Microglia Depletion Attenuates the Pro-Resolving Activity of the Formyl Peptide Receptor 2 Agonist AMS21 Related to Inhibition of Inflammasome NLRP3 Signalling Pathway: A Study of Organotypic Hippocampal Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Establishment of Organotypic Hippocampal Cultures (OHCs)
2.4. Treatment
2.5. Lactate Dehydrogenase (LDH) Assay
2.6. Nitric Oxide (NO) Assay
2.7. RNA Extraction and cDNA Preparation
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Western Blot Analyses
2.11. Immunofluorescence Staining of Organotypic Hippocampal Cultures
2.12. Quantitative Analyses of Confocal Fluorescence Images of Organotypic Hippocampal Cultures
2.13. Statistical Analysis
3. Results
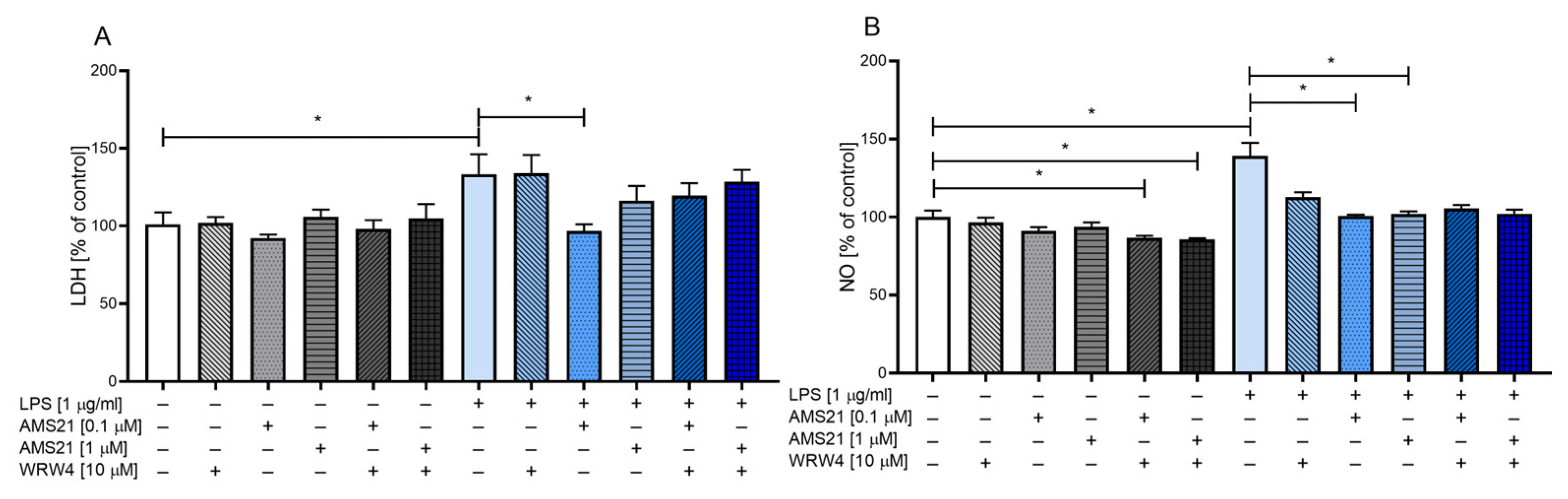
3.1. The Effect of WRW4 and AMS21 Treatment on Lactate Dehydrogenase and Nitric Oxide Release in OHCs
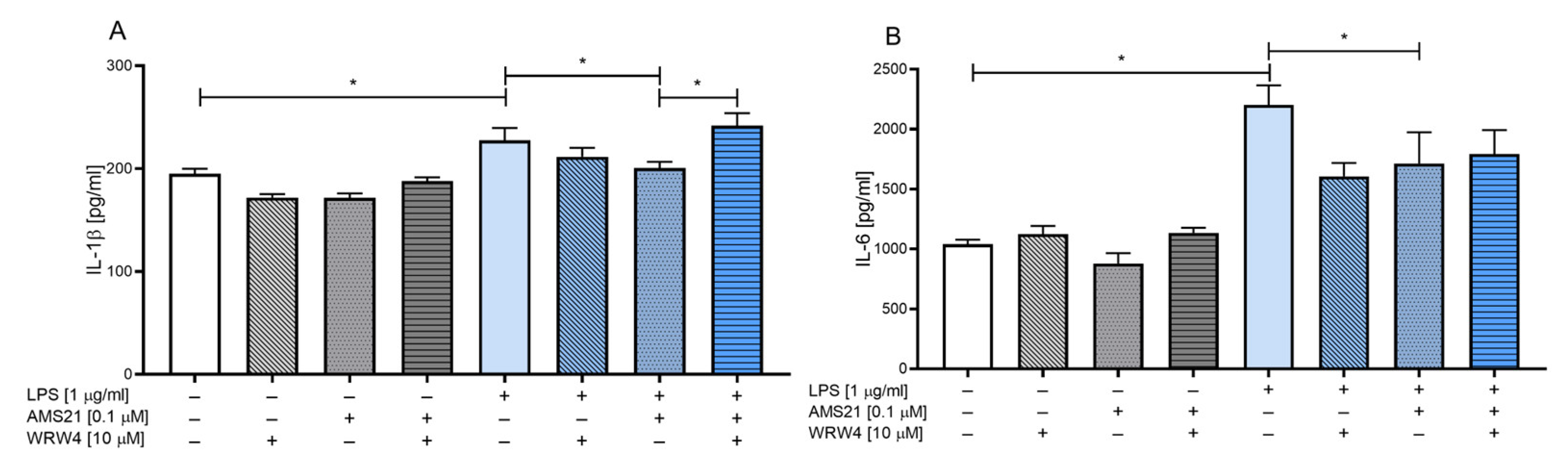
3.2. The Effect of WRW4 and AMS21 Treatment on the Release of the Proinflammatory Cytokines IL-1β and IL-6 in OHCs
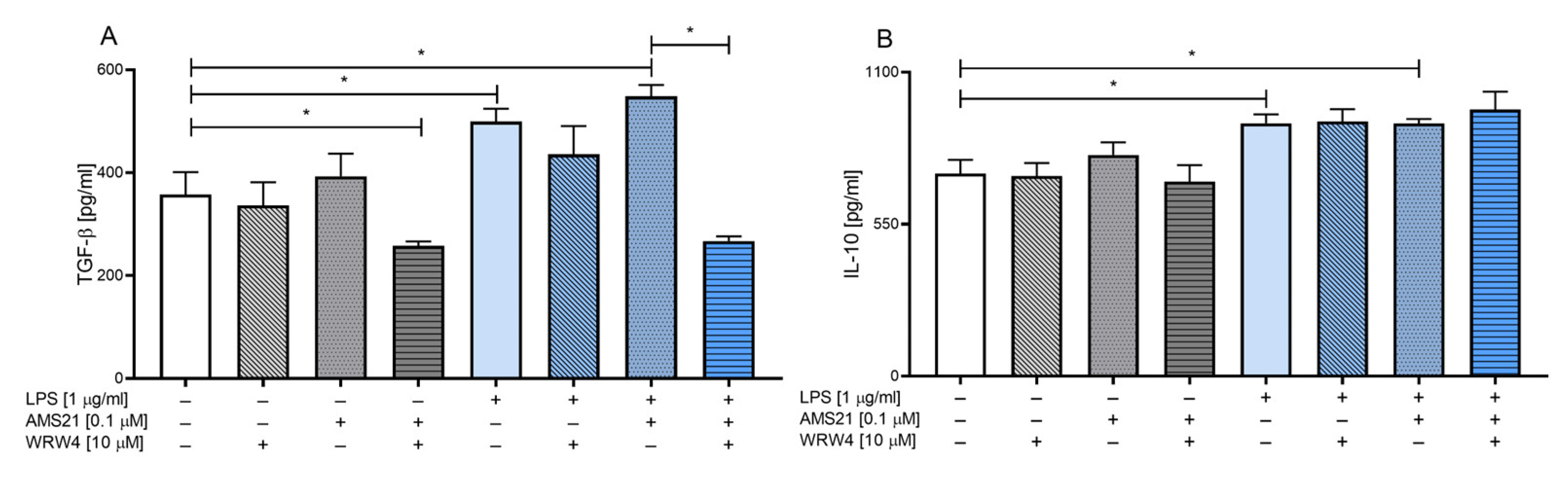
3.3. The Effect of WRW4 and AMS21 Treatment on the Release of Anti-Inflammatory Cytokines TGF-β and IL-10 in OHCs
3.4. The Effect of AMS21 on the mRNA Expression of Proinflammatory and Anti-Inflammatory Genes
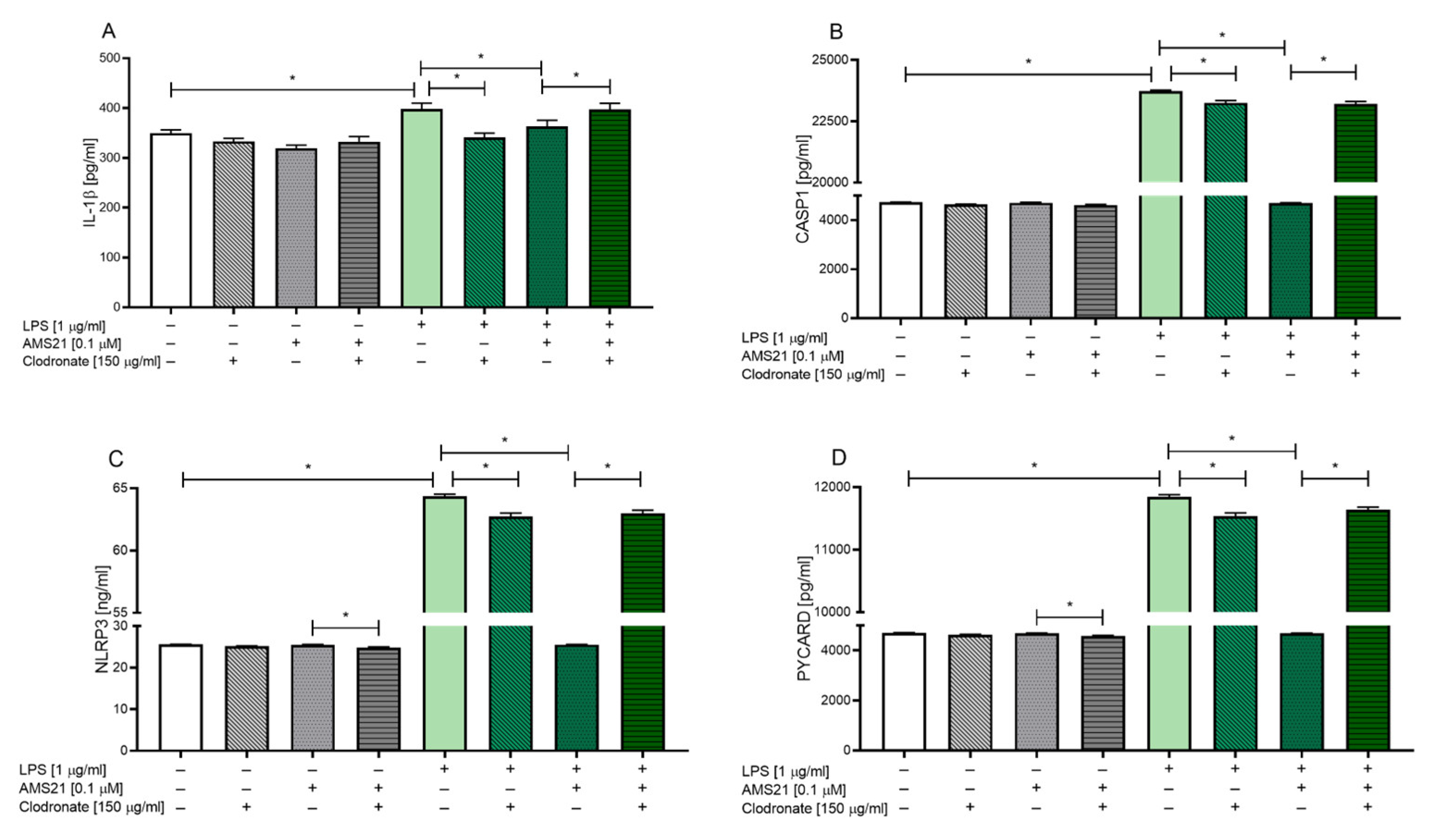
3.5. The Effect of WRW4 and AMS21 Treatment on the NLRP3-Related Pathway in OHCs
3.6. The Effect of Clodronate Treatment on Microglia in OHCs
3.7. The Effect of AMS21 on the Protein Level of NLRP3 Inflammasome Pathway-Related Factors in Microglia-Depleted OHCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and Non-Immune Functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Napoli, I.; Neumann, H. Microglial Clearance Function in Health and Disease. Neuroscience 2009, 158, 1030–1038. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Trojan, E.; Głombik, K.; Piotrowska, A.; Budziszewska, B.; Kubera, M.; Popiołek-Barczyk, K.; Lasoń, W.; Mika, J.; Basta-Kaim, A. Targeting the NLRP3 Inflammasome-Related Pathways via Tianeptine Treatment-Suppressed Microglia Polarization to the M1 Phenotype in Lipopolysaccharide-Stimulated Cultures. Int. J. Mol. Sci. 2018, 19, 1965. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Chamera, K.; Trojan, E.; Kotarska, K.; Szuster-Głuszczak, M.; Bryniarska, N.; Tylek, K.; Basta-Kaim, A. Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia. Int. J. Mol. Sci. 2021, 22, 1558. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.G.; Hoffos, M.; Vecchiarelli, H.A.; Tremblay, M.-È. Microglia: A Pharmacological Target for the Treatment of Age-Related Cognitive Decline and Alzheimer’s Disease. Front. Pharmacol. 2023, 14, 1125982. [Google Scholar] [CrossRef]
- Carvalho-Paulo, D.; Neto, J.B.T.; Filho, C.S.; de Oliveira, T.C.G.; de Sousa, A.A.; dos Reis, R.R.; dos Santos, Z.A.; de Lima, C.M.; de Oliveira, M.A.; Said, N.M.; et al. Microglial Morphology Across Distantly Related Species: Phylogenetic, Environmental and Age Influences on Microglia Reactivity and Surveillance States. Front. Immunol. 2021, 12, 683026. [Google Scholar] [CrossRef] [PubMed]
