Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale
Abstract
:1. Introduction
2. Capsaicinoids: In Situ Synthesis and Bioactivity

3. Molecular Mechanisms behind the Selective Cytotoxicity of Capsaicin in Cancer Cells
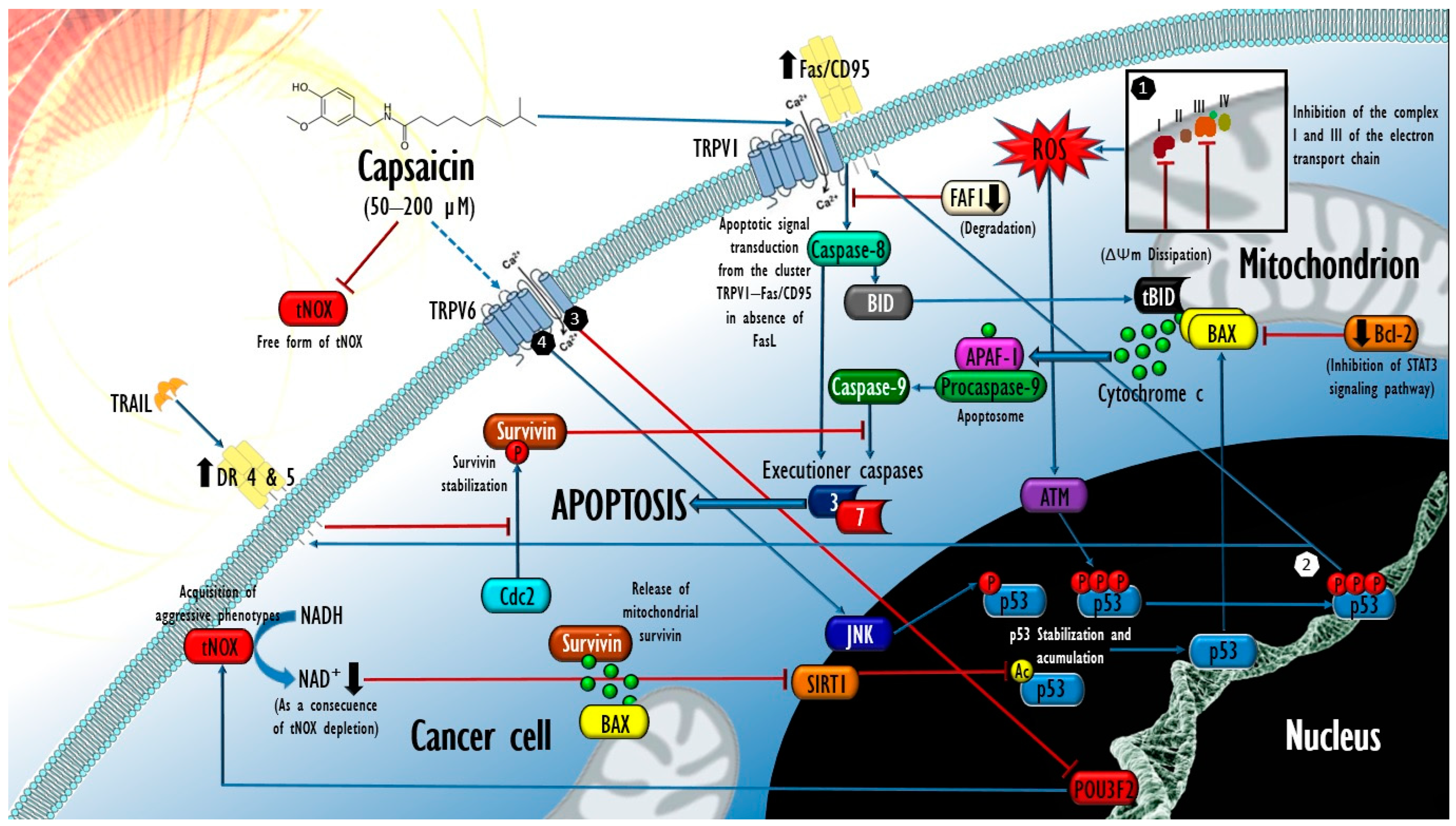
4. Mechanisms Underlying Capsaicin-Induced Apoptosis in Cancer: Brief State of the Art
4.1. Contribution of Oxidative Stress in the Proapoptotic Activity of Capsaicin in Cancer
