The Adaptive Role of Carotenoids and Anthocyanins in Solanum lycopersicum Pigment Mutants under High Irradiance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Determination of Water Content, Photosynthetic and UV-Absorbing Pigments, Anthocyanins and Trolox Equivalent Antioxidant Capacity
2.3. HPLC Analysis of Carotenoids
2.4. Measurements of CO2 Gas Exchange and Photochemical Activity
2.5. RNA Extraction and Quantitative RT-PCR
2.6. Light Microscopyver
2.7. Statistical Analysis
3. Results
3.1. Leaf Morphology, Water Content, Photosynthetic Pigments, UV-Absorbing Pigments, Anthocyanins and Trolox Equivalent Antioxidant Capacity
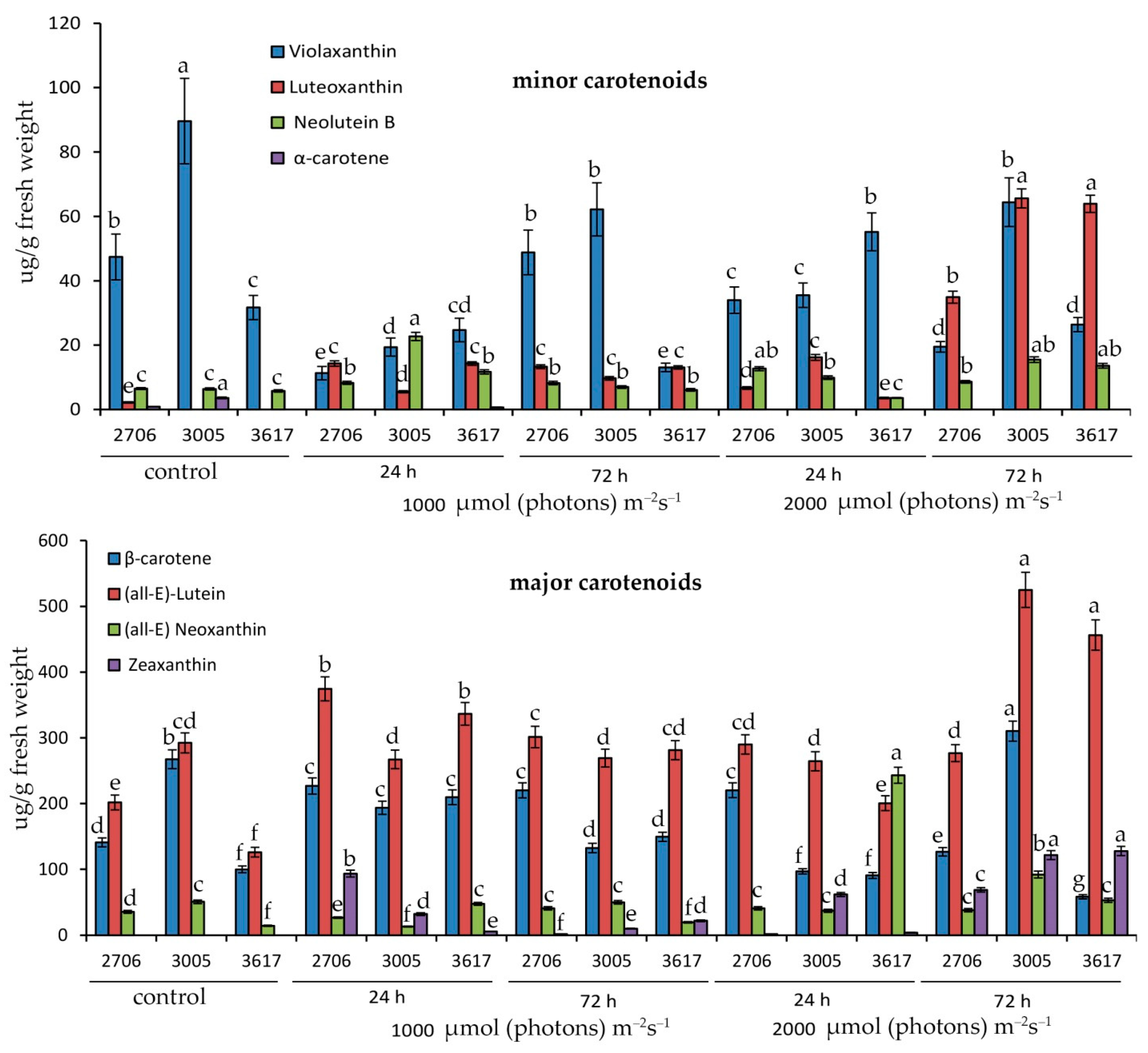
3.2. Qualitative and Quantitative Composition of Carotenoids
3.3. Measurements of CO2 Gas Exchange of Photochemical Activity
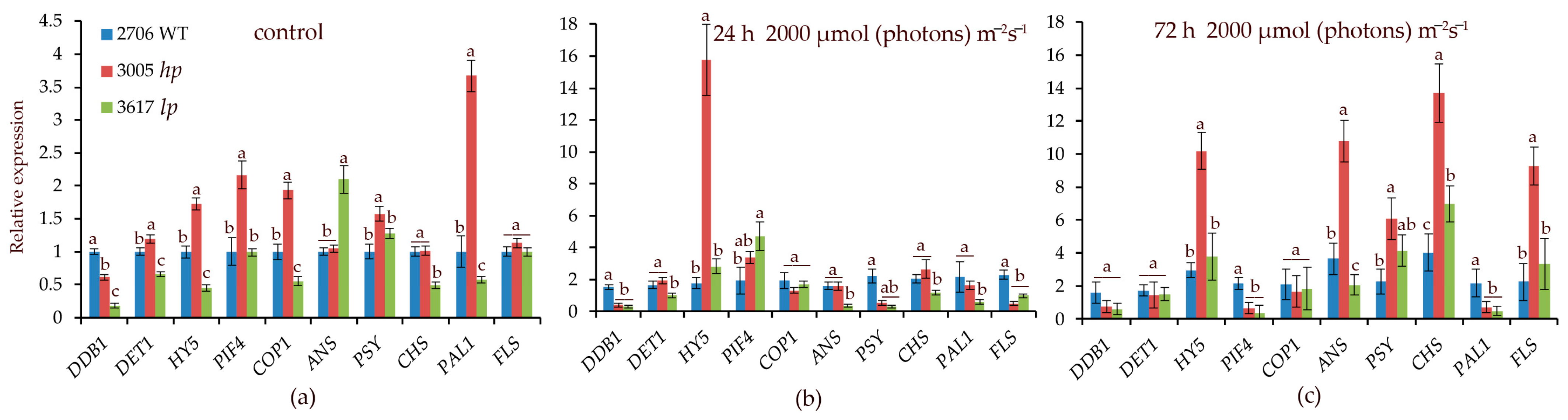
3.4. Transcript Levels of the Studied Genes
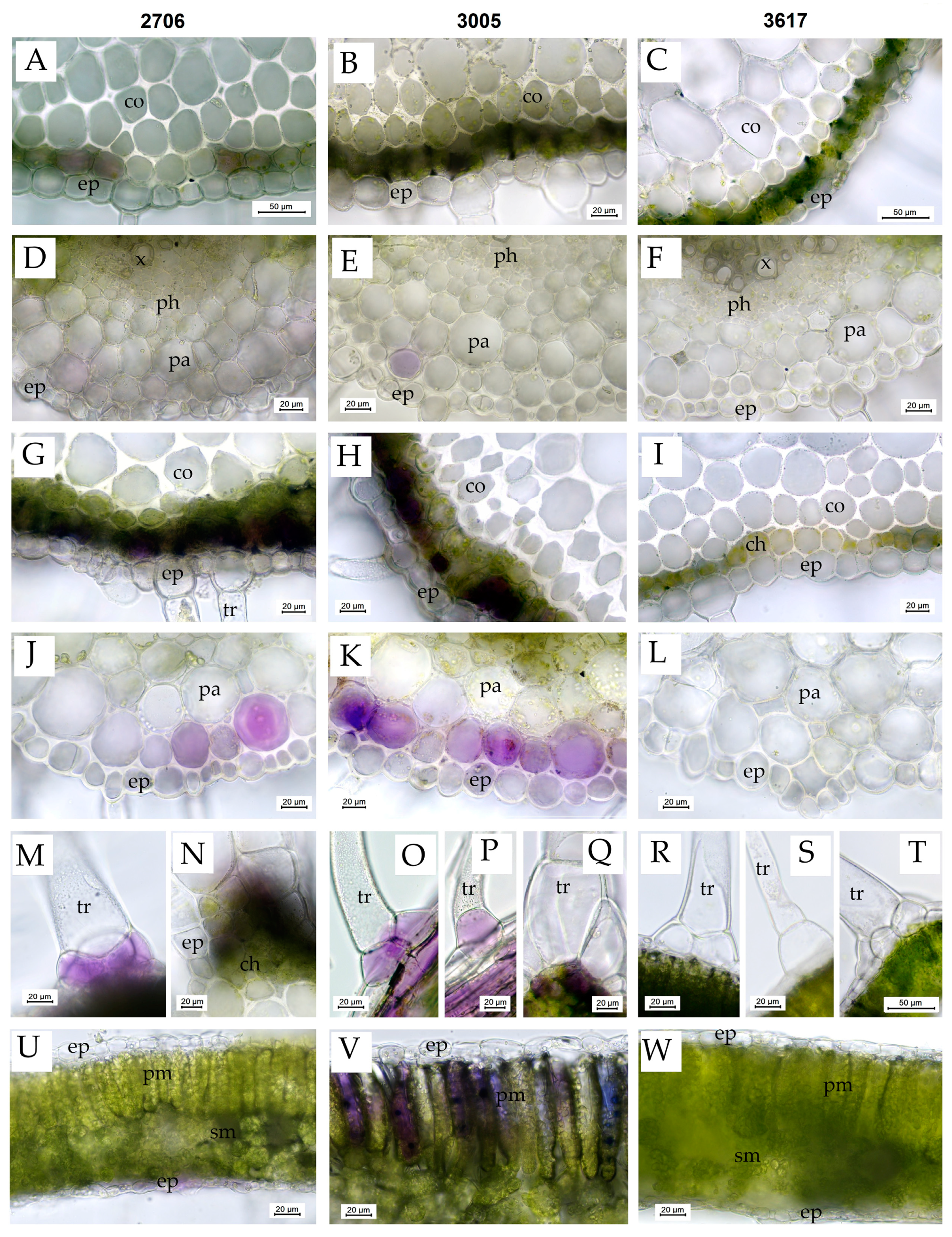
3.5. The Structure of Leaf Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, Y.; Sacharz, J.; Ware, M.A.; Zhang, H.; Ruban, A.V. Effects of Periodic Photoinhibitory Light Exposure on Physiology and Productivity of Arabidopsis Plants Grown under Low Light. J. Exp. Bot. 2017, 68, 4249–4262. [Google Scholar] [CrossRef] [PubMed]
- Tyystjärvi, E. Photoinhibition of Photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Alves, P.L.; Da, C.; Magalhães, A.C.; Barja, P.R. The Phenomenon of Photoinhibition of Photosynthesis and Its Importance in Reforestation. Bot. Rev. 2002, 68, 193–208. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A New Paradigm for the Action of Reactive Oxygen Species in the Photoinhibition of Photosystem II. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 742–749. [Google Scholar] [CrossRef]
- Murata, N.; Allakhverdiev, S.I.; Nishiyama, Y. The Mechanism of Photoinhibition in Vivo: Re-Evaluation of the Roles of Catalase, α-Tocopherol, Non-Photochemical Quenching, and Electron Transport. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 1127–1133. [Google Scholar] [CrossRef]
- Schmitt, F.-J.; Renger, G.; Friedrich, T.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Los, D.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Reactive Oxygen Species: Re-Evaluation of Generation, Monitoring and Role in Stress-Signaling in Phototrophic Organisms. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 835–848. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive Oxygen Species Generation and Signaling in Plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, T.; Feng, B.; Zhang, C.; Peng, S.; Zhang, X.; Fu, G.; Tao, L. Non-Photochemical Quenching Plays a Key Role in Light Acclimation of Rice Plants Differing in Leaf Color. Front. Plant Sci. 2017, 7, 1968. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The Role of the Xanthophyll Cycle and of Lutein in Photoprotection of Photosystem II. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R. When There Is Too Much Light. Plant Physiol. 2001, 125, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A. Photoprotection in Plants: Optical Screening-Based Mechanisms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-13887-4. [Google Scholar]
- Stetsenko, L.A.; Pashkovsky, P.P.; Voloshin, R.A.; Kreslavski, V.D.; Kuznetsov, V.V.; Allakhverdiev, S.I. Role of Anthocyanin and Carotenoids in the Adaptation of the Photosynthetic Apparatus of Purple- and Green-Leaved Cultivars of Sweet Basil (Ocimum Basilicum) to High-Intensity Light. Photosynthetica 2020, 58, 890–901. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Lajayer, B.A.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the Bulwark: Unravelling the Efficient Applications of Plant Phenolics and Tannins against Environmental Stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized Phenolic Compounds in Seeds: Structures, Functions, and Regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef]
- Prabpree, A.; Sangsil, P.; Nualsri, C.; Nakkanong, K. Expression Profile of Phenylalanine Ammonia-Lyase (PAL) and Phenolic Content during Early Stages of Graft Development in Bud Grafted Hevea Brasiliensis. Biocatal. Agric. Biotechnol. 2018, 14, 88–95. [Google Scholar] [CrossRef]
- Jenkins, G.I.; Long, J.C.; Wade, H.K.; Shenton, M.R.; Bibikova, T.N. UV and Blue Light Signalling: Pathways Regulating Chalcone Synthase Gene Expression in Arabidopsis. New Phytol. 2001, 151, 121–131. [Google Scholar] [CrossRef]
- Rai, N.; Kumari, S.; Singh, S.; Saha, P.; Pandey-Rai, S. Genome-Wide Identification of bZIP Transcription Factor Family in Artemisia Annua, Its Transcriptional Profiling and Regulatory Role in Phenylpropanoid Metabolism under Different Light Conditions. Physiol. Mol. Biol. Plants 2023, 29, 905–925. [Google Scholar] [CrossRef] [PubMed]