- Tylek, K.; Trojan, E.; Regulska, M.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Formyl Peptide Receptor 2, as an Important Target for Ligands Triggering the Inflammatory Response Regulation: A Link to Brain Pathology. Pharmacol. Rep. 2021, 74, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Šimončičová, E.; de Andrade, E.G.; Vecchiarelli, H.A.; Awogbindin, I.O.; Delage, C.I.; Tremblay, M.-È. Present and Future of Microglial Pharmacology. Trends Pharmacol. Sci. 2022, 43, 669–685. [Google Scholar] [CrossRef]
- Dokalis, N.; Prinz, M. Resolution of Neuroinflammation: Mechanisms and Potential Therapeutic Option. Semin. Immunopathol. 2019, 41, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, H.; Sakharkar, M.K.; Dhawan, U.; Chidambaram, S.B.; Chandra, R. Neuroinflammation Mechanisms and Phytotherapeutic Intervention: A Systematic Review. ACS Chem. Neurosci. 2020, 11, 3707–3731. [Google Scholar] [CrossRef]
- Moyse, E.; Krantic, S.; Djellouli, N.; Roger, S.; Angoulvant, D.; Debacq, C.; Leroy, V.; Fougere, B.; Aidoud, A. Neuroinflammation: A Possible Link between Chronic Vascular Disorders and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 827263. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Jana, A.; Liu, X.; Ghosh, S.; Pahan, K. Involvement of Phosphatidylinositol 3-Kinase-Mediated Up-Regulation of IκBα in Anti-Inflammatory Effect of Gemfibrozil in Microglia. J. Immunol. 2007, 179, 4142–4152. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Park, J.S.; Jung, J.S.; Kim, D.H.; Kim, H.S. Anti-Inflammatory Effect of Ginsenoside Rg5 in Lipopolysaccharide-Stimulated BV2 Microglial Cells. Int. J. Mol. Sci. 2013, 14, 9820–9833. [Google Scholar] [CrossRef]
- Trojan, E.; Chamera, K.; Bryniarska, N.; Kotarska, K.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. Role of Chronic Administration of Antidepressant Drugs in the Prenatal Stress-Evoked Inflammatory Response in the Brain of Adult Offspring Rats: Involvement of the NLRP3 Inflammasome-Related Pathway. Mol. Neurobiol. 2019, 56, 5365–5380. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, K.L.; Ulland, T.K. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer’s Disease. Front. Neurol. 2020, 11, 570711. [Google Scholar] [CrossRef]
- Kaufmann, F.N.; Costa, A.P.; Ghisleni, G.; Diaz, A.P.; Rodrigues, A.L.S.; Peluffo, H.; Kaster, M.P. NLRP3 Inflammasome-Driven Pathways in Depression: Clinical and Preclinical Findings. Brain. Behav. Immun. 2017, 64, 367–383. [Google Scholar] [CrossRef]