4.2. Extrinsic Apoptosis Events Involved in the Cytotoxic Activity of Capsaicin in Cancer
4.3. In Vivo Antitumor Activity of Capsaicin and Expectations for Its Clinical Evaluation
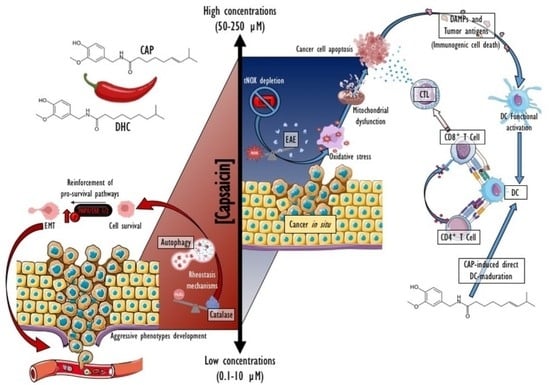
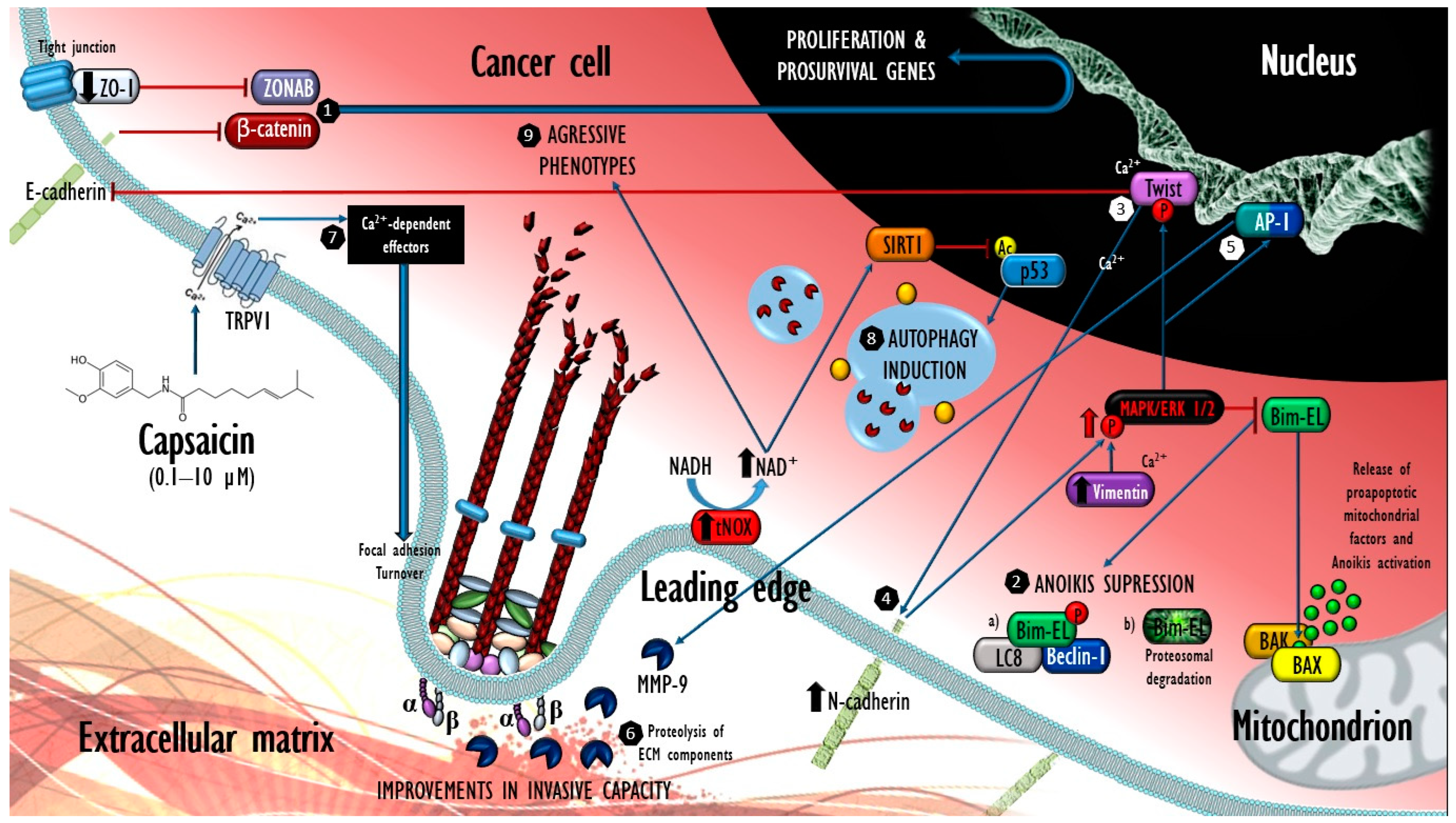
5. An In-Depth Molecular Perspective on the “Double-Edged Sword” Postulate
5.1. Modulation of Intracellular Calcium and Its Possible Involvement in the Promoting Effects of Capsaicin at Low Concentrations
