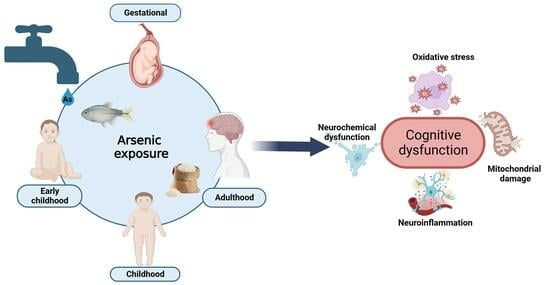
Mechanisms Associated with Cognitive and Behavioral Impairment Induced by Arsenic Exposure
Abstract
1. Arsenic Generalities
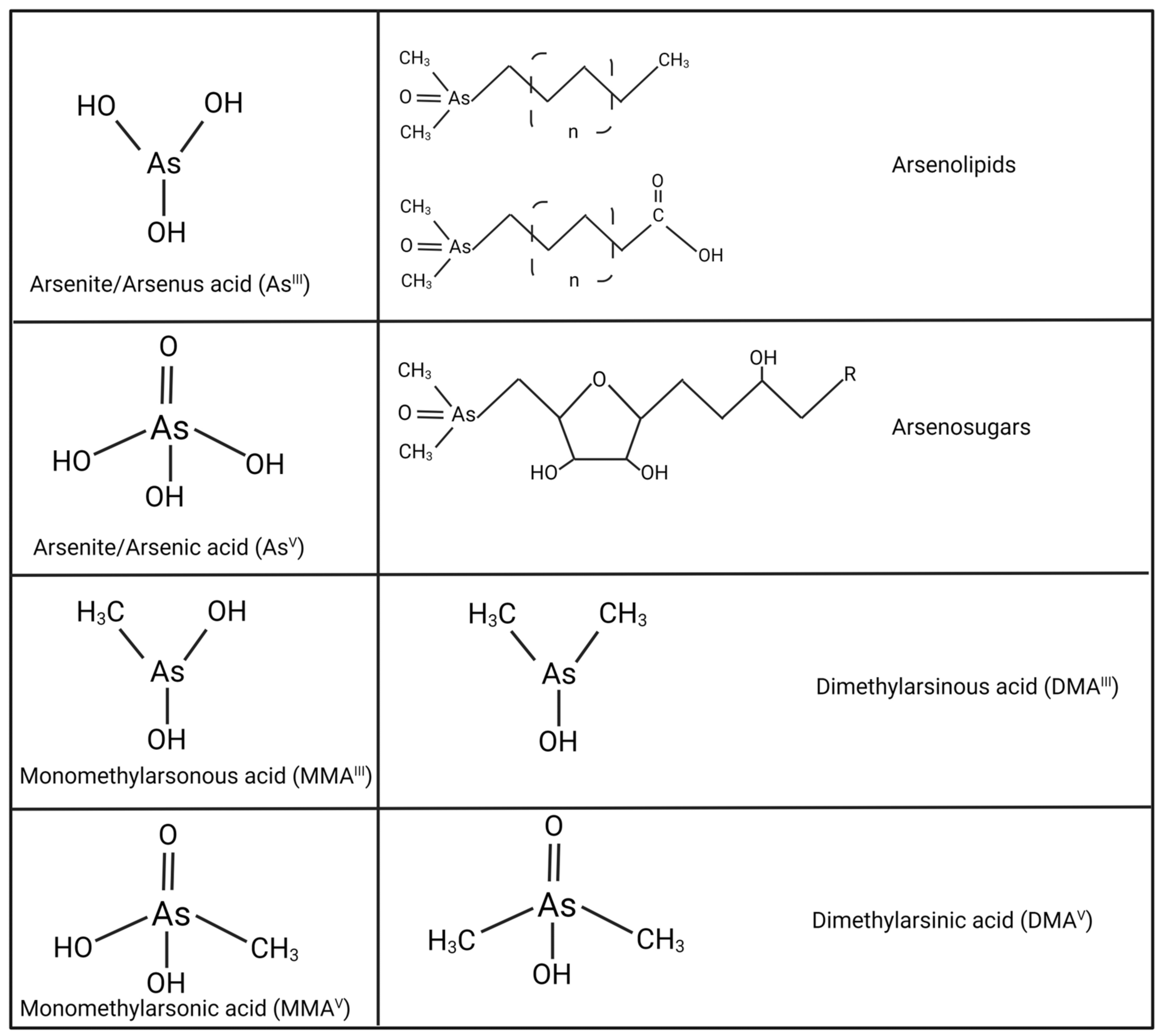
2. Speciation of Arsenic
3. Arsenic Metabolism
4. Cognitive and Behavioral Impairment Induced by As Exposure
5. Morphological Alterations Induced by As Exposure
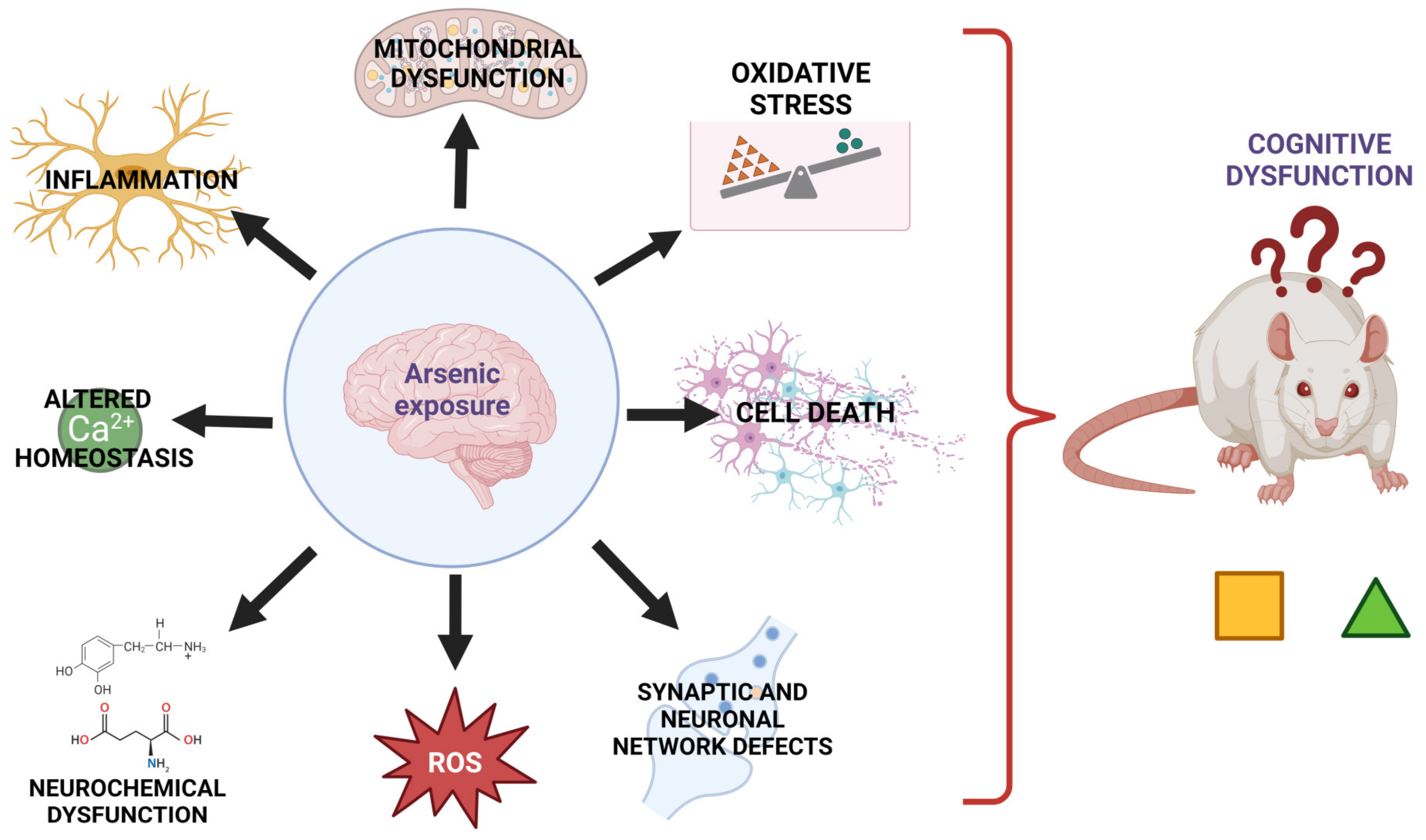
6. Mechanisms Involved in the Neurotoxicity Induced by As Exposure
6.1. Redox and Mitochondrial Alterations Related to Arsenic Exposure
6.2. Neurochemical Dysfunction Induced by Arsenic Exposure
7. Strategies against As Toxicity
7.1. Arsenic Poisoning Treatment
7.2. Nutritional Interventions and Natural Compounds
8. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hollabaugh, C.L. Chapter 2—Modification of Goldschmidt’s geochemical classification of the elements to include arsenic, mercury, and lead as biophile elements. In Developments in Environmental Science; Sarkar, D., Datta, R., Hannigan, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5, pp. 9–31. [Google Scholar]
- Oremland, R.S.; Stolz, J.F. The ecology of arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef]
- National Research Council. Arsenic: Medical and Biologic Effects of Environmental Pollutants; National Research Council: Washington, DC, USA, 1977.
- Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 25 April 2022).
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning—A review. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K. Changing Concept of Arsenic Toxicity with Development of Speciation Techniques. In Handbook of Arsenic Toxicology; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2015; pp. 179–201. [Google Scholar]
- US EPA. 40 CFR parts 9, 141, and 142: National primary drinking water regulations; Arsenic and clarifications to compliance and new source contaminants monitoring; Final rule. Fed. Reg. 2001, 66, 6976–7066. [Google Scholar]
- Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2017.
- Ravenscroft, P.; Brammer, H.; Richards, K. Introduction. In Arsenic Pollution; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–24. [Google Scholar]
- Hindmarsh, J.T.; McLetchle, O.R.; Heffernan, L.P.M.; Hayne, O.A.; Ellenberger, H.A.; McCurdy, R.F.; Thiebaux, H.J. Electromyographic Abnormalities in Chronic Environmental Arsenicalism. J. Anal. Toxicol. 1977, 1, 270–276. [Google Scholar] [CrossRef]
- Mackenzie, C.J.; Kyle, J.H. Two examples of environmental problems occurring in remote sparsely populated areas. Ann. Acad. Med. Singap. 1984, 13, 237–246. [Google Scholar] [PubMed]
- Freund, P.; Al-Shafai, L.; Mankovskii, G.; Howarth, D.; Margolin, E. Clinicopathological Correlates: Chronic Arsenic Toxicity Causing Bilateral Symmetric Progressive Optic Neuropathy. J. Neuroophthalmol. 2020, 40, 423–427. [Google Scholar] [CrossRef]
- Pratt, M.; Wadden, P.; Gulliver, W. Arsenic Keratosis in a Patient from Newfoundland and Labrador, Canada: Case Report and Review. J. Cutan. Med. Surg. 2016, 20, 67–71. [Google Scholar] [CrossRef]
- Feseke, S.K.; St-Laurent, J.; Anassour-Sidi, E.; Ayotte, P.; Bouchard, M.; Levallois, P. Arsenic exposure and type 2 diabetes: Results from the 2007-2009 Canadian Health Measures Survey. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 63–72. [Google Scholar] [CrossRef]
- Senesse, P.; Justrabo, E.; Boschi, F.; Goegebeur, G.; Collet, E.; Boutron, M.C.; Bedenne, L. Cronkhite-Canada syndrome and arsenic poisoning: Fortuitous association or new etiological hypothesis? Gastroenterol. Clin. Biol. 1999, 23, 399–402. [Google Scholar] [PubMed]
- Lampron-Goulet, E.; Gagnon, F.; Langlois, M.F. Association between consumption of private well water contaminated by low levels of arsenic and dysglycemia in a rural region of Quebec, Canada. Environ. Res. 2017, 159, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Infante-Rivard, C.; Olson, E.; Jacques, L.; Ayotte, P. Drinking water contaminants and childhood leukemia. Epidemiology 2001, 12, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Bouchard, M.F.; Shapiro, G.D.; Fisher, M.; Monnier, P.; Morisset, A.S.; Ettinger, A.S. Association between maternal urinary speciated arsenic concentrations and gestational diabetes in a cohort of Canadian women. Environ. Int. 2018, 121, 714–720. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer. Some Drinking-Water Disinfectants and Contaminants, including Arsenic; IARC: Lyon, France, 2004.