- Fan, Z.; Liang, Z.; Yang, H.; Pan, Y.; Zheng, Y.; Wang, X. Tenuigenin Protects Dopaminergic Neurons from Inflammation via Suppressing NLRP3 Inflammasome Activation in Microglia. J. Neuroinflamm. 2017, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Moretti, J.; Blander, J.M. Increasing Complexity of NLRP3 Inflammasome Regulation. J. Leukoc. Biol. 2021, 109, 561–571. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 Inflammasome Activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Weaver, D.F. Microglia and Microglial-Based Receptors in the Pathogenesis and Treatment of Alzheimer’s Disease. Int. Immunopharmacol. 2022, 110, 109070. [Google Scholar] [CrossRef]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as Dynamic Cellular Mediators of Brain Function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Liu, G.J.; Tao, T.; Wang, H.; Zhou, Y.; Gao, X.; Gao, Y.Y.; Hang, C.H.; Li, W. Functions of Resolvin D1-ALX/FPR2 Receptor Interaction in the Hemoglobin-Induced Microglial Inflammatory Response and Neuronal Injury. J. Neuroinflamm. 2020, 17, 239. [Google Scholar] [CrossRef]
- Tylek, K.; Trojan, E.; Leśkiewicz, M.; Regulska, M.; Bryniarska, N.; Curzytek, K.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Time-Dependent Protective and pro-Resolving Effects of Fpr2 Agonists on Lipopolysaccharide-Exposed Microglia Cells Involve Inhibition of Nf-Kb and Mapks Pathways. Cells 2021, 10, 2373. [Google Scholar] [CrossRef]
- Liu, M.; Chen, K.; Yoshimura, T.; Liu, Y.; Gong, W.; Le, Y.; Gao, J.L.; Zhao, J.; Wang, J.M.; Wang, A. Formylpeptide Receptors Mediate Rapid Neutrophil Mobilization to Accelerate Wound Healing. PLoS ONE 2014, 9, e90613. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, D.H.; Li, Q.Q.; Wang, X.Y.; Sun, Y.Y.; Li, L.J. Lipoxin A4 Regulates Lipopolysaccharide-Induced BV2 Microglial Activation and Differentiation via the Notch Signaling Pathway. Front. Cell. Neurosci. 2019, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Chua, J.E. Role of Formyl Peptide Receptor 2 (FPR2) in the Normal Brain and in Neurological Conditions. Neural Regen. Res. 2019, 14, 2071–2072. [Google Scholar] [CrossRef]
- Ho, C.F.Y.; Ismail, N.B.; Koh, J.K.Z.; Gunaseelan, S.; Low, Y.H.; Ng, Y.K.; Chua, J.J.E.; Ong, W.Y. Localisation of Formyl-Peptide Receptor 2 in the Rat Central Nervous System and Its Role in Axonal and Dendritic Outgrowth. Neurochem. Res. 2018, 43, 1587–1598. [Google Scholar] [CrossRef]
- Cattaneo, F.; Parisi, M.; Ammendola, R. Distinct Signaling Cascades Elicited by Different Formyl Peptide Receptor 2 (FPR2) Agonists. Int. J. Mol. Sci. 2013, 14, 7193–7230. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Norling, L.V.; Vecchio, E.A.; Brennan, E.P.; May, L.T.; Wootten, D.; Godson, C.; Perretti, M.; Ritchie, R.H. Formylpeptide Receptor 2: Nomenclature, Structure, Signalling and Translational Perspectives: IUPHAR Review 35. Br. J. Pharmacol. 2022, 179, 4617–4639. [Google Scholar] [CrossRef] [PubMed]