5.2. Promoter Effects of Capsicum Fruits Extracts in Animal Models of Chemically Induced and Spontaneous Tumorigenesis
5.3. Cancer-Promoting Effects at Target Concentrations of Capsaicin: A Potential Risk?
6. Capsaicinoids as Autophagy Inducers in Cancer and Non-Cancer Cells
7. Immunomodulating Properties of Capsaicin in Cancer
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.; Yeom, S.I.; Kim, Y.M.; Lee, J.M.; Lee, H.A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.T.; et al. Genome Sequence of the Hot Pepper Provides Insights into the Evolution of Pungency in Capsicum Species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A.; Araujo, O.E. Capsaicin: Identification, Nomenclature, and Pharmacotherapy. Ann. Pharmacother. 1993, 27, 330–336. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Yahia, E.M. Changes in Capsaicinoids during Development, Maturation, and Senescence of Chile Peppers and Relation with Peroxidase Activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Chung, M.K.; Güler, A.D.; Caterina, M.J. TRPV1 Shows Dynamic Ionic Selectivity during Agonist Stimulation. Nat. Neurosci. 2008, 11, 555–564. [Google Scholar] [CrossRef]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch Fossils and the Domestication and Dispersal of Chili Peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef]
- Halikowski Smith, S. In the Shadow of a Pepper-Centric Historiography: Understanding the Global Diffusion of Capsicums in the Sixteenth and Seventeenth Centuries. J. Ethnopharmacol. 2015, 167, 64–77. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Thorpe, P.A. The Antimicrobial Properties of Chile Peppers (Capsicum species) and Their Uses in Mayan Medicine. J. Ethnopharmacol. 1996, 52, 61–70. [Google Scholar] [CrossRef]
- Meghvansi, M.K.; Siddiqui, S.; Khan, M.H.; Gupta, V.K.; Vairale, M.G.; Gogoi, H.K.; Singh, L. Naga Chilli: A Potential Source of Capsaicinoids with Broad-Spectrum Ethnopharmacological Applications. J. Ethnopharmacol. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Materska, M.; Konopacka, M.; Rogoliński, J.; Ślosarek, K. Antioxidant Activity and Protective Effects against Oxidative Damage of Human Cells Induced by X-Radiation of Phenolic Glycosides Isolated from Pepper Fruits Capsicum annuum L. Food Chem. 2015, 168, 546–553. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary Metabolites of Capsicum Species and Their Importance in the Human Diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite Biodiversity in Pepper (Capsicum) Fruits of Thirty-Two Diverse Accessions: Variation in Health-Related Compounds and Implications for Breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Tundis, R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; Menichini, F.; Loizzo, M.R. Antioxidant and Hypoglycaemic Activities and Their Relationship to Phytochemicals in Capsicum annuum Cultivars during Fruit Development. LWT 2013, 53, 370–377. [Google Scholar] [CrossRef]
- Schwarzlin, R.; Pušenjak, N.; Makuc, D.; Križman, M.; Vovk, I.; Plavec, J.; Švajger, U. Synergistic Complex from Plants Solanaceae Exhibits Cytotoxicity for the Human Hepatocellular Carcinoma Cell Line HepG2. BMC Complement. Altern. Med. 2016, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.R.; Leonardi, B.; Miron, D.; Schapoval, E.; De Oliveira, J.R.; Gosmann, G. Antioxidant and Anti-Inflammatory Properties of Capsicum baccatum: From Traditional Use to Scientific Approach. J. Ethnopharmacol. 2012, 139, 228–233. [Google Scholar] [CrossRef]
- Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Phytochemicals in Capsicum Oleoresin from Different Varieties of Hot Chilli Peppers with Their Antidiabetic and Antioxidant Activities Due to Some Phenolic Compounds. Ultrason. Sonochem. 2017, 38, 629–639. [Google Scholar] [CrossRef]
- Fernández-Bedmar, Z.; Alonso-Moraga, A. In Vivo and in Vitro Evaluation for Nutraceutical Purposes of Capsaicin, Capsanthin, Lutein and Four Pepper Varieties. Food Chem. Toxicol. 2016, 98, 89–99. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Domínguez, F.; Zapata-Morales, J.R.; Carranza-Álvarez, C. Plants Used in the Traditional Medicine of Mesoamerica (Mexico and Central America) and the Caribbean for the Treatment of Obesity. J. Ethnopharmacol. 2015, 175, 335–345. [Google Scholar] [CrossRef]
- Majee, S.K.; Ray, S.; Ghosh, K.; Micard, V.; Ray, B. Isolation and Structural Features of an Antiradical Polysaccharide of Capsicum annuum That Interacts with BSA. Int. J. Biol. Macromol. 2015, 75, 144–151. [Google Scholar] [CrossRef]