- Ayotte, J.D.; Medalie, L.; Qi, S.L.; Backer, L.C.; Nolan, B.T. Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environ. Sci. Technol. 2017, 51, 12443–12454. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.L.; Koestler, D.C.; Li, Z.; Giambelli, C.; Sanchez-Mejias, A.; Gosse, J.A.; Marsit, C.J.; Karagas, M.R.; Robbins, D.J. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: A US birth cohort study. Environ. Health 2013, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Ihrig, M.M.; Shalat, S.L.; Baynes, C. A hospital-based case-control study of stillbirths and environmental exposure to arsenic using an atmospheric dispersion model linked to a geographical information system. Epidemiology 1998, 9, 290–294. [Google Scholar] [CrossRef]
- Aschengrau, A.; Zierler, S.; Cohen, A. Quality of community drinking water and the occurrence of spontaneous abortion. Arch. Environ. Health 1989, 44, 283–290. [Google Scholar] [CrossRef]
- Kilburn, K.H. Neurobehavioral impairment from long-term residential arsenic exposure. In Arsenic: Exposure and Health Effects; Abernathy, C.O., Calderon, R.L., Chappell, W.R., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 159–175. [Google Scholar]
- Knobeloch, L.M.; Zierold, K.M.; Anderson, H.A. Association of arsenic-contaminated drinking-water with prevalence of skin cancer in Wisconsin’s Fox River Valley. J. Health Popul. Nutr. 2006, 24, 206–213. [Google Scholar]
- Karagas, M.R.; Tosteson, T.D.; Blum, J.; Morris, J.S.; Baron, J.A.; Klaue, B. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a U.S. population. Environ. Health Perspect. 1998, 106 (Suppl. S4), 1047–1050. [Google Scholar] [CrossRef]
- Garcia-Esquinas, E.; Pollan, M.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Howard, B.; Farley, J.; Best, L.G.; Navas-Acien, A. Arsenic exposure and cancer mortality in a US-based prospective cohort: The strong heart study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Harter, L.; Milham, S.; Royce, R.; Smith, A.H.; Hartley, J.; Enterline, P. Lung cancer among women residing close to an arsenic emitting copper smelter. Arch. Environ. Health 1987, 42, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.; Higgins, I.; Oh, M.; Burchfiel, C. Arsenic exposure, smoking, and respiratory cancer in copper smelter workers. Arch. Environ. Health 1982, 37, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Burgess, J.L.; Meza, M.M.; Demidenko, E.; Waugh, M.G.; Hamilton, J.W.; Karagas, M.R. Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ. Health Perspect. 2006, 114, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Speer, R.M.; Volk, L.; Hudson, L.G.; Liu, K.J. Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins. Semin. Cancer Biol. 2021, 76, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Del Razo, L.M.; Arellano, M.A.; Cebrian, M.E. The oxidation states of arsenic in well-water from a chronic arsenicism area of northern Mexico. Environ. Pollut. 1990, 64, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Del Razo, L.M.; Garcia-Vargas, G.G.; Valenzuela, O.L.; Castellanos, E.H.; Sanchez-Pena, L.C.; Currier, J.M.; Drobna, Z.; Loomis, D.; Styblo, M. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: A cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ. Health 2011, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Currier, J.M.; Ishida, M.C.; Gonzalez-Horta, C.; Sanchez-Ramirez, B.; Ballinas-Casarrubias, L.; Gutierrez-Torres, D.S.; Ceron, R.H.; Morales, D.V.; Terrazas, F.A.; Del Razo, L.M.; et al. Associations between arsenic species in exfoliated urothelial cells and prevalence of diabetes among residents of Chihuahua, Mexico. Environ. Health Perspect. 2014, 122, 1088–1094. [Google Scholar] [CrossRef]
- Coronado-Gonzalez, J.A.; Del Razo, L.M.; Garcia-Vargas, G.; Sanmiguel-Salazar, F.; Escobedo-de la Pena, J. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ. Res. 2007, 104, 383–389. [Google Scholar] [CrossRef]
- Leke, R.J.; Oduma, J.A.; Bassol-Mayagoitia, S.; Bacha, A.M.; Grigor, K.M. Regional and geographical variations in infertility: Effects of environmental, cultural, and socioeconomic factors. Environ. Health Perspect. 1993, 101 (Suppl. S2), 73–80. [Google Scholar] [CrossRef]
- Rosales-Castillo, J.A.; Acosta-Saavedra, L.C.; Torres, R.; Ochoa-Fierro, J.; Borja-Aburto, V.H.; Lopez-Carrillo, L.; Garcia-Vargas, G.G.; Gurrola, G.B.; Cebrian, M.E.; Calderon-Aranda, E.S. Arsenic exposure and human papillomavirus response in non-melanoma skin cancer Mexican patients: A pilot study. Int. Arch. Occup. Environ. Health 2004, 77, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, O.L.; Germolec, D.R.; Borja-Aburto, V.H.; Contreras-Ruiz, J.; Garcia-Vargas, G.G.; Del Razo, L.M. Chronic arsenic exposure increases TGFalpha concentration in bladder urothelial cells of Mexican populations environmentally exposed to inorganic arsenic. Toxicol. Appl. Pharmacol. 2007, 222, 264–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cebrian, M.E.; Albores, A.; Aguilar, M.; Blakely, E. Chronic arsenic poisoning in the north of Mexico. Hum. Toxicol. 1983, 2, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Calderon, J.; Navarro, M.E.; Jimenez-Capdeville, M.E.; Santos-Diaz, M.A.; Golden, A.; Rodriguez-Leyva, I.; Borja-Aburto, V.; Diaz-Barriga, F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ. Res. 2001, 85, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.T.; Lopez-Carrillo, L.; Gamboa-Loira, B.; Cebrian, M.E. Standards for arsenic in drinking water: Implications for policy in Mexico. J. Public Health Policy 2017, 38, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Zavaleta, A.P.; Garcia-Vargas, G.; Borja-Aburto, V.H.; Acosta-Saavedra, L.C.; Vera Aguilar, E.; Gomez-Munoz, A.; Cebrian, M.E.; Calderon-Aranda, E.S. Nitric oxide and superoxide anion production in monocytes from children exposed to arsenic and lead in region Lagunera, Mexico. Toxicol. Appl. Pharmacol. 2004, 198, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Meza, M.M.; Josyula, A.B.; Poplin, G.S.; Kopplin, M.J.; McClellen, H.E.; Sturup, S.; Lantz, R.C. Environmental Arsenic Exposure and Urinary 8-OHdG in Arizona and Sonora. Clin. Toxicol. 2007, 45, 490–498. [Google Scholar] [CrossRef]
- Gomez-Rubio, P.; Klimentidis, Y.C.; Cantu-Soto, E.; Meza-Montenegro, M.M.; Billheimer, D.; Lu, Z.; Chen, Z.; Klimecki, W.T. Indigenous American ancestry is associated with arsenic methylation efficiency in an admixed population of northwest Mexico. J. Toxicol. Environ. Health A 2012, 75, 36–49. [Google Scholar] [CrossRef][Green Version]
- Gomez-Rubio, P.; Roberge, J.; Arendell, L.; Harris, R.B.; O’Rourke, M.K.; Chen, Z.; Cantu-Soto, E.; Meza-Montenegro, M.M.; Billheimer, D.; Lu, Z.; et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol. Appl. Pharmacol. 2011, 252, 176–182. [Google Scholar] [CrossRef]
- Osorio-Yanez, C.; Ayllon-Vergara, J.C.; Arreola-Mendoza, L.; Aguilar-Madrid, G.; Hernandez-Castellanos, E.; Sanchez-Pena, L.C.; Del Razo, L.M. Blood pressure, left ventricular geometry, and systolic function in children exposed to inorganic arsenic. Environ. Health Perspect. 2015, 123, 629–635. [Google Scholar] [CrossRef]
- Osorio-Yanez, C.; Ayllon-Vergara, J.C.; Aguilar-Madrid, G.; Arreola-Mendoza, L.; Hernandez-Castellanos, E.; Barrera-Hernandez, A.; De Vizcaya-Ruiz, A.; Del Razo, L.M. Carotid intima-media thickness and plasma asymmetric dimethylarginine in Mexican children exposed to inorganic arsenic. Environ. Health Perspect. 2013, 121, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arellano, J.M.; Osorio-Yanez, C.; Sanchez-Pena, L.C.; Ayllon-Vergara, J.C.; Arreola-Mendoza, L.; Aguilar-Madrid, G.; Del Razo, L.M. Natriuretic peptides and echocardiographic parameters in Mexican children environmentally exposed to arsenic. Toxicol. Appl. Pharmacol. 2020, 403, 115164. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, G.; Galvan, M.; Gonzalez-Unzaga, M.; Hernandez Avila, J.; Perez-Labra, M. Blood toxic metals and hemoglobin levels in Mexican children. Environ. Monit. Assess. 2017, 189, 179. [Google Scholar] [CrossRef]
- Rodriguez-Barranco, M.; Lacasana, M.; Aguilar-Garduno, C.; Alguacil, J.; Gil, F.; Gonzalez-Alzaga, B.; Rojas-Garcia, A. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Sci. Total Environ. 2013, 454–455, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.E.; Bailey, K.A.; Rubio-Andrade, M.; Olshan, A.F.; Smeester, L.; Drobna, Z.; Herring, A.H.; Styblo, M.; Garcia-Vargas, G.G.; Fry, R.C. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ. Health Perspect. 2015, 123, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.; Ferreccio, C.; Yuan, Y.; Bates, M.N.; Steinmaus, C.; Selvin, S.; Liaw, J.; Smith, A.H. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J. Natl. Cancer Inst. 2007, 99, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Marshall, G.; Roh, T.; Ferreccio, C.; Liaw, J.; Steinmaus, C. Lung, Bladder, and Kidney Cancer Mortality 40 Years After Arsenic Exposure Reduction. J. Natl. Cancer Inst. 2018, 110, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Marshall, G.; Ferreccio, C.; Steinmaus, C.; Liaw, J.; Bates, M.; Smith, A.H. Kidney cancer mortality: Fifty-year latency patterns related to arsenic exposure. Epidemiology 2010, 21, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Marshall, G.; Liaw, J.; Yuan, Y.; Ferreccio, C.; Steinmaus, C. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ. Health Perspect. 2012, 120, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Balmes, J.R.; Liaw, J.; Troncoso, P.; Dauphine, D.C.; Nardone, A.; Smith, A.H. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol. Appl. Pharmacol. 2016, 313, 10–15. [Google Scholar] [CrossRef]
- Nardone, A.; Ferreccio, C.; Acevedo, J.; Enanoria, W.; Blair, A.; Smith, A.H.; Balmes, J.; Steinmaus, C. The impact of BMI on non-malignant respiratory symptoms and lung function in arsenic exposed adults of Northern Chile. Environ. Res. 2017, 158, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.M.; Acevedo, J.; Lopez, F.G.; Cortes, S.; Ferreccio, C.; Smith, A.H.; Steinmaus, C.M. Hypertension among adults exposed to drinking water arsenic in Northern Chile. Environ. Res. 2017, 153, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Moore, L.E.; Shipp, M.; Kalman, D.; Rey, O.A.; Biggs, M.L.; Hopenhayn, C.; Bates, M.N.; Zheng, S.; Wiencke, J.K.; et al. Genetic Polymorphisms in MTHFR 677 and 1298, GSTM1 and T1, and Metabolism of Arsenic. J. Toxicol. Environ. Health Part A 2007, 70, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Hopenhayn-Rich, C.; Biggs, M.L.; Fuchs, A.; Bergoglio, R.; Tello, E.E.; Nicolli, H.; Smith, A.H. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology 1996, 7, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hopenhayn-Rich, C.; Biggs, M.L.; Smith, A.H. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int. J. Epidemiol. 1998, 27, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Besuschio, S.C.; Perez Desanzo, A.C.; Croci, M. Epidemiological associations between arsenic and cancer in Argentina. Biol. Trace Elem. Res. 1980, 2, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, X.; Wang, J.; Liu, Z.; Gaile, D.; Wu, H.; Yu, G.; Mao, G.; Yang, Z.; Di, Z.; et al. Multi-generational impacts of arsenic exposure on genome-wide DNA methylation and the implications for arsenic-induced skin lesions. Environ. Int. 2018, 119, 250–263. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Z.; Huang, C.; You, L. Levels of arsenic in drinking-water and cutaneous lesions in Inner Mongolia. J. Health Popul. Nutr. 2006, 24, 214–220. [Google Scholar]