- Maciuszek, M.; Cacace, A.; Brennan, E.; Godson, C.; Chapman, T.M. Recent Advances in the Design and Development of Formyl Peptide Receptor 2 (FPR2/ALX) Agonists as pro-Resolving Agents with Diverse Therapeutic Potential. Eur. J. Med. Chem. 2021, 213, 113167. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Langmead, C.J.; Riddy, D.M. New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery. ACS Pharmacol. Transl. Sci. 2020, 3, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Raabe, C.A.; Gröper, J.; Rescher, U. Biased Perspectives on Formyl Peptide Receptors. Biochim. Biophys. Acta BBA Mol. Cell Res. 2019, 1866, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.D.; Boulay, F.; Ji, M.W.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the Formyl Peptide Receptor (FPR) Family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Ye, R.D. Structural and Conformational Studies of Biased Agonism through Formyl Peptide Receptors. Am. J. Physiol. Cell Physiol. 2022, 322, C939–C947. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Guerra, G.; Ammendola, R. Expression and Signaling of Formyl-Peptide Receptors in the Brain. Neurochem. Res. 2010, 35, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 Binds Human Phagocytes with Evidence for Proresolving Receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, M.; Favia, M.; Schepetkin, I.A.; Kirpotina, L.N.; Trojan, E.; Niso, M.; Carrieri, A.; Leśkiewicz, M.; Regulska, M.; Darida, M.; et al. Design, Synthesis, Biological Evaluation, and Computational Studies of Novel Ureidopropanamides as Formyl Peptide Receptor 2 (FPR2) Agonists to Target the Resolution of Inflammation in Central Nervous System Disorders. J. Med. Chem. 2022, 65, 5004–5028. [Google Scholar] [CrossRef] [PubMed]
- Stoppini, L.; Buchs, P.A.; Muller, D. A Simple Method for Organotypic Cultures of Nervous Tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Ślusarczyk, J.; Szczepanowicz, K.; Warszyński, P.; Leśkiewicz, M.; Regulska, M.; Trojan, E.; Lasoń, W. Protective Effects of Polydatin in Free and Nanocapsulated Form on Changes Caused by Lipopolysaccharide in Hippocampal Organotypic Cultures. Pharmacol. Rep. 2019, 71, 603–613. [Google Scholar] [CrossRef]
- Trojan, E.; Tylek, K.; Schröder, N.; Kahl, I.; Brandenburg, L.O.; Mastromarino, M.; Leopoldo, M.; Basta-Kaim, A.; Lacivita, E. The N-Formyl Peptide Receptor 2 (FPR2) Agonist MR-39 Improves Ex Vivo and In Vivo Amyloid Beta (1–42)-Induced Neuroinflammation in Mouse Models of Alzheimer’s Disease. Mol. Neurobiol. 2021, 2, 6203–6221. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Trojan, E.; Głombik, K.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Popiołek-Barczyk, K.; Mika, J.; Wędzony, K.; Basta-Kaim, A. Prenatal Stress Is a Vulnerability Factor for Altered Morphology and Biological Activity of Microglia Cells. Front. Cell. Neurosci. 2015, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Trojan, E.; Tylek, K.; Leśkiewicz, M.; Lasoń, W.; Brandenburg, L.O.; Leopoldo, M.; Lacivita, E.; Basta-Kaim, A. The N-Formyl Peptide Receptor 2 (Fpr2) Agonist Mr-39 Exhibits Anti-Inflammatory Activity in Lps-Stimulated Organotypic Hippocampal Cultures. Cells 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P. A Reagent for the Single-Step Simultaneous Isolation of RNA, DNA and Proteins from Cell and Tissue Samples. Biotechniques 1993, 15, 532–537. [Google Scholar] [PubMed]