- Sung, J.; Jeong, H.S.; Lee, J. Effect of the Capsicoside G-Rich Fraction from Pepper (Capsicum annuum L.) Seeds on High-Fat Diet-Induced Obesity in Mice. Phytother. Res. 2016, 30, 1848–1855. [Google Scholar] [CrossRef]
- Allemand, A.; Leonardi, B.F.; Zimmer, A.R.; Moreno, S.; Romão, P.R.T.; Gosmann, G. Red Pepper (Capsicum baccatum) Extracts Present Anti-Inflammatory Effects In Vivo and Inhibit the Production of TNF-α and NO In Vitro. J. Med. Food 2016, 19, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Brito-Argáez, L.; Tamayo-Sansores, J.A.; Madera-Piña, D.; García-Villalobos, F.J.; Moo-Puc, R.E.; Kú-González, Á.; Villanueva, M.A.; Islas-Flores, I. Biochemical Characterization and Immunolocalization Studies of a Capsicum Chinense Jacq. Protein Fraction Containing DING Proteins and Anti-Microbial Activity. Plant Physiol. Biochem. 2016, 109, 502–514. [Google Scholar] [CrossRef]
- Lv, J.; Qi, L.; Yu, C.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Sun, D.; Du, J.; Ge, P.; et al. Consumption of Spicy Foods and Total and Cause Specific Mortality: Population Based Cohort Study. BMJ 2015, 351, h3942. [Google Scholar] [CrossRef] [PubMed]
- Chopan, M.; Littenberg, B. The Association of Hot Red Chili Pepper Consumption and Mortality: A Large Population-Based Cohort Study. PLoS ONE 2017, 12, e0169876. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; He, T.; Yu, K.; Zhao, A.; Zheng, W.; Zhang, Y.; Zhu, B. Association between Spicy Food Consumption and Lipid Profiles in Adults: A Nationwide Population-Based Study. Br. J. Nutr. 2017, 118, 144–153. [Google Scholar] [CrossRef]
- Asnin, L.; Park, S.W. Isolation and Analysis of Bioactive Compounds in Capsicum Peppers. Crit. Rev. Food Sci. Nutr. 2015, 55, 254–289. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and Clinical Uses of Capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef]
- Abdel Salam, O. Capsaicin as a Therapeutic Molecule; Springer: Basel, Switzerland, 2014; Volume 68, p. 321. [Google Scholar] [CrossRef]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Akhtar, F.; Sharif, H.; Mallick, M.; Zahoor, F.; Abdulmalik, A.; Baig, W.; Shujaat, N.; Gul, S.; Bibi, G.; Ramzan, R.; et al. Capsaicin: Its biological activities and in silico target fishing. Acta Pol. Pharm. 2017, 74, 321–329. [Google Scholar] [PubMed]
- Adaszek, Ł.; Gadomska, D.; Mazurek, Ł.; Łyp, P.; Madany, J.; Winiarczyk, S. Properties of Capsaicin and Its Utility in Veterinary and Human Medicine. Res. Vet. Sci. 2019, 123, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Sanati, S.; Razavi, B.M.; Hosseinzadeh, H. A Review of the Effects of Capsicum annuum L. And Its Constituent, Capsaicin, in Metabolic Syndrome. Iran J. Basic Med. Sci. 2018, 21, 439–448. [Google Scholar] [CrossRef]
- Galati, G.; O’brien, P.J. Cytoprotective and Anticancer Properties of Coenzyme Q versus Capsaicin; IOS Press: Amsterdam, The Netherlands, 2003; Volume 18. [Google Scholar]
- Impheng, H.; Pongcharoen, S.; Richert, L.; Pekthong, D.; Srisawang, P. The Selective Target of Capsaicin on FASN Expression and de Novo Fatty Acid Synthesis Mediated through ROS Generation Triggers Apoptosis in HepG2 Cells. PLoS ONE 2014, 9, e107842. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, E.H.; Kim, S.U.; Kwon, T.K.; Choi, K.S. Capsaicin Sensitizes Malignant Glioma Cells to TRAIL-Mediated Apoptosis via DR5 Upregulation and Survivin Downregulation. Carcinogenesis 2010, 31, 367–375. [Google Scholar] [CrossRef]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G.; et al. Capsaicin Induces Apoptosis in Human Small Cell Lung Cancer via the TRPV6 Receptor and the Calpain Pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.C.; Lu, W.H.; Huang, P.; Hu, Y. Selective Killing of K-Ras–Transformed Pancreatic Cancer Cells by Targeting NAD(P)H Oxidase. Chin. J. Cancer 2015, 34, 1–11. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chen, H.Y.; Su, L.J.; Chueh, P.J. Sirtuin 1 (SIRT1) Deacetylase Activity and NAD+/NADH Ratio Are Imperative for Capsaicin-Mediated Programmed Cell Death. J. Agric. Food Chem. 2015, 63, 7361–7370. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Basu, S. Fas-Associated Factor 1 Is a Negative Regulator in Capsaicin Induced Cancer Cell Apoptosis. Cancer Lett. 2010, 287, 142–149. [Google Scholar] [CrossRef]
- Beltran, J.; Ghosh, A.K.; Basu, S. Immunotherapy of Tumors with Neuroimmune Ligand Capsaicin. J. Immunol. 2007, 178, 3260–3264. [Google Scholar] [CrossRef]