- Guan, H.; Piao, F.; Zhang, X.; Li, X.; Li, Q.; Xu, L.; Kitamura, F.; Yokoyama, K. Prenatal exposure to arsenic and its effects on fetal development in the general population of Dalian. Biol. Trace Elem. Res. 2012, 149, 10–15. [Google Scholar] [CrossRef]
- Hsu, C.H.; Yang, S.A.; Wang, J.Y.; Yu, H.S.; Lin, S.R. Mutational spectrum of p53 gene in arsenic-related skin cancers from the blackfoot disease endemic area of Taiwan. Br. J. Cancer 1999, 80, 1080–1086. [Google Scholar] [CrossRef]
- Fan, Y.; Su, Z.; Wei, M.; Liang, H.; Jiang, Y.; Li, X.; Meng, Z.; Wang, Y.; Pan, H.; Song, J.; et al. Long-term Lung Cancer Risk Associated with Sputum Atypia: A 27-Year Follow-up Study of an Occupational Lung Screening Cohort in Yunnan, China. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2122–2129. [Google Scholar] [CrossRef]
- Wade, T.J.; Xia, Y.; Wu, K.; Li, Y.; Ning, Z.; Le, X.C.; Lu, X.; Feng, Y.; He, X.; Mumford, J.L. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. Int. J. Environ. Res. Public Health 2009, 6, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chiou, H.Y.; Hsu, L.I.; Hsueh, Y.M.; Wu, M.M.; Chen, C.J. Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan. Environ. Res. 2010, 110, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Huang, C.J.; Pu, Y.S.; Su, C.T.; Huang, Y.K.; Chen, Y.T.; Hsueh, Y.M. Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol. Appl. Pharmacol. 2008, 226, 14–21. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Gao, Z.; Wang, Z.; Yang, S.; Liang, J.; Feng, Y.; Kato, K.; Nakano, M.; Okada, S.; Yamanaka, K. Immunohistochemical analysis of oxidative DNA damage in arsenic-related human skin samples from arsenic-contaminated area of China. Cancer Lett. 2004, 214, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Wang, Y.; Li, X.; He, M.; Xue, P.; Fu, J.Q.; Wang, H.H.; Sun, G.F. Variations in arsenic methylation capacity and oxidative DNA lesions over a 2-year period in a high arsenic-exposed population. Int. Arch. Occup. Environ. Health 2009, 82, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mao, G.; Zhang, H.; Zhang, C.; Qiu, W.; Guo, X. Association between dyslipidemia and 8-OHdG/Cr among a population exposed to chronic arsenic. Chin. J. Epidemiol. 2014, 35, 802–805. [Google Scholar]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Berg, M.; Stengel, C.; Pham, T.K.; Pham, H.V.; Sampson, M.L.; Leng, M.; Samreth, S.; Fredericks, D. Magnitude of arsenic pollution in the Mekong and Red River Deltas--Cambodia and Vietnam. Sci. Total Environ. 2007, 372, 413–425. [Google Scholar] [CrossRef]
- Navasumrit, P.; Chaisatra, K.; Ruchirawat, M. Arsenic projects in SE Asia. Rev. Environ. Health 2016, 31, 11–12. [Google Scholar] [CrossRef]
- Fujihara, J.; Soejima, M.; Yasuda, T.; Koda, Y.; Kunito, T.; Iwata, H.; Tanabe, S.; Takeshita, H. Polymorphic trial in oxidative damage of arsenic exposed Vietnamese. Toxicol. Appl. Pharmacol. 2011, 256, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Phung, D.; Connell, D.; Rutherford, S.; Chu, C. Cardiovascular risk from water arsenic exposure in Vietnam: Application of systematic review and meta-regression analysis in chemical health risk assessment. Chemosphere 2017, 177, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Agusa, T.; Kunito, T.; Kubota, R.; Inoue, S.; Fujihara, J.; Minh, T.B.; Ha, N.N.; Tu, N.P.; Trang, P.T.; Chamnan, C.; et al. Exposure, metabolism, and health effects of arsenic in residents from arsenic-contaminated groundwater areas of Vietnam and Cambodia: A review. Rev. Environ. Health 2010, 25, 193–220. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Giri, A.; Chakraborty, S.; Bhattacharjee, P. Association of single nucleotide polymorphism with arsenic-induced skin lesions and genetic damage in exposed population of West Bengal, India. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 809, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Das, N.; Chatterjee, D.; Banerjee, A.; Das, J.K.; Basu, S.; Banerjee, S.; Majumder, P.; Goswami, P.; Giri, A.K. Association of NALP2 polymorphism with arsenic induced skin lesions and other health effects. Mutat. Res. 2013, 755, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.K.; Guha Mazumder, D.N.; Ghose, N.; Ghose, A.; Lahiri, S. Systemic manifestations in chronic arsenic toxicity in absence of skin lesions in West Bengal, Indian. J. Med. Res. 2009, 129, 75–82. [Google Scholar]
- Nriagu, J.; Lin, T.S.; Mazumder, D.G.; Chatterjee, D. E-cadherin polymorphisms and susceptibility to arsenic-related skin lesions in West Bengal, India. Sci. Total Environ. 2012, 420, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, D.N.; Haque, R.; Ghosh, N.; De, B.K.; Santra, A.; Chakraborti, D.; Smith, A.H. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int. J. Epidemiol. 2000, 29, 1047–1052. [Google Scholar] [CrossRef]
- Hudock, G.A.; Levine, R.P. Regulation of Photosynthesis in Chlamydomonas reinhardi. Plant Physiol. 1964, 39, 889–897. [Google Scholar] [CrossRef]
- Chattopadhyay, B.P.; Mukherjee, A.K.; Gangopadhyay, P.K.; Alam, J.; Roychowdhury, A. Respiratory effect related to exposure of different concentrations of arsenic in drinking water in West Bengal, India. J. Environ. Sci. Eng. 2010, 52, 147–154. [Google Scholar]
- Cherry, N.; Shaik, K.; McDonald, C.; Chowdhury, Z. Manganese, arsenic, and infant mortality in Bangladesh: An ecological analysis. Arch. Environ. Occup. Health 2010, 65, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Persson, L.A.; Nermell, B.; El Arifeen, S.; Ekstrom, E.C.; Smith, A.H.; Vahter, M. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology 2010, 21, 797–804. [Google Scholar] [CrossRef]
- Rahman, A.; Vahter, M.; Ekstrom, E.C.; Rahman, M.; Golam Mustafa, A.H.; Wahed, M.A.; Yunus, M.; Persson, L.A. Association of arsenic exposure during pregnancy with fetal loss and infant death: A cohort study in Bangladesh. Am. J. Epidemiol. 2007, 165, 1389–1396. [Google Scholar] [CrossRef]
- Milton, A.H.; Smith, W.; Rahman, B.; Hasan, Z.; Kulsum, U.; Dear, K.; Rakibuddin, M.; Ali, A. Chronic arsenic exposure and adverse pregnancy outcomes in bangladesh. Epidemiology 2005, 16, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Chen, Y.; Kibriya, M.G.; Slavkovich, V.; Parvez, F.; Jasmine, F.; Gamble, M.V.; Graziano, J.H. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Kile, M.L.; Faraj, J.M.; Ronnenberg, A.G.; Quamruzzaman, Q.; Rahman, M.; Mostofa, G.; Afroz, S.; Christiani, D.C. A cross sectional study of anemia and iron deficiency as risk factors for arsenic-induced skin lesions in Bangladeshi women. BMC Public Health 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.J.; Pan, W.C.; Kile, M.L.; Tong, L.; Baccarelli, A.A.; Quamruzzaman, Q.; Rahman, M.; Mostofa, G.; Rakibuz-Zaman, M.; Kibriya, M.; et al. A distinct and replicable variant of the squamous cell carcinoma gene inositol polyphosphate-5-phosphatase modifies the susceptibility of arsenic-associated skin lesions in Bangladesh. Cancer 2015, 121, 2222–2229. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.M.; Ahsan, H.; Shea, C.R. Arsenical keratoses in Bangladesh--update and prevention strategies. Dermatol. Clin. 2011, 29, 45–51. [Google Scholar] [CrossRef]
- McDonald, C.; Hoque, R.; Huda, N.; Cherry, N. Prevalence of arsenic-related skin lesions in 53 widely-scattered villages of Bangladesh: An ecological survey. J. Health Popul. Nutr. 2006, 24, 228–235. [Google Scholar]
- Islam, M.R.; Khan, I.; Attia, J.; Hassan, S.M.; McEvoy, M.; D’Este, C.; Azim, S.; Akhter, A.; Akter, S.; Shahidullah, S.M.; et al. Association between hypertension and chronic arsenic exposure in drinking water: A cross-sectional study in Bangladesh. Int. J. Environ. Res. Public Health 2012, 9, 4522–4536. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Khatun, F.; Sayed, M.H.; Khan, M.H.; Aziz, R.; Hossain, M.Z.; Faruquee, M.H. Electrocardiographic abnormalities among arsenic-exposed persons through groundwater in Bangladesh. J. Health Popul. Nutr. 2006, 24, 221–227. [Google Scholar] [PubMed]
- Huda, N.; Hossain, S.; Rahman, M.; Karim, M.R.; Islam, K.; Mamun, A.A.; Hossain, M.I.; Mohanto, N.C.; Alam, S.; Aktar, S.; et al. Elevated levels of plasma uric acid and its relation to hypertension in arsenic-endemic human individuals in Bangladesh. Toxicol. Appl. Pharmacol. 2014, 281, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Graziano, J.H.; Parvez, F.; Liu, M.; Slavkovich, V.; Kalra, T.; Argos, M.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 2011, 342, d2431. [Google Scholar] [CrossRef] [PubMed]
- Milton, A.H.; Hasan, Z.; Rahman, A.; Rahman, M. Non-cancer effects of chronic arsenicosis in Bangladesh: Preliminary results. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2003, 38, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Eunus, H.M.; Haque, S.E.; Sarwar, G.; Hasan, A.R.; Wu, F.; Islam, T.; Ahmed, A.; Shahriar, M.; Jasmine, F.; et al. Arsenic exposure from drinking water and endothelial dysfunction in Bangladeshi adolescents. Environ. Res. 2022, 208, 112697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Shen, S.; Sun, H.; Wu, F.; Kluz, T.; Kibriya, M.G.; Chen, Y.; Ahsan, H.; Costa, M. PBMC gene expression profiles of female Bangladeshi adults chronically exposed to arsenic-contaminated drinking water. Environ. Pollut. 2020, 259, 113672. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.M.; Srivastava, R.K.; Elmets, C.A.; Athar, M. The mechanistic basis of arsenicosis: Pathogenesis of skin cancer. Cancer Lett. 2014, 354, 211–219. [Google Scholar] [CrossRef]
- Medrano, M.A.; Boix, R.; Pastor-Barriuso, R.; Palau, M.; Damian, J.; Ramis, R.; Del Barrio, J.L.; Navas-Acien, A. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ. Res. 2010, 110, 448–454. [Google Scholar] [CrossRef]
- Paiva, L.; Hernandez, A.; Martinez, V.; Creus, A.; Quinteros, D.; Marcos, R. Association between GSTO2 polymorphism and the urinary arsenic profile in copper industry workers. Environ. Res. 2010, 110, 463–468. [Google Scholar] [CrossRef]
- Fernandez Fernandez, N.; Estevez Boullosa, P.; Gomez Rodriguez, A.; Rodriguez Prada, J.I. A Rare Cause of Gastric Injury: Arsenic Intake. Am. J. Gastroenterol. 2019, 114, 1193. [Google Scholar] [CrossRef]
- Berbel-Garcia, A.; Gonzalez-Aguirre, J.M.; Botia-Paniagua, E.; Orts-Castro, E.; Lopez-Zuazo, I.; Rodriguez-Garcia, J.L.; Gil-Madre, J. Acute polyneuropathy and encephalopathy caused by arsenic poisoning. Rev. Neurol. 2004, 38, 928–930. [Google Scholar] [PubMed]
- Mochizuki, H. Arsenic Neurotoxicity in Humans. Int. J. Mol. Sci. 2019, 20, 3418. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Brauner, E.V.; Nordsborg, R.B.; Andersen, Z.J.; Tjonneland, A.; Loft, S.; Raaschou-Nielsen, O. Long-term exposure to low-level arsenic in drinking water and diabetes incidence: A prospective study of the diet, cancer and health cohort. Environ. Health Perspect. 2014, 122, 1059–1065. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Silbergeld, E.K.; Pastor-Barriuso, R.; Guallar, E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 2008, 300, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Sayed, M.H.; Barua, S.; Khan, M.H.; Faruquee, M.H.; Jalil, A.; Hadi, S.A.; Talukder, H.K. Arsenic in drinking water and pregnancy outcomes. Environ. Health Perspect. 2001, 109, 629–631. [Google Scholar] [CrossRef] [PubMed]