- Chamera, K.; Szuster-Głuszczak, M.; Trojan, E.; Basta-Kaim, A. Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200-CD200R and CX3CL1-CX3CR1 Systems. Cells 2020, 9, 1676. [Google Scholar] [CrossRef] [PubMed]
- Gogolla, N.; Galimberti, I.; DePaola, V.; Caroni, P. Staining Protocol for Organotypic Hippocampal Slice Cultures. Nat. Protoc. 2006, 1, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 Inflammasome Activation and Cell Death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Noraberg, J.; Poulsen, F.R.; Blaabjerg, M.; Kristensen, B.W.; Bonde, C.; Montero, M.; Meyer, M.; Gramsbergen, J.B.; Zimmer, J. Organotypic Hippocampal Slice Cultures for Studies of Brain Damage, Neuroprotection and Neurorepair. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Leroy, X.; Bland, E.J.; Montero-Melendez, T. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef]
- Trojan, E.; Leśkiewicz, M.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. The Formyl Peptide Receptor 2 as a Target For Promotion of Resolution of Inflammation. Curr. Neuropharmacol. 2022, 20, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Maderna, P.; Godson, C. Lipoxins: Resolutionary Road. Br. J. Pharmacol. 2009, 158, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.; Yao, H.; Xu, J. Insights into Drug Discovery from Natural Products through Structural Modification. Fitoterapia 2015, 103, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Stama, M.L.; Lacivita, E.; Kirpotina, L.N.; Niso, M.; Perrone, R.; Schepetkin, I.A.; Quinn, M.T.; Leopoldo, M. Functional N-Formyl Peptide Receptor 2 (FPR2) Antagonists Based on the Ureidopropanamide Scaffold Have Potential To Protect against Inflammation-Associated Oxidative Stress. ChemMedChem 2017, 12, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-Induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-Clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef]
- Facchin, B.M.; dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory Biomarkers on an LPS-Induced RAW 264.7 Cell Model: A Systematic Review and Meta-Analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 Inhibits Interleukin-1β Production and Inflammasome Activation of Microglia in Epileptic Seizures. J. Neuroinflamm. 2019, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, L.; Xiao, J.; Zhang, S.; Liu, G.; Sun, X. IL-1ra Treatment Prevents Chronic Social Defeat Stress-Induced Depression-like Behaviors and Glutamatergic Dysfunction via the Upregulation of CREB-BDNF. J. Affect. Disord. 