- Macho, A.; Calzado, M.A.; Muñoz-Blanco, J.; Gómez-Díaz, C.; Gajate, C.; Mollinedo, F.; Navas, P.; Muñoz, E. Selective Induction of Apoptosis by Capsaicin in Transformed Cells: The Role of Reactive Oxygen Species and Calcium. Cell Death Differ. 1999, 6, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A Comprehensive Review of the Carcinogenic and Anticarcinogenic Potential of Capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Lee, S.S. Capsaicin in Hot Chili Pepper: Carcinogen, Co-Carcinogen or Anticarcinogen? Food Chem. Toxicol. 1996, 34, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The Two Faces of Capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.J.; Kim, G.E.; Cho, M.H.; Yoon, S.Y.; Davies, A.J.; Oh, S.B.; Lee, H.; Cho, Y.K.; Joo, C.H.; et al. Attenuation of Natural Killer Cell Functions by Capsaicin through a Direct and TRPV1-Independent Mechanism. Carcinogenesis 2014, 35, 1652–1660. [Google Scholar] [CrossRef]
- Djian-Caporalino, C.; Fazari, A.; Arguel, M.J.; Vernie, T.; VandeCasteele, C.; Faure, I.; Brunoud, G.; Pijarowski, L.; Palloix, A.; Lefebvre, V.; et al. Root-Knot Nematode (Meloidogyne Spp.) Me Resistance Genes in Pepper (Capsicum annuum L.) Are Clustered on the P9 Chromosome. Theor. Appl. Genet. 2007, 114, 473–486. [Google Scholar] [CrossRef]
- Keyhaninejad, N.; Curry, J.; Romero, J.; O’Connell, M.A. Fruit Specific Variability in Capsaicinoid Accumulation and Transcription of Structural and Regulatory Genes in Capsicum Fruit. Plant Sci. 2014, 215–216, 59–68. [Google Scholar] [CrossRef]
- Sánchez-Segura, L.; Téllez-Medina, D.I.; Evangelista-Lozano, S.; García-Armenta, E.; Alamilla-Beltrán, L.; Hernández-Sánchez, H.; Jiménez-Aparicio, A.R.; Gutiérrez-López, G.F. Morpho-Structural Description of Epidermal Tissues Related to Pungency of Capsicum Species. J. Food Eng. 2015, 152, 95–104. [Google Scholar] [CrossRef]
- Thiele, R.; Mueller-Seitz, E.; Petz, M. Chili Pepper Fruits: Presumed Precursors of Fatty Acids Characteristic for Capsaicinoids. J. Agric. Food Chem. 2008, 56, 4219–4224. [Google Scholar] [CrossRef]
- Mazourek, M.; Pujar, A.; Borovsky, Y.; Paran, I.; Mueller, L.; Jahn, M.M. A Dynamic Interface for Capsaicinoid Systems Biology. Plant Physiol. 2009, 150, 1806–1821. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N.; Ramirez-Malagon, R. In Vitro Chili Pepper Biotechnology. Vitr. Cell. Dev. Biol.—Plant 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Reddy, U.K.; Almeida, A.; Abburi, V.L.; Alaparthi, S.B.; Unselt, D.; Hankins, G.; Park, M.; Choi, D.; Nimmakayala, P. Identification of Gene-Specific Polymorphisms and Association with Capsaicin Pathway Metabolites in Capsicum annuum L. Collections. PLoS ONE 2014, 9, e86393. [Google Scholar] [CrossRef] [PubMed]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. Silencing AT3 Gene Reduces the Expression of PAmt, BCAT, Kas, and Acl Genes Involved in Capsaicinoid Biosynthesis in Chili Pepper Fruits. Biol. Plant. 2015, 59, 477–484. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, D.; Zhang, L.; Zhao, C.; Shu, H.; Cheng, S.; Wang, Z.; Zhu, J.; Liu, P. Identification and Expression Analysis of Capsaicin Biosynthesis Pathway Genes at Genome Level in Capsicum Chinense. Biotechnol. Biotechnol. Equip. 2022, 36, 232–244. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhao, S.N.; Liu, G.F.; Huang, Z.M.; Cao, Z.M.; Cheng, S.H.; Lin, S. Sen Discovery of Putative Capsaicin Biosynthetic Genes by RNA-Seq and Digital Gene Expression Analysis of Pepper. Sci. Rep. 2016, 6, 34121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, J.; Xie, J.; Gan, Y.; Coulter, J.A.; Yu, J.; Li, J.; Wang, J.; Zhang, X. Nitrogen Source Affects the Composition of Metabolites in Pepper (Capsicum annuum L.) and Regulates the Synthesis of Capsaicinoids through the GOGAT-GS Pathway. Foods 2020, 9, 150. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Velez-Haro, J.M.; Buitimea-Cantúa, N.E.; Molina-Torres, J.; Rosas-Burgos, E.C. GC-EIMS Analysis, Antifungal and Anti-Aflatoxigenic Activity of Capsicum Chinense and Piper Nigrum Fruits and Their Bioactive Compounds Capsaicin and Piperine upon Aspergillus Parasiticus. Nat. Prod. Res. 2018, 34, 1452–1455. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Nabhan, G.P. Directed Deterrence by Capsaicin in Chillies. Nature 2001, 412, 403–404. [Google Scholar] [CrossRef]
- Levey, D.J.; Tewksbury, J.J.; Cipollini, M.L.; Carlo, T.A. A Field Test of the Directed Deterrence Hypothesis in Two Species of Wild Chili. Oecologia 2006, 150, 61–68. [Google Scholar] [CrossRef]