- Perea-Resa, C.; Rodríguez-Milla, M.A.; Iniesto, E.; Rubio, V.; Salinas, J. Prefoldins Negatively Regulate Cold Acclimation in Arabidopsis Thaliana by Promoting Nuclear Proteasome-Mediated HY5 Degradation. Mol. Plant 2017, 10, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering Anthocyanin Biosynthesis in Plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, U.; Sagasser, M.; Mehrtens, F.; Stracke, R.; Weisshaar, B. Differential Combinatorial Interactions of Cis-Acting Elements Recognized by R2R3-MYB, BZIP, and BHLH Factors Control Light-Responsive and Tissue-Specific Activation of Phenylpropanoid Biosynthesis Genes. Plant Mol. Biol. 2005, 57, 155–171. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light Absorption and Energy Transfer in the Antenna Complexes of Photosynthetic Organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Do, V.G.; Lee, Y.; Kweon, H.; Kim, S. Light Induces Carotenoid Biosynthesis-Related Gene Expression, Accumulation of Pigment Content, and Expression of the Small Heat Shock Protein in Apple Fruit. Int. J. Mol. Sci. 2022, 23, 6153. [Google Scholar] [CrossRef]
- Tattini, M.; Landi, M.; Brunetti, C.; Giordano, C.; Remorini, D.; Gould, K.S.; Guidi, L. Epidermal Coumaroyl Anthocyanins Protect Sweet Basil against Excess Light Stress: Multiple Consequences of Light Attenuation. Physiol. Plant. 2014, 152, 585–598. [Google Scholar] [CrossRef]
- Mahdavian, K.; Ghorbanli, M.; Kalantari, K. The Effects of Ultraviolet Radiation on the Contents of Chlorophyll, Flavonoid, Anthocyanin and Proline in Capsicum annuum L. Turk. J. Bot. 2008, 32, 25–33. [Google Scholar]
- Wimalasekera, R. Effect of Light Intensity on Photosynthesis. In Photosynthesis, Productivity and Environmental Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 65–73. ISBN 978-1-119-50180-0. [Google Scholar]
- Liu, Y.; Roof, S.; Ye, Z.; Barry, C.; van Tuinen, A.; Vrebalov, J.; Bowler, C.; Giovannoni, J. Manipulation of Light Signal Transduction as a Means of Modifying Fruit Nutritional Quality in Tomato. Proc. Natl. Acad. Sci. USA 2004, 101, 9897–9902. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Senapati, D.; Srivastava, A.; Chakraborty, M.; Gangappa, S.N.; Chattopadhyay, S. Short Hypocotyl in White Light1 Interacts with Elongated Hypocotyl5 (HY5) and Constitutive Photomorphogenic1 (COP1) and Promotes COP1-Mediated Degradation of HY5 during Arabidopsis Seedling Development. Plant Physiol. 2015, 169, 2922–2934. [Google Scholar]
- Davuluri, G.R.; van Tuinen, A.; Mustilli, A.C.; Manfredonia, A.; Newman, R.; Burgess, D.; Brummell, D.A.; King, S.R.; Palys, J.; Uhlig, J.; et al. Manipulation of DET1 Expression in Tomato Results in Photomorphogenic Phenotypes Caused by Post-Transcriptional Gene Silencing. Plant J. 2004, 40, 344–354. [Google Scholar] [CrossRef]