2023, 335, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Ataeinia, B.; Keynejad, K.; Abdolalizadeh, A.; Hirbod-Mobarakeh, A.; Rezaei, N. A Meta-Analysis of pro-Inflammatory Cytokines in Autism Spectrum Disorders: Effects of Age, Gender, and Latitude. J. Psychiatr. Res. 2019, 115, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Ding, J.; Huang, J.; Shao, A.; Tang, B. The Role of Formyl Peptide Receptors in Neurological Diseases via Regulating Inflammation. Front. Cell. Neurosci. 2021, 15, 753832. [Google Scholar] [CrossRef] [PubMed]
- Wickstead, E.S.; Irving, M.A.; Getting, S.J.; McArthur, S. Exploiting Formyl Peptide Receptor 2 to Promote Microglial Resolution: A New Approach to Alzheimer’s Disease Treatment. FEBS J. 2022, 289, 1801–1822. [Google Scholar] [CrossRef]
- Brandenburg, L.O.; Konrad, M.; Wruck, C.J.; Koch, T.; Lucius, R.; Pufe, T. Functional and Physical Interactions between Formyl-Peptide-Receptors and Scavenger Receptor MARCO and Their Involvement in Amyloid Beta 1-42-Induced Signal Transduction in Glial Cells. J. Neurochem. 2010, 113, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of Microglia and Inhibition of Exosome Synthesis Halt Tau Propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Seong, J.; Kim, S.H.; Lee, S.J.; Cho, Y.J.; An, J.; Nam, D.H.; Joo, K.M.; Cha, C.I. Replacement of Microglial Cells Using Clodronate Liposome and Bone Marrow Transplantation in the Central Nervous System of SOD1G93A Transgenic Mice as an in Vivo Model of Amyotrophic Lateral Sclerosis. Biochem. Biophys. Res. Commun. 2012, 418, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, N.; Hirvonen, M.R.; Teräväinen, T.; Savolainen, K.; Mönkkönen, J. Different Effects of Three Bisphosphonates on Nitric Oxide Production by RAW 264 Macrophage-like Cells In Vitro. J. Pharmacol. Exp. Ther. 1996, 277, 1097–1102. [Google Scholar] [PubMed]
- Dehghani, F.; Conrad, A.; Kohl, A.; Korf, H.; Hailer, N. Clodronate Inhibits the Secretion of Proinflammatory Cytokines and NO by Isolated Microglial Cells and Reduces the Number of Proliferating Glial Cells in Excitotoxically Injured Organotypic Hippocampal Slice Cultures. Exp. Neurol. 2004, 189, 241–251. [Google Scholar] [CrossRef]
- Tylek, K.; Trojan, E.; Leśkiewicz, M.; Francavilla, F.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Stimulation of Formyl Peptide Receptor-2 by the New Agonist CMC23 Protects against Endotoxin-Induced Neuroinflammatory Response: A Study in Organotypic Hippocampal Cultures. ACS Chem. Neurosci. 2023, 14, 3869–3882. [Google Scholar] [CrossRef]
- Makkonen, N.; Salminen, A.; Rogers, M.J.; Frith, J.C.; Urtti, A.; Azhayeva, E.; Mönkkönen, J. Contrasting Effects of Alendronate and Clodronate on RAW 264 Macrophages: The Role of a Bisphosphonate Metabolite. Eur. J. Pharm. Sci. 1999, 8, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.C.; Mönkkönen, J.; Blackburn, G.M.; Russell, R.G.G.; Rogers, M.J. Clodronate and Liposome-Encapsulated Clodronate Are Metabolized to a Toxic ATP Analog, Adenosine 5′-(β,γ-Dichloromethylene) Triphosphate, by Mammalian Cells In Vitro. J. Bone Miner. Res. 1997, 12, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, Q.; Lan, X.; EL-Mufti, L.; Ren, H.; Wang, J. Microglial Depletion with Clodronate Liposomes Increases Proinflammatory Cytokine Levels, Induces Astrocyte Activation, and Damages Blood Vessel Integrity. Mol. Neurobiol. 2019, 56, 6184–6196. [Google Scholar] [CrossRef] [PubMed]