- Khan, A.L.; Shin, J.H.; Jung, H.Y.; Lee, I.J. Regulations of Capsaicin Synthesis in Capsicum annuum L. by Penicillium Resedanum LK6 during Drought Conditions. Sci. Hortic. 2014, 175, 167–173. [Google Scholar] [CrossRef]
- Chu, Y.; Cohen, B.E.; Chuang, H. hu A Single TRPV1 Amino Acid Controls Species Sensitivity to Capsaicin. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural Mechanism Underlying Capsaicin Binding and Activation of the TRPV1 Ion Channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Xue, J.-Y.; Jiang, A.-Q.; Zhu, H.-L. Capsaicin and Its Analogues: Structure-Activity Relationship Study. Curr. Med. Chem. 2013, 20, 2661–2672. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Gordon-Shaag, A.; Munari, M.; Gordon, S.E. Ca2+/Calmodulin Modulates TRPV1 Activation by Capsaicin. J. Gen. Physiol. 2004, 123, 53–62. [Google Scholar] [CrossRef]
- Cortright, D.W.; Szallasi, A. Biochemical Pharmacology of the Vanilloid Receptor TRPV1. An Update. Eur. J. Biochem. 2004, 271, 1814–1819. [Google Scholar] [CrossRef]
- Burks, T.F.; Buck, S.H.; Miller, M.S. Mechanisms of Depletion of Substance P by Capsaicin. Fed. Proc. 1985, 44, 2531–2534. [Google Scholar]
- Sághy, É.; Szoke, É.; Payrits, M.; Helyes, Z.; Börzsei, R.; Erostyák, J.; Jánosi, T.Z.; Sétáló, G.; Szolcsányi, J. Evidence for the Role of Lipid Rafts and Sphingomyelin in Ca2+-Gating of Transient Receptor Potential Channels in Trigeminal Sensory Neurons and Peripheral Nerve Terminals. Pharmacol. Res. 2015, 100, 101–116. [Google Scholar] [CrossRef]
- Weng, H.J.; Patel, K.N.; Jeske, N.A.; Bierbower, S.M.; Zou, W.; Tiwari, V.; Zheng, Q.; Tang, Z.; Mo, G.C.H.; Wang, Y.; et al. Tmem100 Is a Regulator of TRPA1-TRPV1 Complex and Contributes to Persistent Pain. Neuron 2015, 85, 833–846. [Google Scholar] [CrossRef]
- Ravishankar, G.A.; Suresh, B.; Giridhar, P.; Rao, S.R.; Johnson, T.S. (11) Biotechnological Studies on Capsicum for Metabolite Production and Plant Improvement|Request PDF. In Capsicum: The Genus Capsicum; De, A.K., Ed.; Taylor & Francis Publishers: Abingdon, UK, 2003; p. 33. ISBN 9780429220548. [Google Scholar]
- Pramanik, K.C.; Srivastava, S.K. Role of Capsaicin in Cancer Prevention. In Role Capsaicin Oxidative Stress Cancer; Springer: Dordrecht, The Netherlands, 2013; pp. 1–18. [Google Scholar] [CrossRef]
- Clark, R.; Lee, S.-H. Anticancer Properties of Capsaicin Against Human Cancer. Anticancer Res. 2016, 36, 837–843. [Google Scholar]
- Kim, C.S.; Park, W.H.; Park, J.Y.; Kang, J.H.; Kim, M.O.; Kawada, T.; Yoo, H.; Han, I.S.; Yu, R. Capsaicin, a Spicy Component of Hot Pepper, Induces Apoptosis by Activation of the Peroxisome Proliferator-Activated Receptor Gamma in HT-29 Human Colon Cancer Cells. J. Med. Food 2004, 7, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Yoshino, M. Involvement of Peroxynitrite in Capsaicin-Induced Apoptosis of C6 Glioma Cells. Neurosci. Res. 2005, 51, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Sánchez, M.G.; Malagarie-Cazenave, S.; Olea, N.; Díaz-Laviada, I. Induction of Apoptosis in Prostate Tumor PC-3 Cells and Inhibition of Xenograft Prostate Tumor Growth by the Vanilloid Capsaicin. Apoptosis 2006, 11, 89–99. [Google Scholar] [CrossRef]
- Athanasiou, A.; Smith, P.A.; Vakilpour, S.; Kumaran, N.M.; Turner, A.E.; Bagiokou, D.; Layfield, R.; Ray, D.E.; Westwell, A.D.; Alexander, S.P.H.; et al. Vanilloid Receptor Agonists and Antagonists Are Mitochondrial Inhibitors: How Vanilloids Cause Non-Vanilloid Receptor Mediated Cell Death. Biochem. Biophys. Res. Commun. 2007, 354, 50–55. [Google Scholar] [CrossRef]
- Wang, S.; Morré, D.M.; Morré, D.J. Sera from Cancer Patients Contain Two Oscillating ECTO-NOX Activities with Different Period Lengths. Cancer Lett. 2003, 190, 135–141. [Google Scholar] [CrossRef]
- Cho, N.; Morré, D.J. Early Developmental Expression of a Normally Tumor-Associated and Drug-Inhibited Cell Surface-Located NADH Oxidase (ENOX2) in Non-Cancer Cells. Cancer Immunol. Immunother. 2009, 58, 547–552. [Google Scholar] [CrossRef]
- Morré, D.J.; Morré, D.M. ECTO-NOX Proteins: Growth, Cancer, and Aging; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; pp. 1–507. [Google Scholar] [CrossRef]
- Morré, D.J.; Hostetler, B.; Weston, N.; Kim, C.; Morré, D.M. Cancer Type-Specific TNOX Isoforms: A Putative Family of Redox Protein Splice Variants with Cancer Diagnostic and Prognostic Potential. Biofactors 2008, 34, 201–207. [Google Scholar] [CrossRef]