- LaMontagne, L.L.; Hepworth, J.T.; Pawlak, R.; Chiafery, M. Parental coping and activities during pediatric critical care. Am. J. Crit. Care 1992, 1, 76–80. [Google Scholar] [CrossRef]
- Neff, J.M. Ecotoxicology of arsenic in the marine environment. Environ. Toxicol. Chem. 1997, 16, 917–927. [Google Scholar] [CrossRef]
- Flora, S.J.S. Arsenic: Chemistry, Occurrence, and Exposure. In Handbook of Arsenic Toxicology; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2015; pp. 1–49. [Google Scholar]
- Thomas, D.J. Arsenic methylation—Lessons from three decades of research. Toxicology 2021, 457, 152800. [Google Scholar] [CrossRef]
- Rose, M.; Lewis, J.; Langford, N.; Baxter, M.; Origgi, S.; Barber, M.; MacBain, H.; Thomas, K. Arsenic in seaweed—Forms, concentration and dietary exposure. Food Chem. Toxicol. 2007, 45, 1263–1267. [Google Scholar] [CrossRef]
- Chávez-Capilla, T. The Need to Unravel Arsenolipid Transformations in Humans. DNA Cell Biol. 2022, 41, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, J.; Carbrey, J.M.; Mukhopadhyay, R.; Agre, P.; Rosen, B.P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA 2002, 99, 6053–6058. [Google Scholar] [CrossRef] [PubMed]
- Styblo, M.; Douillet, C.; Bangma, J.; Eaves, L.A.; de Villena, F.P.; Fry, R. Differential metabolism of inorganic arsenic in mice from genetically diverse Collaborative Cross strains. Arch. Toxicol. 2019, 93, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.M.; Wildfang, E.; Zakharyan, R.A.; Aposhian, H.V. Diversity of inorganic arsenite biotransformation. Biol. Trace Elem. Res. 1999, 68, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Nemeti, B.; Gregus, Z. Reduction of dimethylarsinic acid to the highly toxic dimethylarsinous acid by rats and rat liver cytosol. Chem. Res. Toxicol. 2013, 26, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Mandal, B.K.; Ogra, Y. Speciation of arsenic in body fluids. Talanta 2002, 58, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gebel, T.W. Arsenic methylation is a process of detoxification through accelerated excretion. Int. J. Hyg. Environ. Health 2002, 205, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Saha, A. Metabolism and toxicity of arsenic: A human carcinogen. Curr. Sci. 2002, 82, 38–45. [Google Scholar]
- Vahter, M. Variation in Human Metabolism of Arsenic. In Arsenic Exposure and Health Effects III; Chappell, W.R., Abernathy, C.O., Calderon, R.L., Eds.; Elsevier Science Ltd.: Oxford, UK, 1999; pp. 267–279. [Google Scholar]
- Kala, S.V.; Neely, M.W.; Kala, G.; Prater, C.I.; Atwood, D.W.; Rice, J.S.; Lieberman, M.W. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J. Biol. Chem. 2000, 275, 33404–33408. [Google Scholar] [CrossRef]
- Hayakawa, T.; Kobayashi, Y.; Cui, X.; Hirano, S. A new metabolic pathway of arsenite: Arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch. Toxicol. 2005, 79, 183–191. [Google Scholar] [CrossRef]
- Aposhian, H.V. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 397–419. [Google Scholar] [CrossRef]
- Vahter, M. What are the chemical forms of arsenic in urine, and what can they tell us about exposure? Clin. Chem. 1994, 40, 679–680. [Google Scholar] [CrossRef]
- Wildfang, E.; Radabaugh, T.R.; Vasken Aposhian, H. Enzymatic methylation of arsenic compounds. IX. Liver arsenite methyltransferase and arsenate reductase activities in primates. Toxicology 2001, 168, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci. Prog. 1999, 82 Pt 1, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Zakharyan, R.A.; Wildfang, E.; Aposhian, H.V. Enzymatic methylation of arsenic compounds. III. The marmoset and tamarin, but not the rhesus, monkeys are deficient in methyltransferases that methylate inorganic arsenic. Toxicol. Appl. Pharmacol. 1996, 140, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.M.; Casarez, E.A.; Ayala-Fierro, F.; Aposhian, H. Enzymatic methylation of arsenic compounds. V. Arsenite methyltransferase activity in tissues of mice. Toxicol. Appl. Pharmacol. 1998, 148, 65–70. [Google Scholar] [CrossRef]
- Vahter, M.; Marafante, E. Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits. Chem. Biol. Interact. 1983, 47, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Bertolero, F.; Marafante, E.; Rade, J.E.; Pietra, R.; Sabbioni, E. Biotransformation and intracellular binding of arsenic in tissues of rabbits after intraperitoneal administration of 74As labelled arsenite. Toxicology 1981, 20, 35–44. [Google Scholar] [CrossRef]
- Vahter, M.; Marafante, E. Reduction and binding of arsenate in marmoset monkeys. Arch. Toxicol. 1985, 57, 119–124. [Google Scholar] [CrossRef]
- Zhang, X.; Cornelis, R.; de Kimpe, J.; Mees, L.; Lameire, N. Study of arsenic-protein binding in serum of patients on continuous ambulatory peritoneal dialysis. Clin. Chem. 1998, 44, 141–147. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, X.; Cooper, K.L.; Wang, F.; Liu, K.J.; Hudson, L.G. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J. Biol. Chem. 2011, 286, 22855–22863. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Ju, M.; Zhu, X.; Gan, H.; Gu, R.; Wu, Z.; Meng, Z.; Dou, G. Pharmacokinetic Properties of Arsenic Species after Intravenous and Intragastrical Administration of Arsenic Trioxide Solution in Cynomolgus Macaques Using HPLC-ICP-MS. Molecules 2019, 24, 241. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Niu, Q.; Xu, M.; Rui, D.; Xu, S.; Feng, G.; Ding, Y.; Li, S.; Jing, M. Factors Affecting Arsenic Methylation in Arsenic-Exposed Humans: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2016, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, L.; Lopez-Carrillo, L.; Rosado, J.L.; Rodriguez, V.M.; Vera-Aguilar, E.; Kordas, K.; Garcia-Vargas, G.G.; Cebrian, M.E. Sex differences in the reduction of arsenic methylation capacity as a function of urinary total and inorganic arsenic in Mexican children. Environ. Res. 2016, 151, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular Mechanism of Arsenic-Induced Neurotoxicity including Neuronal Dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef]
- Loffredo, C.A.; Aposhian, H.V.; Cebrian, M.E.; Yamauchi, H.; Silbergeld, E.K. Variability in human metabolism of arsenic. Environ. Res. 2003, 92, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Yasuda, T.; Kato, H.; Yuasa, I.; Panduro, A.; Kunito, T.; Takeshita, H. Genetic variants associated with arsenic metabolism within human arsenic (+3 oxidation state) methyltransferase show wide variation across multiple populations. Arch. Toxicol. 2011, 85, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Guo, D. Comparison of the accumulation of toxic metal biomarkers in Asian subgroups and other races in the United States: NHANES 2015–2016. Chemosphere 2023, 336, 139319. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, B.; Aposhian, H.V.; Zhou, Y.; Chen, M.L.; Zhang, A.; Waalkes, M.P. Chronic arsenic poisoning from burning high-arsenic-containing coal in Guizhou, China. Environ. Health Perspect. 2002, 110, 119–122. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, T.; Zhang, A. Associations between and risks of trace elements related to skin and liver damage induced by arsenic from coal burning. Ecotoxicol. Environ. Saf. 2021, 208, 111719. [Google Scholar] [CrossRef]
- Yao, M.; Zeng, Q.; Luo, P.; Yang, G.; Li, J.; Sun, B.; Liang, B.; Zhang, A. Assessing the health risks of coal-burning arsenic-induced skin damage: A 22-year follow-up study in Guizhou, China. Sci. Total Environ. 2023, 905, 167236. [Google Scholar] [CrossRef]
- Thomas, D.J.; Styblo, M.; Lin, S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol. 2001, 176, 127–144. [Google Scholar] [CrossRef]
- Dopp, E.; Hartmann, L.M.; Florea, A.M.; von Recklinghausen, U.; Pieper, R.; Shokouhi, B.; Rettenmeier, A.W.; Hirner, A.V.; Obe, G. Uptake of inorganic and organic derivatives of arsenic associated with induced cytotoxic and genotoxic effects in Chinese hamster ovary (CHO) cells. Toxicol. Appl. Pharmacol. 2004, 201, 156–165. [Google Scholar] [CrossRef]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Styblo, M.; Del Razo, L.M.; LeCluyse, E.L.; Hamilton, G.A.; Wang, C.; Cullen, W.R.; Thomas, D.J. Metabolism of arsenic in primary cultures of human and rat hepatocytes. Chem. Res. Toxicol. 1999, 12, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Concha, G.; Vogler, G.; Lezcano, D.; Nermell, B.; Vahter, M. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 1998, 44, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Vaishnavi, C.; Singh, H. Toxicity and metabolism of arsenic in vertebrates. Adv. Environ. Sci. Technol. 1994, 27, 55. [Google Scholar]
- Sanchez-Pena, L.C.; Petrosyan, P.; Morales, M.; Gonzalez, N.B.; Gutierrez-Ospina, G.; Del Razo, L.M.; Gonsebatt, M.E. Arsenic species, AS3MT amount, and AS3MT gene expression in different brain regions of mouse exposed to arsenite. Environ. Res. 2010, 110, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, Y.; Liu, H.; Sun, J.; Liu, Y.; Wu, J.; Li, D.; Sun, D. Assessment of relationship on excess arsenic intake from drinking water and cognitive impairment in adults and elders in arsenicosis areas. Int. J. Hyg. Environ. Health 2017, 220, 424–430. [Google Scholar] [CrossRef]
- Rodriguez-Barranco, M.; Gil, F.; Hernandez, A.F.; Alguacil, J.; Lorca, A.; Mendoza, R.; Gomez, I.; Molina-Villalba, I.; Gonzalez-Alzaga, B.; Aguilar-Garduno, C.; et al. Postnatal arsenic exposure and attention impairment in school children. Cortex 2016, 74, 370–382. [Google Scholar] [CrossRef]
- Tyler, C.R.; Allan, A.M. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014, 1, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Parvez, F.; Wasserman, G.A.; Factor-Litvak, P.; Liu, X.; Slavkovich, V.; Siddique, A.B.; Sultana, R.; Sultana, R.; Islam, T.; Levy, D.; et al. Arsenic exposure and motor function among children in Bangladesh. Environ. Health Perspect. 2011, 119, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Chou, H.Y.; The, H.W.; Chen, C.M.; Chen, C.J. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 2003, 24, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Factor-Litvak, P.; Kline, J.; van Geen, A.; Slavkovich, V.; Loiacono, N.J.; Levy, D.; et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ. Health Perspect. 2007, 115, 285–289. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Edwards, M.; Menon, C.V.; Gong, G.; Barber, R. Long-term low-level arsenic exposure is associated with poorer neuropsychological functioning: A Project FRONTIER study. Int. J. Environ. Res. Public Health 2011, 8, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Zhu, C.H.; Su, C.C. Increased prevalence of Parkinson’s disease in soils with high arsenic levels. Park. Relat. Disord. 2021, 88, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Babic Leko, M.; Mihelcic, M.; Jurasovic, J.; Nikolac Perkovic, M.; Spanic, E.; Sekovanic, A.; Orct, T.; Zubcic, K.; Langer Horvat, L.; Pleic, N.; et al. Heavy Metals and Essential Metals Are Associated with Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 467. [Google Scholar] [CrossRef]