- Christopher, D.A.; Hoffer, P.H. DET1 Represses a Chloroplast Blue Light-Responsive Promoter in a Developmental and Tissue-Specific Manner in Arabidopsis Thaliana. Plant J. 1998, 14, 1–11. [Google Scholar] [CrossRef]
- Genschik, P.; Molinier, J.; Lechner, E.; Dumbliauskas, E. Regulation and Role of Arabidopsis CUL4–DDB1A–DDB2 in Maintaining Genome Integrity upon UV Stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 150, S176. [Google Scholar] [CrossRef]
- Levin, I.; De Vos, C.; Tadmor, Y.; Bovy, A.; Lieberman, M.; Oren-Shamir, M.; Segev, O.; Kolotilin, I.; Keller, M.; Ovadia, R.; et al. High Pigment Tomato Mutants—More than Just Lycopene (a Review). Isr. J. Plant Sci. 2006, 54, 179–190. [Google Scholar] [CrossRef]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a Tomato Landraces Collection for Fruit-Related Traits by the Aid of a High-Throughput Genomic Platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef]
- Kurup, S.; Jones, H.D.; Holdsworth, M.J. Interactions of the Developmental Regulator ABI3 with Proteins Identified from Developing Arabidopsis Seeds. Plant J. 2000, 21, 143–155. [Google Scholar] [CrossRef]
- Pan, Y.; Bradley, G.; Pyke, K.; Ball, G.; Lu, C.; Fray, R.; Marshall, A.; Jayasuta, S.; Baxter, C.; van Wijk, R. Network Inference Analysis Identifies an APRR2-like Gene Linked to Pigment Accumulation in Tomato and Pepper Fruits. Plant Physiol. 2013, 161, 1476–1485. [Google Scholar] [CrossRef]
- Kumordzi, B.B.; Wardle, D.A.; Freschet, G.T. Plant Assemblages Do Not Respond Homogenously to Local Variation in Environmental Conditions: Functional Responses Differ with Species Identity and Abundance. J. Veg. Sci. 2015, 26, 32–45. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Mirecki, R.M.; Teramura, A.H. Effects of Ultraviolet-B Irradiance on Soybean 1: V. The Dependence of Plant Sensitivity on the Photosynthetic Photon Flux Density during and after Leaf Expansion. Plant Physiol. 1984, 74, 475–480. [Google Scholar] [CrossRef]
- Liu, C.-C.; Chi, C.; Jin, L.-J.; Zhu, J.; Yu, J.-Q.; Zhou, Y.-H. The bZip Transcription Factor HY5 Mediates CRY1a-Induced Anthocyanin Biosynthesis in Tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Makhneva, Z.; Bolshakov, M.; Moskalenko, A. Heterogeneity of Carotenoid Content and Composition in LH2 of the Purple Sulphur Bacterium Allochromatium Minutissimum Grown under Carotenoid-Biosynthesis Inhibition. Photosynth. Res. 2008, 98, 633–641. [Google Scholar] [CrossRef]