- Cheng, H.L.; Lee, Y.H.; Yuan, T.M.; Chen, S.W.; Chueh, P.J. Update on a Tumor-Associated NADH Oxidase in Gastric Cancer Cell Growth. World J. Gastroenterol. 2016, 22, 2900–2905. [Google Scholar] [CrossRef]
- Morré, D.J. NADH Oxidase: A Multifunctional Ectoprotein of the Eukaryotic Cell Surface. In Plasma Membrane Redox Systems and Their Role in Biological Stress and Disease; Springer: Dordrecht, The Netherlands, 1998; pp. 121–156. [Google Scholar] [CrossRef]
- Liu, S.C.; Yang, J.J.; Shao, K.N.; Chueh, P.J. RNA Interference Targeting TNOX Attenuates Cell Migration via a Mechanism That Involves Membrane Association of Rac. Biochem. Biophys. Res. Commun. 2008, 365, 672–677. [Google Scholar] [CrossRef]
- Liu, N.C.; Hsieh, P.F.; Hsieh, M.K.; Zeng, Z.M.; Cheng, H.L.; Liao, J.W.; Chueh, P.J. Capsaicin-Mediated TNOX (ENOX2) up-Regulation Enhances Cell Proliferation and Migration In Vitro and In Vivo. J. Agric. Food Chem. 2012, 60, 2758–2765. [Google Scholar] [CrossRef]
- Zeng, Z.M.; Chuang, S.M.; Chang, T.C.; Hong, C.W.; Chou, J.C.; Yang, J.J.; Chueh, P.J. Phosphorylation of Serine-504 of TNOX (ENOX2) Modulates Cell Proliferation and Migration in Cancer Cells. Exp. Cell Res. 2012, 318, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Su, A.J.; Zeng, Z.M.; Chueh, P.J.; Lin, M.H. Capsaicin Targets TNOX (ENOX2) to Inhibit G1 Cyclin/CDK Complex, as Assessed by the Cellular Thermal Shift Assay (CETSA). Cells 2019, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Morré, D.J.; Caldwell, S.; Mayorga, A.; Wu, L.Y.; Morré, D.M. NADH Oxidase Activity from Sera Altered by Capsaicin Is Widely Distributed among Cancer Patients. Arch. Biochem. Biophys. 1997, 342, 224–230. [Google Scholar] [CrossRef]
- Morré, D.J.; Bridge, A.; Wu, L.Y.; Morré, D.M. Preferential Inhibition by (-)-Epigallocatechin-3-Gallate of the Cell Surface NADH Oxidase and Growth of Transformed Cells in Culture. Biochem. Pharmacol. 2000, 60, 937–946. [Google Scholar] [CrossRef]
- Chueh, P.J.; Kim, C.; Cho, N.M.; Morré, D.M.; Morré, D.J. Molecular Cloning and Characterization of a Tumor-Associated, Growth-Related, and Time-Keeping Hydroquinone (NADH) Oxidase (TNOX) of the HeLa Cell Surface. Biochemistry 2002, 41, 3732–3741. [Google Scholar] [CrossRef]
- Chueh, P.-J.; Wu, L.-Y.; Morré, D.M.; Morré, D.J. TNOX Is Both Necessary and Sufficient as a Cellular Target for the Anticancer Actions of Capsaicin and the Green Tea Catechin (-)-Epigallocatechin-3-Gallate. Biofactors 2004, 20, 235–249. [Google Scholar]
- Wang, H.M.; Chueh, P.J.; Chang, S.P.; Yang, C.L.; Shao, K.N. Effect of Ccapsaicin on TNOX (ENOX2) Protein Expression in Stomach Cancer Cells. Biofactors 2008, 34, 209–217. [Google Scholar] [CrossRef]
- Lin, M.H.; Lee, Y.H.; Cheng, H.L.; Chen, H.Y.; Jhuang, F.H.; Chueh, P.J. Capsaicin Inhibits Multiple Bladder Cancer Cell Phenotypes by Inhibiting Tumor-Associated NADH Oxidase (TNOX) and Sirtuin1 (SIRT1). Molecules 2016, 21, 849. [Google Scholar] [CrossRef]
- Wang, H.M.; Chuang, S.M.; Su, Y.C.; Li, Y.H.; Chueh, P.J. Down-Regulation of Tumor-Associated NADH Oxidase, TNOX (ENOX2), Enhances Capsaicin-Induced Inhibition of Gastric Cancer Cell Growth. Cell Biochem. Biophys. 2011, 61, 355–366. [Google Scholar] [CrossRef]
- Chang, C.-F.; Islam, A.; Liu, P.-F.; Zhan, J.-H.; Chueh, J. Capsaicin Acts through TNOX (ENOX2) to Induce Autophagic Apoptosis in P53-Mutated HSC-3 Cells but Autophagy in P53-Functional SAS Oral Cancer Cells. Am. J. Cancer Res. 2020, 10, 3230. [Google Scholar]
- Hu, Y.L.; Lu, S.; Szeto, K.W.; Sun, J.; Wang, Y.; Lasheras, J.C.; Chien, S. FAK and Paxillin Dynamics at Focal Adhesions in the Protrusions of Migrating Cells. Sci. Rep. 2014, 4, srep06024. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Srivastava, S.K. CBP-Mediated FOXO-1 Acetylation Inhibits Pancreatic Tumor Growth by Targeting SirT. Mol. Cancer Ther. 2014, 13, 687–698. [Google Scholar] [CrossRef]
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 Mediates Capsaicin-Induced Apoptosis in Gastric Cancer Cells--Mechanisms behind a Possible New “Hot” Cancer Treatment. Biochim. Biophys. Acta 2007, 1773, 565–576. [Google Scholar] [CrossRef]