- Aung, K.H.; Kyi-Tha-Thu, C.; Sano, K.; Nakamura, K.; Tanoue, A.; Nohara, K.; Kakeyama, M.; Tohyama, C.; Tsukahara, S.; Maekawa, F. Prenatal Exposure to Arsenic Impairs Behavioral Flexibility and Cortical Structure in Mice. Front. Neurosci. 2016, 10, 137. [Google Scholar] [CrossRef]
- Htway, S.M.; Sein, M.T.; Nohara, K.; Win-Shwe, T.T. Effects of Developmental Arsenic Exposure on the Social Behavior and Related Gene Expression in C3H Adult Male Mice. Int. J. Environ. Res. Public Health 2019, 16, 174. [Google Scholar] [CrossRef]
- Htway, S.M.; Suzuki, T.; Kyaw, S.; Nohara, K.; Win-Shwe, T.T. Effects of maternal exposure to arsenic on social behavior and related gene expression in F2 male mice. Environ. Health Prev. Med. 2021, 26, 34. [Google Scholar] [CrossRef]
- Cronican, A.A.; Fitz, N.F.; Carter, A.; Saleem, M.; Shiva, S.; Barchowsky, A.; Koldamova, R.; Schug, J.; Lefterov, I. Genome-wide alteration of histone H3K9 acetylation pattern in mouse offspring prenatally exposed to arsenic. PLoS ONE 2013, 8, e53478. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Sun, W.; Wang, F.; Jin, Y.; Sun, G. Transplacental and early life exposure to inorganic arsenic affected development and behavior in offspring rats. Arch. Toxicol. 2009, 83, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.J.; Kolb, B.L.; Bell, A.; Savage, D.D.; Allan, A.M. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology 2008, 29, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Ali, A.M.; Allan, A.M. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: Consequence of lower corticosterone receptor levels? Pharmacol. Biochem. Behav. 2009, 94, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Goggin, S.L.; Labrecque, M.T.; Allan, A.M. Reduced expression of MAPK/ERK genes in perinatal arsenic-exposed offspring induced by glucocorticoid receptor deficits. Neurotoxicol. Teratol. 2011, 33, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mei, D.; Li, Y.; You, M.; Wang, D.; Yao, D.; Xu, Y.; Zhai, L.; Wang, Y. Arsenic exposure via drinking water during pregnancy and lactation induces autism-like behaviors in male offspring mice. Chemosphere 2022, 290, 133338. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Chavez, L.A.; Rendon-Lopez, C.R.; Zepeda, A.; Silva-Adaya, D.; Del Razo, L.M.; Gonsebatt, M.E. Neurological effects of inorganic arsenic exposure: Altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front. Cell Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Mora, J.; Escobar, M.L.; Rodriguez-Duran, L.; Massieu, L.; Montiel, T.; Rodriguez, V.M.; Hernandez-Mercado, K.; Gonsebatt, M.E. Gestational exposure to inorganic arsenic (iAs3+) alters glutamate disposition in the mouse hippocampus and ionotropic glutamate receptor expression leading to memory impairment. Arch. Toxicol. 2018, 92, 1037–1048. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Z.; Liao, Y.; Wang, G.; Jin, Y. Alterations of NMDA and AMPA receptors and their signaling apparatus in the hippocampus of mouse offspring induced by developmental arsenite exposure. J. Toxicol. Sci. 2019, 44, 777–788. [Google Scholar] [CrossRef]
- Zhao, F.; Liao, Y.; Tang, H.; Piao, J.; Wang, G.; Jin, Y. Effects of developmental arsenite exposure on hippocampal synapses in mouse offspring. Metallomics 2017, 9, 1394–1412. [Google Scholar] [CrossRef]
- Monaco, N.M.; Bartos, M.; Dominguez, S.; Gallegos, C.; Bras, C.; Esandi, M.D.C.; Bouzat, C.; Giannuzzi, L.; Minetti, A.; Gumilar, F. Low arsenic concentrations impair memory in rat offpring exposed during pregnancy and lactation: Role of alpha7 nicotinic receptor, glutamate and oxidative stress. Neurotoxicology 2018, 67, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.L.; Flanigan, T.J.; Law, C.D.; Loukotkova, L.; Woodling, K.A.; da Costa, G.G.; Fitzpatrick, S.C.; Ferguson, S.A. Developmental neurotoxicity of inorganic arsenic exposure in Sprague-Dawley rats. Neurotoxicol. Teratol. 2019, 72, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chandravanshi, L.P.; Gupta, R.; Shukla, R.K. Arsenic-Induced Neurotoxicity by Dysfunctioning Cholinergic and Dopaminergic System in Brain of Developing Rats. Biol. Trace Elem. Res. 2019, 189, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Mehra, R.D.; Dhar, P. Effect of alpha-lipoic acid on spatial memory and structural integrity of developing hippocampal neurons in rats subjected to sodium arsenite exposure. Environ. Toxicol. Pharmacol. 2020, 75, 103323. [Google Scholar] [CrossRef]
- Luo, J.H.; Qiu, Z.Q.; Shu, W.Q.; Zhang, Y.Y.; Zhang, L.; Chen, J.A. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 2009, 184, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Reddy, G.R. Influence of age on arsenic-induced behavioral and cholinergic perturbations: Amelioration with zinc and alpha-tocopherol. Hum. Exp. Toxicol. 2018, 37, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Bardullas, U.; Limon-Pacheco, J.H.; Giordano, M.; Carrizales, L.; Mendoza-Trejo, M.S.; Rodriguez, V.M. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol. Appl. Pharmacol. 2009, 239, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Kaur, B.; Pandey, K.K.; Kaler, S.; Dhar, P. Curcumin supplementation shows modulatory influence on functional and morphological features of hippocampus in mice subjected to arsenic trioxide exposure. Anat. Cell Biol. 2020, 53, 355–365. [Google Scholar] [CrossRef]
- Mehta, K.; Pandey, K.K.; Kaur, B.; Dhar, P.; Kaler, S. Resveratrol attenuates arsenic-induced cognitive deficits via modulation of Estrogen-NMDAR-BDNF signalling pathway in female mouse hippocampus. Psychopharmacology 2021, 238, 2485–2502. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, J.; Gao, Y.; Gao, Y.; Wu, C.; He, S.; Zou, L.; Yang, D.; Han, Y.; Yuan, Q.; et al. Sodium arsenite induces spatial learning and memory impairment associated with oxidative stress and activates the Nrf2/PPARgamma pathway against oxidative injury in mice hippocampus. Toxicol. Res. 2021, 10, 277–283. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Liu, X.; Jiang, L.; Yang, G.; Shi, X.; Zhang, C.; Piao, F. Inhibition of miR-219 Alleviates Arsenic-Induced Learning and Memory Impairments and Synaptic Damage Through Up-regulating CaMKII in the Hippocampus. Neurochem. Res. 2018, 43, 948–958. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Sun, Y.; Dong, W.; Piao, F.; Piao, Y.; Liu, S.; Guan, H.; Yu, S. Arsenic downregulates gene expression at the postsynaptic density in mouse cerebellum, including genes responsible for long-term potentiation and depression. Toxicol. Lett. 2014, 228, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Qiu, Z.; Zhou, X.; Li, S.; Liu, X.; Zhang, C.; Piao, F. Protection of Taurine Against Impairment in Learning and Memory in Mice Exposed to Arsenic. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huo, T.; Li, A.; Wu, X.; Feng, C.; Liu, J.; Jiang, H. Identification of neurotoxicity markers induced by realgar exposure in the mouse cerebral cortex using lipidomics. J. Hazard. Mater. 2020, 389, 121567. [Google Scholar] [CrossRef]
- Chang, C.Y.; Guo, H.R.; Tsai, W.C.; Yang, K.L.; Lin, L.C.; Cheng, T.J.; Chuu, J.J. Subchronic Arsenic Exposure Induces Anxiety-Like Behaviors in Normal Mice and Enhances Depression-Like Behaviors in the Chemically Induced Mouse Model of Depression. Biomed. Res. Int. 2015, 2015, 159015. [Google Scholar] [CrossRef] [PubMed]
- Moreno Avila, C.L.; Limon-Pacheco, J.H.; Giordano, M.; Rodriguez, V.M. Chronic Exposure to Arsenic in Drinking Water Causes Alterations in Locomotor Activity and Decreases Striatal mRNA for the D2 Dopamine Receptor in CD1 Male Mice. J. Toxicol. 2016, 2016, 4763434. [Google Scholar] [CrossRef] [PubMed]
- Aktar, S.; Jahan, M.; Alam, S.; Mohanto, N.C.; Arefin, A.; Rahman, A.; Haque, A.; Himeno, S.; Hossain, K.; Saud, Z.A. Individual and Combined Effects of Arsenic and Lead on Behavioral and Biochemical Changes in Mice. Biol. Trace Elem. Res. 2017, 177, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Anjum, A.; Banna, H.U.; Rahman, M.; Siddique, A.E.; Karim, Y.; Nikkon, F.; Haque, A.; Hossain, K.; Saud, Z.A. Manganese attenuates the effects of arsenic on neurobehavioral and biochemical changes in mice co-exposed to arsenic and manganese. Environ. Sci. Pollut. Res. Int. 2019, 26, 29257–29266. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Tu, J.B.; Ran, R.T.; Zhang, W.X.; Tan, Q.; Tang, P.; Kuang, T.; Cheng, S.Q.; Chen, C.Z.; Jiang, X.J.; et al. Using the Metabolome to Understand the Mechanisms Linking Chronic Arsenic Exposure to Microglia Activation, and Learning and Memory Impairment. Neurotox. Res. 2021, 39, 720–739. [Google Scholar] [CrossRef]
- Rodriguez, V.M.; Carrizales, L.; Jimenez-Capdeville, M.E.; Dufour, L.; Giordano, M. The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res. Bull. 2001, 55, 301–308. [Google Scholar] [CrossRef]
- Flora, S.J.; Bhatt, K.; Mehta, A. Arsenic moiety in gallium arsenide is responsible for neuronal apoptosis and behavioral alterations in rats. Toxicol. Appl. Pharmacol. 2009, 240, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.M.; Limon-Pacheco, J.H.; Carrizales, L.; Mendoza-Trejo, M.S.; Giordano, M. Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol. Teratol. 2010, 32, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Ram Kumar, M.; Flora, S.J.; Reddy, G.R. Monoisoamyl 2,3-dimercaptosuccinic acid attenuates arsenic induced toxicity: Behavioral and neurochemical approach. Environ. Toxicol. Pharmacol. 2013, 36, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Sarkozi, K.; Papp, A.; Mate, Z.; Horvath, E.; Paulik, E.; Szabo, A. Rutin, a flavonoid phytochemical, ameliorates certain behavioral and electrophysiological alterations and general toxicity of oral arsenic in rats. Acta Biol. Hung. 2015, 66, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Peruru, R.; Dodoala, S. Therapeutic potential of diosmin, a citrus flavonoid against arsenic-induced neurotoxicity via suppression of NOX 4 and its subunits. Indian J. Pharmacol. 2021, 53, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Taheri Zadeh, Z.; Esmaeilpour, K.; Aminzadeh, A.; Heidari, M.R.; Joushi, S. Resveratrol Attenuates Learning, Memory, and Social Interaction Impairments in Rats Exposed to Arsenic. Biomed. Res. Int. 2021, 2021, 9993873. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Xi, S.H.; Li, M.Y.; Ding, T.T.; Liu, N.; Cao, F.Y.; Zeng, Y.; Liu, X.J.; Tong, J.W.; Jiang, S.F. Fluoride and arsenic exposure affects spatial memory and activates the ERK/CREB signaling pathway in offspring rats. Neurotoxicology 2017, 59, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, W.; Ye, L.; Chen, Z.; Cui, Y. Postnatal low-concentration arsenic exposure induces autism-like behavior and affects frontal cortex neurogenesis in rats. Environ. Toxicol. Pharmacol. 2018, 62, 188–198. [Google Scholar] [CrossRef]
- Luo, J.; Qiu, Z.; Chen, J.; Zhang, L.; Liu, W.; Tan, Y.; Shu, W. Maternal and early life arsenite exposure impairs neurodevelopment and increases the expression of PSA-NCAM in hippocampus of rat offspring. Toxicology 2013, 311, 99–106. [Google Scholar] [CrossRef]
- Pandey, R.; Garg, A.; Gupta, K.; Shukla, P.; Mandrah, K.; Roy, S.; Chattopadhyay, N.; Bandyopadhyay, S. Arsenic Induces Differential Neurotoxicity in Male, Female, and E2-Deficient Females: Comparative Effects on Hippocampal Neurons and Cognition in Adult Rats. Mol. Neurobiol. 2022, 59, 2729–2744. [Google Scholar] [CrossRef]