- Ashikhmin, A.; Makhneva, Z.; Bolshakov, M.; Moskalenko, A. Incorporation of Spheroidene and Spheroidenone into Light-Harvesting Complexes from Purple Sulfur Bacteria. J. Photochem. Photobiol. B Biol. 2017, 170, 99–107. [Google Scholar] [CrossRef]
- Britton, G. Carotenoids. 1, B. Spectroscopy; Birkhäuser: Basel, Switzerland, 1995. [Google Scholar]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Strokina, V.V.; Khudyakova, A.Y.; Shirshikova, G.N.; Kosobryukhov, A.A.; Pashkovskiy, P.P.; Alwasel, S.; Allakhverdiev, S.I. Effect of high-intensity light and UV-B on photosynthetic activity and the expression of certain light-responsive genes in A. thaliana phyA and phyB mutants. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2021, 1862, 148445. [Google Scholar] [CrossRef]
- Solovchenko, A. Buildup of Screening Pigments and Resistance of Plants to Photodamage. In Photoprotection in Plants: Optical Screening-Based Mechanisms; Solovchenko, A., Ed.; Springer Series in Biophysics; Springer: Berlin/Heidelberg, Germany, 2010; pp. 143–163. ISBN 978-3-642-13887-4. [Google Scholar]
- Kim, J.Y.H.; Kwak, H.S.; Sung, Y.J.; Choi, H.I.; Hong, M.E.; Lim, H.S.; Lee, J.-H.; Lee, S.Y.; Sim, S.J. Microfluidic High-Throughput Selection of Microalgal Strains with Superior Photosynthetic Productivity Using Competitive Phototaxis. Sci. Rep. 2016, 6, 21155. [Google Scholar] [CrossRef]
- Horton, P. Optimization of Light Harvesting and Photoprotection: Molecular Mechanisms and Physiological Consequences. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3455–3465. [Google Scholar] [CrossRef] [PubMed]
- van Bezouwen, L.S.; Caffarri, S.; Kale, R.S.; Kouřil, R.; Thunnissen, A.-M.W.; Oostergetel, G.T.; Boekema, E.J. Subunit and Chlorophyll Organization of the Plant Photosystem II Supercomplex. Nat. Plants 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gunell, S.; Lempiäinen, T.; Rintamäki, E.; Aro, E.-M.; Tikkanen, M. Enhanced Function of Non-Photoinhibited Photosystem II Complexes upon PSII Photoinhibition. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2023, 1864, 148978. [Google Scholar] [CrossRef] [PubMed]
- Ballottari, M.; Mozzo, M.; Girardon, J.; Hienerwadel, R.; Bassi, R. Chlorophyll Triplet Quenching and Photoprotection in the Higher Plant Monomeric Antenna Protein Lhcb5. J. Phys. Chem. B 2013, 117, 11337–11348. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Fiore, A.; Cazzaniga, S.; Giuliano, G.; Bassi, R. Different Roles of α- and β-Branch Xanthophylls in Photosystem Assembly and Photoprotection. J. Biol. Chem. 2007, 282, 35056–35068. [Google Scholar] [CrossRef]
