; Vila, M.M.; Tolosa, P.; Bofill, F.S.; Jurado, J.C.; Gonzalez-Farre, B. LBA3 Patritumab deruxtecan (HER3-DXd) in early-stage HR+/HER2-breast cancer: Final results of the SOLTI TOT-HER3 window of opportunity trial. Ann. Oncol. 2022, 33, S164. [Google Scholar] [CrossRef]
- Steuer, C.E.; Hayashi, H.; Su, W.-C.; Nishio, M.; Johnson, M.L.; Kim, D.-W.; Koczywas, M.; Felip, E.; Gold, K.A.; Murakami, H. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in advanced/metastatic non-small cell lung cancer (NSCLC) without EGFR-activating mutations. J. Clin. Oncol. 2022, 40, 9017. [Google Scholar] [CrossRef]
- Majumder, A. Targeting Homocysteine and Hydrogen Sulfide Balance as Future Therapeutics in Cancer Treatment. Antioxidants 2023, 12, 1520. [Google Scholar] [CrossRef] [PubMed]
- Sedillo, J.C.; Cryns, V.L. Targeting the methionine addiction of cancer. Am. J. Cancer Res. 2022, 12, 2249–2276. [Google Scholar]
- Sun, X.; Gao, H.; Yang, Y.; He, M.; Wu, Y.; Song, Y.; Tong, Y.; Rao, Y. PROTACs: Great opportunities for academia and industry. Signal Transduct. Target. Ther. 2019, 4, 64. [Google Scholar] [CrossRef]
- Gaborit, N.; Abdul-Hai, A.; Mancini, M.; Lindzen, M.; Lavi, S.; Leitner, O.; Mounier, L.; Chentouf, M.; Dunoyer, S.; Ghosh, M.; et al. Examination of HER3 targeting in cancer using monoclonal antibodies. Proc. Natl. Acad. Sci. USA 2015, 112, 839–844. [Google Scholar] [CrossRef]
- Wymant, J.M.; Sayers, E.J.; Muir, D.; Jones, A.T. Strategic Trastuzumab Mediated Crosslinking Driving Concomitant HER2 and HER3 Endocytosis and Degradation in Breast Cancer. J. Cancer 2020, 11, 3288–3302. [Google Scholar] [CrossRef]
- Xie, T.; Lim, S.M.; Westover, K.D.; Dodge, M.E.; Ercan, D.; Ficarro, S.B.; Udayakumar, D.; Gurbani, D.; Tae, H.S.; Riddle, S.M.; et al. Pharmacological targeting of the pseudokinase Her3. Nat. Chem. Biol. 2014, 10, 1006–1012. [Google Scholar] [CrossRef]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Expression Analysis of the Circular RNA Molecules in the Human Retinal Cells Treated with Homocysteine. Curr. Eye Res. 2019, 44, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Circular RNAs profiling in the cystathionine-β-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp. Eye Res. 2018, 174, 80–92. [Google Scholar] [CrossRef]
- George, A.K.; Master, K.; Majumder, A.; Homme, R.P.; Laha, A.; Sandhu, H.S.; Tyagi, S.C.; Singh, M. Circular RNAs constitute an inherent gene regulatory axis in the mammalian eye and brain (1). Can. J. Physiol. Pharmacol. 2019, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Wang, M.; Li, Q.; Qu, Z.; Shi, V.; Kraft, P.; Kim, S.; Gao, Y.; Pak, J.; et al. Downregulation of HER3 by a novel antisense oligonucleotide, EZN-3920, improves the antitumor activity of EGFR and HER2 tyrosine kinase inhibitors in animal models. Mol. Cancer Ther. 2013, 12, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qu, Z.; Kim, S.; Shi, V.; Liao, B.; Kraft, P.; Bandaru, R.; Wu, Y.; Greenberger, L.M.; Horak, I.D. Down-modulation of cancer targets using locked nucleic acid (LNA)-based antisense oligonucleotides without transfection. Gene Ther. 2011, 18, 326–333. [Google Scholar] [CrossRef]

| Antibody–Drug Conjugates | Study Population | Clinical Trial Phase | Adverse Events | Status Finding | Reference |
|---|---|---|---|---|---|
| U3-1402 | Advanced or metastatic CRC | NCT04479436 phase II | Terminated | ||
| Naïve patients with HR + /HER2-early BC | NCT04610528 phase I | Active, not recruiting | |||
| Metastatic or unresectable NSCLC | NCT03260491 phase I | Nausea, vomiting, fatigue, decreased appetite, and alopecia | Active, not recruiting U3-1402 has antitumor activity and a manageable safety profile | [186,187,188] | |
| HER3 + metastatic BC | NCT02980341 phase I + II | Nausea, vomiting, and decreased appetite | Active, not recruiting In a preliminary analysis, U3-1402 demonstrated antitumor activity and a manageable safety profile | [189,190] | |
| Metastatic or locally advanced EGFR-mutated NSCLC | NCT04619004 phase II | Active, not recruiting | |||
| Locally advanced or metastatic EGFR-mutated NSCLC | NCT04676477 phase I | Recruiting | |||
| Metastatic BC | NCT04699630 phase II | Recruiting | |||
| Advanced BC | NCT04965766 phase II | Recruiting | |||
| Metastatic or locally advanced EGFR-mutated NSCLC after failure of EGFR TKI therapy | NCT05338970 phase III | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majumder, A. HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer. Cells 2023, 12, 2517. https://doi.org/10.3390/cells12212517
Majumder A. HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer. Cells. 2023; 12(21):2517. https://doi.org/10.3390/cells12212517
Chicago/Turabian StyleMajumder, Avisek. 2023. "HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer" Cells 12, no. 21: 2517. https://doi.org/10.3390/cells12212517
APA StyleMajumder, A. (2023). HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer. Cells, 12(21), 2517. https://doi.org/10.3390/cells12212517