- Kumamaru, H.; Saiwai, H.; Kobayakawa, K.; Kubota, K.; van Rooijen, N.; Inoue, K.; Iwamoto, Y.; Okada, S. Liposomal Clodronate Selectively Eliminates Microglia from Primary Astrocyte Cultures. J. Neuroinflamm. 2012, 9, 116. [Google Scholar] [CrossRef]
- Ji, K.; Akgul, G.; Wollmuth, L.P.; Tsirka, S.E. Microglia Actively Regulate the Number of Functional Synapses. PLoS ONE 2013, 8, e56293. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic Reactive Astrocytes Induce Cell Death via Saturated Lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Guttikonda, S.R.; Sikkema, L.; Tchieu, J.; Saurat, N.; Walsh, R.M.; Harschnitz, O.; Ciceri, G.; Sneeboer, M.; Mazutis, L.; Setty, M.; et al. Fully Defined Human Pluripotent Stem Cell-Derived Microglia and Tri-Culture System Model C3 Production in Alzheimer’s Disease. Nat. Neurosci. 2021, 24, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Tarassishin, L.; Suh, H.-S.; Lee, S.C. LPS and IL-1 Differentially Activate Mouse and Human Astrocytes: Role of CD14. Glia 2014, 62, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- John, G.R.; Lee, S.C.; Brosnan, C.F. Cytokines: Powerful Regulators of Glial Cell Activation. Neuroscientist 2003, 9, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Jin, S.; Li, E.; Doi, Y.; Parajuli, B.; Noda, M.; Sonobe, Y.; Mizuno, T.; Suzumura, A. The Neurotoxic Effect of Astrocytes Activated with Toll-like Receptor Ligands. J. Neuroimmunol. 2013, 254, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gorina, R.; Font-Nieves, M.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 Activation Induces a Proinflammatory Environment through the Interplay between MyD88-Dependent NFκB Signaling, MAPK, and Jak1/Stat1 Pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef] [PubMed]
- Abulafia, D.P.; De Rivero Vaccari, J.P.; Lozano, J.D.; Lotocki, G.; Keane, R.W.; Dietrich, W.D. Inhibition of the Inflammasome Complex Reduces the Inflammatory Response after Thromboembolic Stroke in Mice. J. Cereb. Blood Flow Metab. 2009, 29, 534–544. [Google Scholar] [CrossRef]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 Inflammasome Is Involved in the Innate Immune Response to Amyloid-β. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Feng, Y.; Xiong, G.; Whyte, S.; Duan, J.; Yang, Y.; Wang, K.; Yang, S.; Geng, Y.; Ou, Y.; et al. Caspase-11, a Specific Sensor for Intracellular Lipopolysaccharide Recognition, Mediates the Non-Canonical Inflammatory Pathway of Pyroptosis. Cell Biosci. 