- Chandravanshi, L.P.; Shukla, R.K.; Sultana, S.; Pant, A.B.; Khanna, V.K. Early life arsenic exposure and brain dopaminergic alterations in rats. Int. J. Dev. Neurosci. 2014, 38, 91–104. [Google Scholar] [CrossRef]
- Wheater, E.; Shenkin, S.D.; Munoz Maniega, S.; Valdes Hernandez, M.; Wardlaw, J.M.; Deary, I.J.; Bastin, M.E.; Boardman, J.P.; Cox, S.R. Birth weight is associated with brain tissue volumes seven decades later but not with MRI markers of brain ageing. Neuroimage Clin. 2021, 31, 102776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, X.; Dong, D.; Zhang, Y.; Zhao, L.; Li, W.; Chen, J.; Sun, G.; Li, B. Tissue-specific distributions of inorganic arsenic and its methylated metabolites, especially in cerebral cortex, cerebellum and hippocampus of mice after a single oral administration of arsenite. J. Trace Elem. Med. Biol. 2017, 43, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Zhao, L.; Hu, S.; Piao, F. Effect of subchronic exposure to arsenic on levels of essential trace elements in mice brain and its gender difference. Biometals 2013, 26, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, J.; Li, J.; Zhang, X.; Wang, Y.; Chen, X.; Luo, P.; Hu, T.; Cao, X.; Zhuang, H.; et al. Effects of arsenic exposure on trace element levels in the hippocampus and cortex of rats and their gender differences. J. Trace Elem. Med. Biol. 2023, 80, 127289. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzade, N.; Babri, S.; Majidinia, M. Attenuation of chronic arsenic neurotoxicity via melatonin in male offspring of maternal rats exposed to arsenic during conception: Involvement of oxidative DNA damage and inflammatory signaling cascades. Life Sci. 2021, 266, 118876. [Google Scholar] [CrossRef] [PubMed]
- Manthari, R.K.; Tikka, C.; Ommati, M.M.; Niu, R.; Sun, Z.; Wang, J.; Zhang, J.; Wang, J. Arsenic induces autophagy in developmental mouse cerebral cortex and hippocampus by inhibiting PI3K/Akt/mTOR signaling pathway: Involvement of blood-brain barrier’s tight junction proteins. Arch. Toxicol. 2018, 92, 3255–3275. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Bhaumik, S.; Purkayastha, M.; Basu, S.; Nag Chaudhuri, A.; Das Gupta, S. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol. Lett. 2002, 136, 65–76. [Google Scholar] [CrossRef]
- Dhar, P.; Mohari, N.; Mehra, R.D. Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology 2007, 234, 10–20. [Google Scholar] [CrossRef]
- Frankel, S.; Concannon, J.; Brusky, K.; Pietrowicz, E.; Giorgianni, S.; Thompson, W.D.; Currie, D.A. Arsenic exposure disrupts neurite growth and complexity in vitro. Neurotoxicology 2009, 30, 529–537. [Google Scholar] [CrossRef]
- Nino, S.A.; Chi-Ahumada, E.; Ortiz, J.; Zarazua, S.; Concha, L.; Jimenez-Capdeville, M.E. Demyelination associated with chronic arsenic exposure in Wistar rats. Toxicol. Appl. Pharmacol. 2020, 393, 114955. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Chao, P.L.; Fang, S.F.; Chi, C.W.; Yang, C.H. Endoplasmic reticulum stress is involved in arsenite-induced oxidative injury in rat brain. Toxicol. Appl. Pharmacol. 2007, 224, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Maurya, S.K.; Khare, P.; Srivastava, A.; Bandyopadhyay, S. Characterization of developmental neurotoxicity of As, Cd, and Pb mixture: Synergistic action of metal mixture in glial and neuronal functions. Toxicol. Sci. 2010, 118, 586–601. [Google Scholar] [CrossRef]
- Kushwaha, R.; Mishra, J.; Tripathi, S.; Raza, W.; Mandrah, K.; Roy, S.K.; Bandyopadhyay, S. Arsenic Attenuates Heparin-Binding EGF-Like Growth Factor/EGFR Signaling That Promotes Matrix Metalloprotease 9-Dependent Astrocyte Damage in the Developing Rat Brain. Toxicol. Sci. 2018, 162, 406–428. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, R.; Mishra, J.; Tripathi, S.; Khare, P.; Bandyopadhyay, S. Arsenic, Cadmium, and Lead Like Troglitazone Trigger PPARgamma-Dependent Poly (ADP-Ribose) Polymerase Expression and Subsequent Apoptosis in Rat Brain Astrocytes. Mol. Neurobiol. 2018, 55, 2125–2149. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Wang, X.; Wu, Y.; Zhang, Y.; Chen, S.; Zhang, W.; Sun, X.; Zheng, T.; Xia, W.; et al. Prenatal arsenic exposure, arsenic metabolism and neurocognitive development of 2-year-old children in low-arsenic areas. Environ. Int. 2023, 174, 107918. [Google Scholar] [CrossRef] [PubMed]
- Soler-Blasco, R.; Murcia, M.; Lozano, M.; Sarzo, B.; Esplugues, A.; Riutort-Mayol, G.; Vioque, J.; Lertxundi, N.; Santa Marina, L.; Lertxundi, A.; et al. Prenatal arsenic exposure, arsenic methylation efficiency, and neuropsychological development among preschool children in a Spanish birth cohort. Environ. Res. 2022, 207, 112208. [Google Scholar] [CrossRef]
- Vahter, M.; Skroder, H.; Rahman, S.M.; Levi, M.; Derakhshani Hamadani, J.; Kippler, M. Prenatal and childhood arsenic exposure through drinking water and food and cognitive abilities at 10 years of age: A prospective cohort study. Environ. Int. 2020, 139, 105723. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef]
- Goekoop, J.G. Unfavorable prognosis of long-term depression. Ned. Tijdschr. Geneeskd. 1992, 136, 2356–2358. [Google Scholar] [PubMed]
- Aposhian, H.V.; Zakharyan, R.A.; Avram, M.D.; Kopplin, M.J.; Wollenberg, M.L. Oxidation and detoxification of trivalent arsenic species. Toxicol. Appl. Pharmacol. 2003, 193, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Hasegawa, A.; Sawamura, R.; Okada, S. Dimethylated arsenics induce DNA strand breaks in lung via the production of active oxygen in mice. Biochem. Biophys. Res. Commun. 1989, 165, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Hoshino, M.; Okamoto, M.; Sawamura, R.; Hasegawa, A.; Okada, S. Induction of DNA damage by dimethylarsine, a metabolite of inorganic arsenics, is for the major part likely due to its peroxyl radical. Biochem. Biophys. Res. Commun. 1990, 168, 58–64. [Google Scholar] [CrossRef]
- Yamanaka, K.; Okada, S. Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ. Health Perspect. 1994, 102 (Suppl. S3), 37–40. [Google Scholar] [CrossRef] [PubMed]
- Naranmandura, H.; Xu, S.; Sawata, T.; Hao, W.H.; Liu, H.; Bu, N.; Ogra, Y.; Lou, Y.J.; Suzuki, N. Mitochondria are the main target organelle for trivalent monomethylarsonous acid (MMA(III))-induced cytotoxicity. Chem. Res. Toxicol. 2011, 24, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Kitchin, K.T.; Cullen, W.R. Arsenic species that cause release of iron from ferritin and generation of activated oxygen. Arch. Biochem. Biophys. 2000, 382, 195–202. [Google Scholar] [CrossRef]
- Dwivedi, D.; Megha, K.; Mishra, R.; Mandal, P.K. Glutathione in Brain: Overview of Its Conformations, Functions, Biochemical Characteristics, Quantitation and Potential Therapeutic Role in Brain Disorders. Neurochem. Res. 2020, 45, 1461–1480. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Scott, N.; Hatlelid, K.M.; MacKenzie, N.E.; Carter, D.E. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem. Res. Toxicol. 1993, 6, 102–106. [Google Scholar] [CrossRef]
- Styblo, M.; Serves, S.V.; Cullen, W.R.; Thomas, D.J. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem. Res. Toxicol. 1997, 10, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.M.; Del Razo, L.M.; Limon-Pacheco, J.H.; Giordano, M.; Sanchez-Pena, L.C.; Uribe-Querol, E.; Gutierrez-Ospina, G.; Gonsebatt, M.E. Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol. Sci. 2005, 84, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J. Unraveling arsenic--glutathione connections. Toxicol. Sci. 2009, 107, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Koehler, Y.; Luther, E.M.; Meyer, S.; Schwerdtle, T.; Dringen, R. Uptake and toxicity of arsenite and arsenate in cultured brain astrocytes. J. Trace Elem. Med. Biol. 2014, 28, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liao, Y.; Jin, Y.; Li, G.; Lv, X.; Sun, G. Effects of arsenite on glutamate metabolism in primary cultured astrocytes. Toxicol. Vitr. 2012, 26, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Del Razo, L.M.; Styblo, M.; Wang, C.; Cullen, W.R.; Thomas, D.J. Arsenicals inhibit thioredoxin reductase in cultured rat hepatocytes. Chem. Res. Toxicol. 2001, 14, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M.; Chouchane, S.; Snow, E.T. Upregulation of glutathione-related genes and enzyme activities in cultured human cells by sublethal concentrations of inorganic arsenic. Toxicol. Sci. 2002, 70, 183–192. [Google Scholar] [CrossRef][Green Version]
- Petrick, J.S.; Jagadish, B.; Mash, E.A.; Aposhian, H.V. Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem. Res. Toxicol. 2001, 14, 651–656. [Google Scholar] [CrossRef]
- Guidarelli, A.; Cerioni, L.; Fiorani, M.; Catalani, A.; Cantoni, O. Arsenite-Induced Mitochondrial Superoxide Formation: Time and Concentration Requirements for the Effects of the Metalloid on the Endoplasmic Reticulum and Mitochondria. J. Pharmacol. Exp. Ther. 2020, 373, 62–71. [Google Scholar] [CrossRef]
- Binet, F.; Chiasson, S.; Girard, D. Arsenic trioxide induces endoplasmic reticulum stress-related events in neutrophils. Int. Immunopharmacol. 2010, 10, 508–512. [Google Scholar] [CrossRef]
- Spina, A.; Guidarelli, A.; Fiorani, M.; Varone, E.; Catalani, A.; Zito, E.; Cantoni, O. Crosstalk between ERO1alpha and ryanodine receptor in arsenite-dependent mitochondrial ROS formation. Biochem. Pharmacol. 2022, 198, 114973. [Google Scholar] [CrossRef]
- King, Y.A.; Chiu, Y.J.; Chen, H.P.; Kuo, D.H.; Lu, C.C.; Yang, J.S. Endoplasmic reticulum stress contributes to arsenic trioxide-induced intrinsic apoptosis in human umbilical and bone marrow mesenchymal stem cells. Environ. Toxicol. 2016, 31, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, O.; Zito, E.; Guidarelli, A.; Fiorani, M.; Ghezzi, P. Mitochondrial ROS, ER Stress, and Nrf2 Crosstalk in the Regulation of Mitochondrial Apoptosis Induced by Arsenite. Antioxidants 2022, 11, 1034. [Google Scholar] [CrossRef] [PubMed]
- Chandravanshi, L.P.; Gupta, R.; Shukla, R.K. Developmental Neurotoxicity of Arsenic: Involvement of Oxidative Stress and Mitochondrial Functions. Biol. Trace Elem. Res. 2018, 186, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Kumar, V. Arsenic-induced mitochondrial oxidative damage is mediated by decreased PGC-1alpha expression and its downstream targets in rat brain. Chem. Biol. Interact. 2016, 256, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Luo, L.; Huang, Q.; Zhang, J. Cortex metabolome and proteome analysis reveals chronic arsenic exposure via drinking water induces developmental neurotoxicity through hnRNP L mediated mitochondrial dysfunction in male rats. Sci. Total Environ. 2022, 820, 153325. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Qiu, Z.Q.; Zhang, L.; Shu, W.Q. Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol. Lett. 2012, 211, 39–44. [Google Scholar] [CrossRef]
- Lin, A.M.; Fang, S.F.; Chao, P.L.; Yang, C.H. Melatonin attenuates arsenite-induced apoptosis in rat brain: Involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J. Pineal Res. 2007, 43, 163–171. [Google Scholar] [CrossRef]
- Yoshinaga-Sakurai, K.; Shinde, R.; Rodriguez, M.; Rosen, B.P.; El-Hage, N. Comparative Cytotoxicity of Inorganic Arsenite and Methylarsenite in Human Brain Cells. ACS Chem. Neurosci. 2020, 11, 743–751. [Google Scholar] [CrossRef]