- Xi, Y.; Bian, J.; Luo, G.; Kong, F.; Chi, Z. Enhanced β-Carotene Production in Dunaliella Salina under Relative High Flashing Light. Algal Res. 2022, 67, 102857. [Google Scholar] [CrossRef]
- Dhami, N.; Cazzonelli, C.I. Environmental Impacts on Carotenoid Metabolism in Leaves. Plant Growth Regul. 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.; Khudyakova, A.; Ashikhmin, A.; Bolshakov, M.; Kozhevnikova, A.; Kosobryukhov, A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Effect of High-Intensity Light on the Photosynthetic Activity, Pigment Content and Expression of Light-Dependent Genes of Photomorphogenetic Solanum Lycopersicum Hp Mutants. Plant Physiol. Biochem. 2021, 167, 91–100. [Google Scholar] [CrossRef]
- Foyer, C.H.; Trebst, A.; Noctor, G. Signaling and Integration of Defense Functions of Tocopherol, Ascorbate and Glutathione. In Photoprotection, Photoinhibition, Gene Regulation, and Environment; Demmig-Adams, B., Adams, W.W., Mattoo, A.K., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2006; pp. 241–268. ISBN 978-1-4020-3579-1. [Google Scholar]
- Zeng, X.-Q.; Chow, W.S.; Su, L.-J.; Peng, X.-X.; Peng, C.-L. Protective Effect of Supplemental Anthocyanins on Arabidopsis Leaves under High Light. Physiol. Plant. 2010, 138, 215–225. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Xiao, Y.; Chu, L.; Zhang, Y.; Bian, Y.; Xiao, J.; Xu, D. HY5: A Pivotal Regulator of Light-Dependent Development in Higher Plants. Front. Plant Sci. 2022, 12, 800989. [Google Scholar] [CrossRef] [PubMed]

| Variant | Light Intensity | Time, h | Chl a | Chl b | Chl a + b | Car | TEAC | Leaf Thickness, µm | Antocyanin µg g−1 FW | UAPs | H2O, % | DW, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2706 | 1760 ± 70b | 657 ± 26b | 2417 ± 97b | 435 ± 17b | 25.5 ± 1.0a | 108 ± 10a | 0.45 ± 0.15b | 4.4 ± 1.4c | 84.4 ± 0.3b | 15.6 ± 0.5b | ||
| 3005 | Control | 0 | 2317 ± 93a | 788 ± 32a | 3105 ± 124a | 711 ± 28a | 20.3 ± 0.8b | 110 ± 8a | 1.33 ± 0.07a | 6.7 ± 0.9b | 87.8 ± 0.5a | 12.2 ± 0.6c |
| 3617 | 1120 ± 45c | 380 ± 15c | 1500 ± 60c | 278 ± 11c | 15.3 ± 0.6c | 111 ± 12a | 0.15 ± 0.02c | 12.1 ± 2.1a | 83.2 ± 0.8c | 16.8 ± 0.3a | ||
| 2706 | 2120 ± 85a | 839 ± 34a | 2959 ± 118a | 753 ± 30a | 53.1 ± 2.1b | 115 ± 11a | 1.88 ± 0.09b | 7.1 ± 0.8b | ||||
| 3005 | 24 | 1757 ± 70b | 658 ± 26c | 2415 ± 97b | 553 ± 22b | 66.5 ± 2.7a | 121 ± 9a | 2.41 ± 0.21a | 4.3 ± 0.7c | |||