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Viganò, E.; Diamond, C.E.; Spreafico, R.; Balachander, A.; Sobota, R.M.; Mortellaro, A. Human Caspase-4 and Caspase-5 Regulate the One-Step Non-Canonical Inflammasome Activation in Monocytes. Nat. Commun. 2015, 6, 8761. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, M.; Lin, K.; Xiang, X.; Zheng, Y.; Zhu, S. Inhibiting Caspase-12 Mediated Inflammasome Activation Protects against Oxygen-Glucose Deprivation Injury in Primary Astrocytes. Int. J. Med. Sci. 2020, 17, 1936–1945. [Google Scholar] [CrossRef] [PubMed]


| Control | LPS | AMS21 | AMS21 + LPS | |
|---|---|---|---|---|
| A | ||||
| Proinflammatory factors | ||||
| Cd40 | 1.04 ± 0.18 | 2.83 ± 1.51 * | 1.02 ± 0.25 | 0.96 ± 0.14 # |
| Cd68 | 1.06 ± 0.18 | 1.07 ± 0.39 | 0.92 ± 0.20 | 0.94 ± 0.16 |
| Il-1β | 1.06 ± 0.15 | 27.05 ± 3.70 * | 1.02 ± 0.18 | 27.58 ± 1.90 * |
| Il-6 | 1.07 ± 0.27 | 3.57 ± 0.33 * | 3.23 ± 1.19 | 3.42 ± 0.24 |
| Il-18 | 1.05 ± 0.18 | 2.52 ± 0.86 * | 1.04 ± 0.29 | 0.97 ± 0.18 # |
| B | ||||
| Anti-inflammatory factors | ||||
| Igf-1 | 1.04 ± 0.15 | 0.33 ± 0.02 * | 1.06 ± 0.18 | 0.47 ± 0.10 |
| Il-1Ra | 1.06 ± 0.28 | 7.18 ± 1.63 * | 1.59 ± 0.25 | 11.74 ± 1.98 # |
| Tgf-β | 1.03 ± 0.16 | 0.45 ± 0.14 | 1.15 ± 0.21 | 0.84 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tylek, K.; Trojan, E.; Leśkiewicz, M.; Ghafir El Idrissi, I.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Microglia Depletion Attenuates the Pro-Resolving Activity of the Formyl Peptide Receptor 2 Agonist AMS21 Related to Inhibition of Inflammasome NLRP3 Signalling Pathway: A Study of Organotypic Hippocampal Cultures. Cells 2023, 12, 2570. https://doi.org/10.3390/cells12212570
Tylek K, Trojan E, Leśkiewicz M, Ghafir El Idrissi I, Lacivita E, Leopoldo M, Basta-Kaim A. Microglia Depletion Attenuates the Pro-Resolving Activity of the Formyl Peptide Receptor 2 Agonist AMS21 Related to Inhibition of Inflammasome NLRP3 Signalling Pathway: A Study of Organotypic Hippocampal Cultures. Cells. 2023; 12(21):2570. https://doi.org/10.3390/cells12212570
Chicago/Turabian StyleTylek, Kinga, Ewa Trojan, Monika Leśkiewicz, Imane Ghafir El Idrissi, Enza Lacivita, Marcello Leopoldo, and Agnieszka Basta-Kaim. 2023. "Microglia Depletion Attenuates the Pro-Resolving Activity of the Formyl Peptide Receptor 2 Agonist AMS21 Related to Inhibition of Inflammasome NLRP3 Signalling Pathway: A Study of Organotypic Hippocampal Cultures" Cells 12, no. 21: 2570. https://doi.org/10.3390/cells12212570
APA StyleTylek, K., Trojan, E., Leśkiewicz, M., Ghafir El Idrissi, I., Lacivita, E., Leopoldo, M., & Basta-Kaim, A. (2023). Microglia Depletion Attenuates the Pro-Resolving Activity of the Formyl Peptide Receptor 2 Agonist AMS21 Related to Inhibition of Inflammasome NLRP3 Signalling Pathway: A Study of Organotypic Hippocampal Cultures. Cells, 12(21), 2570. https://doi.org/10.3390/cells12212570