- Maekawa, F.; Tsuboi, T.; Oya, M.; Aung, K.H.; Tsukahara, S.; Pellerin, L.; Nohara, K. Effects of sodium arsenite on neurite outgrowth and glutamate AMPA receptor expression in mouse cortical neurons. Neurotoxicology 2013, 37, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Y.; Tan, X.J.; Wang, Y.C.; Liu, K.Y.; Cui, Y.X. Inhibitory effect of arsenic trioxide on neuronal migration in vitro and its potential molecular mechanism. Environ. Toxicol. Pharmacol. 2015, 40, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.C.; Jeng, C.J.; Huang, H.J.; Lin, A.M. Role of autophagy in arsenite-induced neurotoxicity: The involvement of alpha-synuclein. Toxicol. Lett. 2015, 233, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Mao, J.; Zhao, J.; Zhang, Y.; Li, T.; Wang, C.; Xu, L.; Hu, Q.; Wang, X.; Jiang, S.; et al. Arsenic trioxide mediates HAPI microglia inflammatory response and the secretion of inflammatory cytokine IL-6 via Akt/NF-kappaB signaling pathway. Regul. Toxicol. Pharmacol. 2016, 81, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yang, J.; Zhang, Y.; Li, T.; Wang, C.; Xu, L.; Hu, Q.; Wang, X.; Jiang, S.; Nie, X.; et al. Arsenic trioxide mediates HAPI microglia inflammatory response and subsequent neuron apoptosis through p38/JNK MAPK/STAT3 pathway. Toxicol. Appl. Pharmacol. 2016, 303, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Wang, H.; Wei, Y.; Yuan, Y.; Zhou, Q.; Fang, F.; Shi, S.; Jiang, X.; Dong, Y.; et al. Microglia-derived IL-1beta promoted neuronal apoptosis through ER stress-mediated signaling pathway PERK/eIF2alpha/ATF4/CHOP upon arsenic exposure. J. Hazard. Mater. 2021, 417, 125997. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mitra, S.; Sharma, A.K.; Gera, R.; Ghosh, D. Isolation and characterization of microglia from adult mouse brain: Selected applications for ex vivo evaluation of immunotoxicological alterations following in vivo xenobiotic exposure. Chem. Res. Toxicol. 2014, 27, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, F.; Liao, Y.; Jin, Y.; Sun, G. Effects of arsenite in astrocytes on neuronal signaling transduction. Toxicology 2013, 303, 43–53. [Google Scholar] [CrossRef]
- Nino, S.A.; Martel-Gallegos, G.; Castro-Zavala, A.; Ortega-Berlanga, B.; Delgado, J.M.; Hernandez-Mendoza, H.; Romero-Guzman, E.; Rios-Lugo, J.; Rosales-Mendoza, S.; Jimenez-Capdeville, M.E.; et al. Chronic Arsenic Exposure Increases Abeta((1–42)) Production and Receptor for Advanced Glycation End Products Expression in Rat Brain. Chem. Res. Toxicol. 2018, 31, 13–21. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Yu, H.; Wang, H.; Qi, Y.; Geng, M. Effects of arsenic exposure on D-serine metabolism in the hippocampus of offspring mice at different developmental stages. Arch. Toxicol. 2020, 94, 77–87. [Google Scholar] [CrossRef]
- Nagaraja, T.N.; Desiraju, T. Regional alterations in the levels of brain biogenic amines, glutamate, GABA, and GAD activity due to chronic consumption of inorganic arsenic in developing and adult rats. Bull. Environ. Contam. Toxicol. 1993, 50, 100–107. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, X.; Yang, M.; Han, M.; Ommati, M.M.; Ma, Y. Arsenic exposure induced anxiety-like behaviors in male mice via influencing the GABAergic Signaling in the prefrontal cortex. Environ. Sci. Pollut. Res. Int. 2023, 30, 86352–86364. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Bhadauria, S.; Kannan, G.M.; Singh, N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: A review. J. Environ. Biol. 2007, 28, 333–347. [Google Scholar] [PubMed]
- Bjorklund, G.; Rahaman, M.S.; Shanaida, M.; Lysiuk, R.; Oliynyk, P.; Lenchyk, L.; Chirumbolo, S.; Chasapis, C.T.; Peana, M. Natural Dietary Compounds in the Treatment of Arsenic Toxicity. Molecules 2022, 27, 4871. [Google Scholar] [CrossRef] [PubMed]
- Shayan, M.; Barangi, S.; Hosseinzadeh, H.; Mehri, S. The protective effect of natural or chemical compounds against arsenic-induced neurotoxicity: Cellular and molecular mechanisms. Food Chem. Toxicol. 2023, 175, 113691. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharm. Biol. 2017, 55, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Mehrandish, R.; Rahimian, A.; Shahriary, A. Heavy metals detoxification: A review of herbal compounds for chelation therapy in heavy metals toxicity. J. Herbmed Pharmacol. 2019, 8, 69–77. [Google Scholar] [CrossRef]
- Najafi, N.; Rezaee, R.; Hayes, A.W.; Karimi, G. A review of mechanisms underlying the protective effects of natural compounds against arsenic-induced neurotoxicity. Biometals 2023, 36, 799–813. [Google Scholar] [CrossRef]
- Dixit, S.; Dhar, P.; Mehra, R.D. Alpha lipoic acid (ALA) modulates expression of apoptosis associated proteins in hippocampus of rats exposed during postnatal period to sodium arsenite (NaAsO(2)). Toxicol. Rep. 2015, 2, 78–87. [Google Scholar] [CrossRef]
- Hossain, K.F.B.; Akter, M.; Rahman, M.M.; Sikder, M.T.; Rahaman, M.S.; Yamasaki, S.; Kimura, G.; Tomihara, T.; Kurasaki, M.; Saito, T. Amelioration of Metal-Induced Cellular Stress by alpha-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells. Int. J. Environ. Res. Public Health 2021, 18, 2126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.J.; Wang, Y.J.; Kao, W.W.; Chen, R.J.; Ho, Y.S. Protection against arsenic trioxide-induced autophagic cell death in U118 human glioma cells by use of lipoic acid. Food Chem. Toxicol. 2007, 45, 1027–1038. [Google Scholar] [CrossRef]
- Shakeri, A.; Zirak, M.R.; Wallace Hayes, A.; Reiter, R.; Karimi, G. Curcumin and its analogues protect from endoplasmic reticulum stress: Mechanisms and pathways. Pharmacol. Res. 2019, 146, 104335. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Protective effects of curcumin on chemical and drug-induced cardiotoxicity: A review. Naunyn Schmied. Arch. Pharmacol. 2021, 394, 1341–1353. [Google Scholar] [CrossRef]
- Wu, S.; Rao, G.; Wang, R.; Pang, Q.; Zhang, X.; Huang, R.; Li, T.; Tang, Z.; Hu, L. The neuroprotective effect of curcumin against ATO triggered neurotoxicity through Nrf2 and NF-kappaB signaling pathway in the brain of ducks. Ecotoxicol. Environ. Saf. 2021, 228, 112965. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S.; Sankhwar, M.L.; Shukla, R.K.; Chandra, R.; Pant, A.B.; Islam, F.; Khanna, V.K. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol. Appl. Pharmacol. 2009, 240, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.; Banik, S.; Akter, M.; Rahman, M.M.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Curcumin alleviates arsenic-induced toxicity in PC12 cells via modulating autophagy/apoptosis. Ecotoxicol. Environ. Saf. 2020, 200, 110756. [Google Scholar] [CrossRef]
- Firdaus, F.; Zafeer, M.F.; Anis, E.; Ahmad, F.; Hossain, M.M.; Ali, A.; Afzal, M. Evaluation of phyto-medicinal efficacy of thymoquinone against Arsenic induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. Phytomedicine 2019, 54, 224–230. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, J.; Jiang, H.; Wang, M.; Gao, L.; Ma, D.; Zhang, Z. Neuroprotective effect of resveratrol on arsenic trioxide-induced oxidative stress in feline brain. Hum. Exp. Toxicol. 2014, 33, 737–747. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Wang, Y.; Zhang, J.; Zhang, H.; Cao, X.; Hu, T.; Lin, J.; Tang, X.; Yan, X.; et al. Proteomic Study on the Mechanism of Arsenic Neurotoxicity in the Rat Cerebral Cortex and the Protective Mechanism of Dictyophora Polysaccharides against Arsenic Neurotoxicity. ACS Chem. Neurosci. 2023, 14, 2302–2319. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, T.; Wang, Y.; Zhang, X.; Zhang, H.; Lin, J.; Tang, X.; Liu, X.; Chen, M.; Khan, N.U.; et al. Investigating the Neurotoxic Impacts of Arsenic and the Neuroprotective Effects of Dictyophora Polysaccharide Using SWATH-MS-Based Proteomics. Molecules 2022, 27, 1495. [Google Scholar] [CrossRef]
- Srivastava, D.; Subramanian, R.B.; Madamwar, D.; Flora, S.J. Protective effects of selenium, calcium, and magnesium against arsenic-induced oxidative stress in male rats. Arh. Hig. Rada Toksikol. 2010, 61, 153–159. [Google Scholar] [CrossRef][Green Version]

| Reported Alterations Associated with As Exposure | Country | Regulatory Levels (mg/mL) | As Levels Exposure | People Exposed | References |
|---|---|---|---|---|---|
| Canada | 0.01 | 0.0005–3.78 | 1,700,000 | [11,12,13,14,15,16,17,18,19,20,21] |
| United States of America | 0.01 | 0.01–12 | 44,100,000 | [11,21,22,23,24,25,26,27,28,29,30,31,32,33] | |
| Mexico | 0.25 | 0.008–1.1 | 2,000,000 | [11,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] | |
| Chile | 0.05 | 0.05–2 | 2,100,000 | [11,21,54,55,56,57,58,59,60] | |
| Argentina | 0.05 | 0.004–14.969 | 3,500,000 | [61,62,63,64] | |
| China | 0.01 | 0.01–126 | 33,000,000 | [65,66,67,68,69,70,71,72,73,74,75] | |
| Vietnam | 0.05 | 0.001–3.05 | 10,000,000 | [11,76,77,78,79,80,81] | |
| India | 0.05 | 0.0005–3.2 | 45,000,000 to 100,000,000 | [76,82,83,84,85,86,87,88] | |
| Bangladesh | 0.05 | >0.05–4.6 | 85,000,000 | [21,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] | |
| Spain | 0.05 | 0.001–0.29 | 50,000 | [21,76,106,107,108,109] |
| Exposure Model | Doses | Cognitive/Behavioral Alterations | Redox, Neurochemical, and Structural Alterations | References |
|---|---|---|---|---|
Gestational Exposure | Pregnant C3H mice exposed to NaAsO2 (85 ppm) from GD 8 to GD 18. | Impaired adaptation to repetitive reversal tasks in adulthood (60 postnatal weeks) Poor sociability and poor social novelty preference at 74 postnatal weeks on F1, as well as on F2. | Increase in the number of pyramidal neurons in layers V and VI of the prelimbic cortex. | [168,169,170] |
| Decreased neurite length. | ||||
| Decreased in serotonin receptor (5-HT 5B) and BNDF gene expression in brain cortex of F1 and F2 mice. | ||||
| Pregnant C57Bl6/J mice exposed to NaAsO2 (100 mg/L) in drinking water starting 1 week before conception until birth. | Impaired spatial and episodic memory in adulthood. Impairments in fear conditioning performance. | Global hypo-acetylation at H3K9. | [171] | |
| Highly significant representation of KRAB transcription factors. | ||||
| Decreased viability of astroglial cells. | ||||
Perinatal exposure | Pregnant rats—lactating dams—weaned pups exposed to NaAsO2 (100 mg/L) from GD 6 to PND 42 | Alterations in learning and memory function. | Increased expression of glutamate decarboxylase and decrease the γ-aminobutyric acid transferase. | [172] |
| Pregnant C57BL/6J mice—lactating dams and weaned pups exposed to 50 ppb of arsenic in drinking water from GD 1 to PND 23–28 | Increased scape latencies. Increased immobility in a forced swim task. Induced spatial learning and memory impairment. Abnormal social behaviors, restricted interests, and repetitive behaviors (autism-like behaviors). Anxiety-like behaviors. | Reduced protein expression of CRFR1. | [173,174,175,176] | |
| Increased sensitivity of dorsal hippocampal 5HT1A receptor to serotonin. | ||||
| Reduced corticosterone receptor in the hippocampus. | ||||
| Decreased MAPK pathway transcripts, proteins and activity. | ||||
| Decreased synaptic density in cortex, hippocampus, and cerebellum. | ||||
| Decreased expression of markers for presynaptic and postsynaptic membranes (PSD-95 and SYP). | ||||