| 3617 | 1000 µmol (photons) m−2s−1 | 2258 ± 90a | 784 ± 31b | 3042 ± 122a | 651 ± 26b | 44.9 ± 1.8c | 95 ± 11a | 0.38 ± 0.03c | 11.9 ± 1.8a | |||
| 2706 | 1683 ± 67a | 535 ± 21a | 2218 ± 89a | 634 ± 25a | 53.7 ± 2.2c | 109 ± 8b | 9.01 ± 0.52b | 10.3 ± 0.5b | 77.4 ± 0.7b | 22.6 ± 0.7a | ||
| 3005 | 72 | 1675.6 ± 67a | 605 ± 24a | 2280 ± 91a | 541 ± 22b | 113.9 ± 4.6a | 130 ± 8a | 12.24 ± 0.34a | 5.2 ± 0.3c | 79.9 ± 0.8a | 20.1 ± 0.8b | |
| 3617 | 1524 ± 61a | 654 ± 26a | 2177 ± 87a | 505 ± 20b | 71.6 ± 2.9b | 101 ± 10b | 0.54 ± 0.14c | 12.2 ± 0.3a | 77.9 ± 0.4b | 22.1 ± 0.4a | ||
| 2706 | 1860 ± 74a | 661 ± 26a | 2521 ± 101a | 607 ± 24a | 48.5 ± 1.9b | 119 ± 11a | 2.9 ± 0.25b | 8.2 ± 0.6b | ||||
| 3005 | 24 | 1578 ± 63b | 582 ± 23b | 2160 ± 84b | 522 ± 21b | 56.0 ± 2.2a | 123 ± 9a | 5.7 ± 0.91a | 5.8 ± 0.9c | |||
| 3617 | 2000 µmol (photons) m−2s−1 | 1996 ± 80a | 641 ± 26a | 2637 ± 106a | 600 ± 22a | 47.2 ± 1.9b | 107 ± 13a | 0.61 ± 0.05c | 10.5 ± 0.7a | |||
| 2706 | 1428 ± 57b | 579 ± 23ab | 2008 ± 80b | 573 ± 23c | 43.8 ± 1.8b | 97 ± 12b | 11.01 ± 0.50b | 13.4 ± 1.1b | 78.8 ± 0.9b | 21.1 ± 0.9b | ||
| 3005 | 72 | 2405 ± 96a | 617 ± 25a | 3022 ± 121a | 1194 ± 48a | 149.7 ± 6.0a | 197 ± 10a | 18.22 ± 0.88a | 6.1 ± 1.0c | 81.1 ± 0.5a | 18.8 ± 0.5c | |
| 3617 | 1458 ± 58b | 724 ± 29a | 2182 ± 87b | 799 ± 32b | 33.7 ± 1.4c | 111 ± 12b | 0.47 ± 0.02c | 19.1 ± 1.6a | 75.3 ± 0.8c | 24.6 ± 0.8a | ||
| Variant | Light Intensity | Time, h | NPQ | DI0/RC | PIABS | Pn | Tr | Fv/Fm | Y(II) | Y(NO) | Y(NPQ) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2706 | 0.53 ± 0.06a | 0.51 ± 0.02a | 4.3 ± 0.3a | 6.1 ± 0.6b | 3.69 ± 0.16a | 0.83 ± 0.01a | 0.65 ± 0.02a | 0.23 ± 0.01a | 0.12 ± 0.02a | ||
| 3005 | Control | 0 | 0.62 ± 0.06a | 0.52 ± 0.03a | 4.7 ± 0.3a | 5.8 ± 0.7b | 3.36 ± 0.17a | 0.84 ± 0.01a | 0.64 ± 0.02a | 0.22 ± 0.01a | 0.14 ± 0.01a |
| 3617 | 0.53 ± 0.06a | 0.48 ± 0.02b | 5.0 ± 0.3a | 7.9 ± 0.4a | 2.81 ± 0.11b | 0.83 ± 0.01a | 0.60 ± 0.03a | 0.27 ± 0.02a | 0.14 ± 0.02a | ||
| 2706 | 1.05 ± 0.11a | 0.53 ± 0.02b | 3.8 ± 0.4a | 14.9 ± 0.8b | 1.51 ± 0.13a | 0.80 ± 0.03a | 0.49 ± 0.06a | 0.29 ± 0.02a | 0.25 ± 0.03a | ||
| 3005 | 24 | 0.81 ± 0.11a | 0.73 ± 0.07a | 2.8 ± 0.2ab | 21.8 ± 2.2a | 1.31 ± 0.15b | 0.79 ± 0.02a | 0.52 ± 0.03a | 0.26 ± 0.01a | 0.21 ± 0.03a | |
| 3617 | 1000 µmol (photons) m−2s−1 | 0.82 ± 0.12a | 0.72 ± 0.03a | 2.3 ± 0.3a | 16.1 ± 0.7b | 0.76 ± 0.08c | 0.80 ± 0.02a | 0.56 ± 0.04a | 0.26 ± 0.02a | 0.18 ± 0.03a | |