| Pre-mating CD-1 mice–-Pregnant and lactating dams exposed to 20 mg/L of iAs: 1) from 30 days prior mating to postnatal day 15 and 2) from 30 days prior mating to PND 90. | Both iAs-PND15 and iAsPND90 decrease recognition of the object location indicating spatial memory impairment. Impaired learning and memory in the water maze test. | Increased expression of xCT and EAAC1 transporters on PND15. | [177,178] | |
| Down-regulation of NR2B NMDA receptor subunit on PND15. | ||||
| Decreased GSH/GSSG ratio in cortex and hippocampus on PND 90. | ||||
| Decreased GluA1 and GluA2 AMPA receptor subunits in hippocampus. | ||||
| Low capacity to induce long-term potentiation and reduction in basal excitability. | ||||
| Pregnant Kunming albino mice—lactating dams and weaned pups exposed to NaAsO2 (25–100 mg/L) in drinking water from GD 1 to PND 35. | Impaired spatial learning ability | Dose-dependent decreased on NR1, NR2A, NR2B, GluR1, GluR2, GluR3, CaMKII, and p-CaMKII expression in the hippocampus at PND 7, 14, 21 and 35. | [179,180] | |
| Decreased thickness of the postsynaptic density. | ||||
| Decreased postsynaptic density protein-95 and synaptophysin proteins. | ||||
| Pregnant Wistar rats—lactating dams and weaned pups exposed to NaAsO2 (0.05–0.10 mg/L) in drinking water from GD 0 to PND 35. | Impaired retention of long-term memory. | Decreased α7-nAChR expression. | [181] | |
| Decreased aspartate transaminase levels. | ||||
| Increased glutamate levels. | ||||
| Pregnant Sprague-Dawley rats—lactating dams—weaned pups exposed to NaAsO2 (23.6–71 ppm, drinking water) from GD 6 to weaning. | Delayed in bilateral eye opening and incisor eruption. | Decreased catalase activity. | [182] | |
| Poor slat board performance (PND 8–11). | No changes in brain dopamine and its metabolites. | |||
| Pregnant Sprague-Dawley rats—lactating dams—weaned pups exposed to NaAsO2 (2–4 mg/kg) from GD 6 to PND21 | Hypoactivity. | Decreased mRNA and protein expression of dopamine D2 receptor. | [183] | |
| Impairment of spatial learning and memory | Altered dopamine and its metabolites in striatum. | |||
Early Postnatal exposure | Wistar rat pups exposed to NaAsO2 (1.5–2 mg/kg, i.p.) from PND4 to PND17. | Impaired learning and memory | Decreased dendritic arborization and reduced number of spines in pyramidal neurons and granule cells. | [184] |
Early life to adulthood | Weaning male rats exposed to NaAsO2 (68 mg/L) for 3 months. | Spatial memory damage. | Swollen mitochondrion and expanded rough endoplasmic reticulum in hippocampal neurons. | [185] |
| Reduced mRNA expression of NMDA NR2A subunit. | ||||
| Postnatal day 21 Wistar rats exposed to NaAsO2 (10 mg/kg, orally through gavage) for 1 week. | Decreased open-field behavior, locomotor activity, exploratory behavior, and grip strength. | Increased acetylcholine levels in cortex, cerebellum, and hippocampus. | [186] | |
Adult | Male and female C57B1/6J mice exposed to NaAsO2 (0.05–50 mg As/L) in drinking water for 4 months. | Female mice showed hyperactivity each month after being exposed to 0.05, 0.5 and 5 mg As/L. | Dopamine decreased on the striatum and hypothalamus in female mice. | [187] |
| Male mice showed hyperactivity in the group exposed to 0.5 mg As/L and hypoactivity in the group exposed to 50 mg As/L. | Tyrosine hydroxylase and cytosolic thioredoxin mRNA decrease in striatum and nucleus accumbens of male and female mice, respectively. | |||
| Adult male Swiss albino mice administered with As2O3 (2 mg/kg bw) through oral gavage for 45 days. | Enhanced anxiety levels. | Reduction in stratum pyramidale thickness (CA1). | [188] | |
| Reduced exploratory activity. | ||||
| Decrease in density and size of pyramidal neurons. | ||||
| Spatial memory retention impairment. | ||||
| Reduction in hippocampal GSH levels | ||||
| Fear for height and open environment. | ||||
| Adult female Swiss albino mice administered with As2O3 (2 or 4 mg/kg bw) through oral gavage for 45 days. | Increased anxiety levels with both doses in open field test and elevates plus maze test. | Downregulation of estrogen receptor expression | [189] | |
| Reduced locomotion and decreased exploratory ability at both doses. | ||||
| Reduction of BNDF and NMDAR 2B levels in hippocampus. | ||||
| Impaired spatial learning and memory. | ||||
| 4-week-old male Kunming mice were administrated NaAsO2 (50 mg/kg bw) twice a week for 7 weeks. | Reduced spatial learning and memory ability. | Increased ROS levels and SOD activity in hippocampus. | [190] | |
| Loss of pyramidal cell layer. | ||||
| Upregulated expression of SOD, HO-1 and GPx-3 genes. | ||||
| Positive association between Nrf2/PPARγ expression in hippocampus. | ||||
| 9-weeks old male Kunming mice exposed to arsenic trioxide (4 ppm) in drinking water for 90 days. | Impairment of learning and memory. | Thickness postsynaptic density and synaptic cleft width decrease in hippocampus. | [191] | |
| 9-weeks old male SPF mice exposed to As2O3 in drinking water for 60 days. | Poor performance in cognition functions. | Reduced thickness of cerebellar postsynaptic density. | [192,193] | |
| Decreased mRNA expressions of genes related to long-term potentiation and long-term depression. | ||||
| Cellular edema in neurons with metamorphotic nuclei in hippocampus. | ||||
| Reduction in cells body and number of pyramidal cells in CA3 area. | ||||
| Male ICR mice exposed to realgar (90% As2S2; 0.15, 0.45 or 1.35 g/kg) once a day for 12 weeks. | Decreased locomotor activity and exploratory behavior. | Decreased SOD activity and increased MDA levels in brain cortex. | [194] | |
| Lost plasma membrane integrity. Swollen mitochondria, vague structure of rough endoplasmic reticulum and vacuoles structures presence in cortex neurons. | ||||
| Decreased in the number of synaptic structures, in synaptic vesicles and in the curvature of the synaptic interface. Increase in the distance of synaptic cleft and in postsynaptic density. | ||||
| Male C57/BL/6J exposed to As2O3 (10 mg/L) for 8 weeks. | Enhance anxiety-like behavior on elevated plus maze and open field test. | Decrease in BDNF-TrkB pathway. | [195] | |
| Increase depression-like behaviors on tail suspension test and forced swimming test in the reserpine pre-treated mice. | ||||
| 2-months old CD1 mice exposed to 0.5 or 5.0 mg As/L in drinking water for 6 months. | Hypoactivity. | Decrease in the expression of mRNA for dopamine receptors D2 in striatum. | [196] | |
| Alterations on rearing and on-wall rearing and barbering behaviors. | ||||
| Swiss male Albino mice exposed to NaAsO2 (10 mg/kg body weight) in drinking water for 60 days. | Anxiety-like behaviors. | Increased blood indices of alkaline phosphatase, alanine aminotransferase, creatinine, aspartate aminotransferase, and urea. | [197,198] | |
| Deficits in spatial memory and learning. | Decreased butyryl cholinesterase and SOD activity. | |||
| Male C57BL/6J mice exposed to NaAsO2 (50 mg/L) for 24 weeks in drinking water. | Learning and memory impairments. | Activation of microglia in cortex and dentate gyrus in the hippocampus. | [199] | |
| Adult male Sprague Dawley rats exposed to NaAsO2 (5, 10, and 20 mg/Kg, intragastric via) for 2 or 2 weeks. | Reduced locomotor activity. | Alterations in monoamine content in midbrain and cortex. | [200] | |
| Increased numbers of errors in egocentric task. | ||||
| Male Wistar rats exposed to NaAsO2 (1 mg/Kg, oral via) once daily for 6 months. | Loss of memory and learning ability. | Increased ROS levels, nitric oxide generation and lipid peroxidation levels. | [201] | |
| Decreased GSH levels. | ||||
| Decreased GPx, GST, SOD and acetylcholinesterase activity in cortex and hippocampus. | ||||
| Male Sprague-Dawley rats exposed to iAs (50 mg/L) in drinking water for a year. | Hypoactivity. | Increased striatal dopamine content. Up-regulation of mRNA for Mn-SOD and cytosolic thioredoxin enzymes. Down-regulation of dopamine receptor D2, D1 and Nrf2 mRNA expression. | [202] | |
| Adult male Wistar rats exposed to NaAsO2 (2mg/kg, orally through gavage) for 10 weeks. | Decreased exploratory behavior. | Increased lipoperoxidation in cortex, hippocampus, and cerebellum. | [203] | |
| Hypoactivity. | ||||
| Decreased Mn-SOD, Cu/Zn-SOD, CAT, GPx, GR and GST activity in cortex, hippocampus and cerebellum. | ||||
| Decreased grip strength of the fore limb and hind limb. | ||||
| Adult male Wistar rats exposed to NaAsO2 (10 mg/kg, orally through gavage) for 6 weeks. | Decreased open field motility. | Increased latency of the cortical evoked potentials | [204] | |
| Decreased peripheral nerve conduction velocity. | ||||
| Adult and aging Wistar rats exposed to NaAsO2 (10 mg/kg, orally through gavage) for 1 week. | Decreased the open-field behaviour (crossings, rearings, sniffings, and groomings), locomotor activity, grip strength, and exploratory behaviour. | Disturbances in cholinergic system. | [186] | |
| Increased escape latency of all the three phases observed in the water maze test. | ||||
| Adult female Wistar rats exposed to NaAsO2 (13 mg/Kg, orally) for 3 weeks. | Suppressed exploratory behavior. | Induce oxidative stress. | [205] | |
| Decreased motor coordination. | ||||
| Degenerative changes (vacuolization, inflammatory infiltration, karyosis, pyknosis, and necrosis) in the neuronal cells. | ||||
| Decreased immobility period in the forced swim test. | ||||
| Decreased dopamine, noradrenaline, and serotonin levels. | ||||
| Decreased head dips count | ||||
| Adult male Wistar rats exposed to As (10 mg/kg/day, in drinking water) for 21 days. | Impaired motor function, recognition learning and memory, spatial learning, and social interaction. | Decreased GSH and reduced antioxidant power. | [206] | |
| Increased anxiety-like behavior. | Increased lipid peroxidation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez Cervantes, G.I.; González Esquivel, D.F.; Ramírez Ortega, D.; Blanco Ayala, T.; Ramos Chávez, L.A.; López-López, H.E.; Salazar, A.; Flores, I.; Pineda, B.; Gómez-Manzo, S.; et al. Mechanisms Associated with Cognitive and Behavioral Impairment Induced by Arsenic Exposure. Cells 2023, 12, 2537. https://doi.org/10.3390/cells12212537
Vázquez Cervantes GI, González Esquivel DF, Ramírez Ortega D, Blanco Ayala T, Ramos Chávez LA, López-López HE, Salazar A, Flores I, Pineda B, Gómez-Manzo S, et al. Mechanisms Associated with Cognitive and Behavioral Impairment Induced by Arsenic Exposure. Cells. 2023; 12(21):2537. https://doi.org/10.3390/cells12212537
Chicago/Turabian StyleVázquez Cervantes, Gustavo Ignacio, Dinora Fabiola González Esquivel, Daniela Ramírez Ortega, Tonali Blanco Ayala, Lucio Antonio Ramos Chávez, Humberto Emanuel López-López, Alelí Salazar, Itamar Flores, Benjamín Pineda, Saúl Gómez-Manzo, and et al. 2023. "Mechanisms Associated with Cognitive and Behavioral Impairment Induced by Arsenic Exposure" Cells 12, no. 21: 2537. https://doi.org/10.3390/cells12212537
APA StyleVázquez Cervantes, G. I., González Esquivel, D. F., Ramírez Ortega, D., Blanco Ayala, T., Ramos Chávez, L. A., López-López, H. E., Salazar, A., Flores, I., Pineda, B., Gómez-Manzo, S., & Pérez de la Cruz, V. (2023). Mechanisms Associated with Cognitive and Behavioral Impairment Induced by Arsenic Exposure. Cells, 12(21), 2537. https://doi.org/10.3390/cells12212537