| 2706 | 0.67 ± 0.04b | 0.63 ± 0.06c | 1.9 ± 0.2a | 7.5 ± 1.0 c | 1.27 ± 0.16a | 0.82 ± 0.03a | 0.58 ± 0.03a | 0.27 ± 0.04a | 0.14 ± 0.01a | ||
| 3005 | 72 | 0.76 ± 0.07b | 0.72 ± 0.09b | 2.5 ± 0.4a | 13.8 ± 1.3a | 0.5 ± 0.08b | 0.79 ± 0.02a | 0.55 ± 0.03a | 0.26 ± 0.02a | 0.19 ± 0.02a | |
| 3617 | 0.98 ± 0.05a | 1.04 ± 0.09a | 1.9 ± 0.3a | 11.0 ± 0.2b | 0.33 ± 0.06c | 0.76 ± 0.02a | 0.49 ± 0.03a | 0.32 ± 0.01a | 0.19 ± 0.03a | ||
| 2706 | 1.19 ± 0.07a | 0.75 ± 0.06b | 1.7 ± 0.5a | 15.7 ± 1.1a | 0.95 ± 0.15a | 0.77 ± 0.04a | 0.47 ± 0.04a | 0.28 ± 0.01a | 0.22 ± 0.04a | ||
| 3005 | 24 | 0.97 ± 0.02b | 0.81 ± 0.12a | 2.0 ± 0.2a | 15.9 ± 1.0a | 0.38 ± 0.05b | 0.79 ± 0.03a | 0.49 ± 0.04a | 0.25 ± 0.02a | 0.26 ± 0.02a | |
| 3617 | 2000 µmol (photons) m−2s−1 | 0.88 ± 0.07b | 0.81 ± 0.05a | 1.2 ± 0.3a | 12.1 ± 0.3b | 0.94 ± 0.11a | 0.76 ± 0.03a | 0.49 ± 0.03a | 0.28 ± 0.02a | 0.23 ± 0.03a | |
| 2706 | 1.12 ± 0.07a | 1.07 ± 0.06a | 0.9 ± 0.3b | 4.7 ± 0.3c | 0.56 ± 0.07a | 0.65 ± 0.04b | 0.43 ± 0.02b | 0.33 ± 0.03ab | 0.24 ± 0.03a | ||
| 3005 | 72 | 0.87 ± 0.06b | 0.76 ± 0.10b | 2.2 ± 0.2a | 10.9 ± 1.6a | 0.19 ± 0.03c | 0.78 ± 0.02a | 0.53 ± 0.02a | 0.27 ± 0.03b | 0.20 ± 0.03a | |
| 3617 | 0.96 ± 0.06ab | 0.96 ± 0.10ab | 1.1 ± 0.2b | 5.8 ± 0.2b | 0.41 ± 0.06b | 0.69 ± 0.03b | 0.47 ± 0.02ab | 0.37 ± 0.02a | 0.17 ± 0.02a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashikhmin, A.; Bolshakov, M.; Pashkovskiy, P.; Vereshchagin, M.; Khudyakova, A.; Shirshikova, G.; Kozhevnikova, A.; Kosobryukhov, A.; Kreslavski, V.; Kuznetsov, V.; et al. The Adaptive Role of Carotenoids and Anthocyanins in Solanum lycopersicum Pigment Mutants under High Irradiance. Cells 2023, 12, 2569. https://doi.org/10.3390/cells12212569
Ashikhmin A, Bolshakov M, Pashkovskiy P, Vereshchagin M, Khudyakova A, Shirshikova G, Kozhevnikova A, Kosobryukhov A, Kreslavski V, Kuznetsov V, et al. The Adaptive Role of Carotenoids and Anthocyanins in Solanum lycopersicum Pigment Mutants under High Irradiance. Cells. 2023; 12(21):2569. https://doi.org/10.3390/cells12212569
Chicago/Turabian StyleAshikhmin, Aleksandr, Maksim Bolshakov, Pavel Pashkovskiy, Mikhail Vereshchagin, Alexandra Khudyakova, Galina Shirshikova, Anna Kozhevnikova, Anatoliy Kosobryukhov, Vladimir Kreslavski, Vladimir Kuznetsov, and et al. 2023. "The Adaptive Role of Carotenoids and Anthocyanins in Solanum lycopersicum Pigment Mutants under High Irradiance" Cells 12, no. 21: 2569. https://doi.org/10.3390/cells12212569