- Skrzypski, M.; Sassek, M.; Abdelmessih, S.; Mergler, S.; Grötzinger, C.; Metzke, D.; Wojciechowicz, T.; Nowak, K.W.; Strowski, M.Z. Capsaicin Induces Cytotoxicity in Pancreatic Neuroendocrine Tumor Cells via Mitochondrial Action. Cell Signal. 2014, 26, 41–48. [Google Scholar] [CrossRef]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of Vanilloid Transient Receptor Potential Cation Channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.J.; Hartog, A.; Stuiver, M.; Doucet, A.; Willems, P.H.G.M.; Bindels, R.J.M. Localization of the Epithelial Ca(2+) Channel in Rabbit Kidney and Intestine. J. Am. Soc. Nephrol. 2000, 11, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Roderick, H.L.; Cook, S.J. Ca2+ Signalling Checkpoints in Cancer: Remodelling Ca2+ for Cancer Cell Proliferation and Survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef] [PubMed]
- van Abel, M.; Hoenderop, J.G.J.; Bindels, R.J.M. The Epithelial Calcium Channels TRPV5 and TRPV6: Regulation and Implications for Disease. Naunyn. Schmiedebergs Arch. Pharmacol. 2005, 371, 295–306. [Google Scholar] [CrossRef]
- Woudenberg-Vrenken, T.E.; Lameris, A.L.; Weißgerber, P.; Olausson, J.; Flockerzi, V.; Bindels, R.J.M.; Freichel, M.; Hoenderop, J.G.J. Functional TRPV6 Channels Are Crucial for Transepithelial Ca2+ Absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G879–G885. [Google Scholar] [CrossRef] [PubMed]
- Seebohm, G.; Schreiber, J.A. Beyond Hot and Spicy: TRPV Channels and Their Pharmacological Modulation. Cell Physiol. Biochem. 2021, 22, 108–130. [Google Scholar]
- Peng, J.B.; Zhuang, L.; Berger, U.V.; Adam, R.M.; Williams, B.J.; Brown, E.M.; Hediger, M.A.; Freeman, M.R. CaT1 Expression Correlates with Tumor Grade in Prostate Cancer. Biochem. Biophys. Res. Commun. 2001, 282, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Wissenbach, U.; Niemeyer, B.A.; Fixemer, T.; Schneidewind, A.; Trost, C.; Cavalié, A.; Reus, K.; Meese, E.; Bonkhoff, H.; Flockerzi, V. Expression of CaT-like, a Novel Calcium-Selective Channel, Correlates with the Malignancy of Prostate Cancer. J. Biol. Chem. 2001, 276, 19461–19468. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; bin Peng, J.; Tou, L.; Takanaga, H.; Adam, R.M.; Hediger, M.A.; Freeman, M.R. Calcium-Selective Ion Channel, CaT1, Is Apically Localized in Gastrointestinal Tract Epithelia and Is Aberrantly Expressed in Human Malignancies. Lab. Investig. 2002, 82, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Fixemer, T.; Wissenbach, U.; Flockerzi, V.; Bonkhoff, H. Expression of the Ca2+-Selective Cation Channel TRPV6 in Human Prostate Cancer: A Novel Prognostic Marker for Tumor Progression. Oncogene 2003, 22, 7858–7861. [Google Scholar] [CrossRef]
- Lehen’kyi, V.; Flourakis, M.; Skryma, R.; Prevarskaya, N. TRPV6 Channel Controls Prostate Cancer Cell Proliferation via Ca(2+)/NFAT-Dependent Pathways. Oncogene 2007, 26, 7380–7385. [Google Scholar] [CrossRef]
- Raphaël, M.; Lehen’kyi, V.; Vandenberghe, M.; Beck, B.; Khalimonchyk, S.; vanden Abeele, F.; Farsetti, L.; Germain, E.; Bokhobza, A.; Mihalache, A.; et al. TRPV6 Calcium Channel Translocates to the Plasma Membrane via Orai1-Mediated Mechanism and Controls Cancer Cell Survival. Proc. Natl. Acad. Sci. USA 2014, 111, E3870–E3879. [Google Scholar] [CrossRef]
- Lo, Y.C.; Yang, Y.C.; Wu, I.C.; Kuo, F.C.; Liu, C.M.; Wang, H.W.; Kuo, C.H.; Wu, J.Y.; Wu, D.C. Capsaicin-Induced Cell Death in a Human Gastric Adenocarcinoma Cell Line. World J. Gastroenterol. 2005, 11, 6254–6257. [Google Scholar] [CrossRef]
- Jin, J.; Lin, G.; Huang, H.; Xu, D.; Yu, H.; Ma, X.; Zhu, L.; Ma, D.; Jiang, H. Capsaicin Mediates Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells via Stabilizing and Activating P53. Int. J. Biol. Sci. 2014, 10, 285–295. [Google Scholar] [CrossRef]
- Sarkar, A.; Bhattacharjee, S.; Mandal, D.P. Induction of Apoptosis by Eugenol and Capsaicin in Human Gastric Cancer AGS Cells--Elucidating the Role of P53. Asian Pac. J. Cancer Prev. 2015, 16, 6753–6759. [Google Scholar] [CrossRef]
- Kim, M.Y.; Trudel, L.J.; Wogan, G.N. Apoptosis Induced by Capsaicin and Resveratrol in Colon Carcinoma Cells Requires Nitric Oxide Production and Caspase Activation. Anticancer Res. 2009, 29, 3733–3740. [Google Scholar]
- Xu, S.; Cheng, X.; Wu, L.; Zheng, J.; Wang, X.; Wu, J.; Yu, H.; Bao, J.; Zhang, L. Capsaicin Induces Mitochondrial Dysfunction and Apoptosis in Anaplastic Thyroid Carcinoma Cells via TRPV1-Mediated Mitochondrial Calcium Overload. Cell Signal. 2020, 75, 109733. [Google Scholar] [CrossRef] [PubMed]
- Pawar, J.S.; Mustafa, S.; Ghosh, I. Chrysin and Capsaicin Induces Premature Senescence and Apoptosis via Mitochondrial Dysfunction and P53 Elevation in Cervical Cancer Cells. Saudi J. Biol. Sci. 2022, 29, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of Mitochondrial Electron Transport Chain Complexes in Capsaicin Mediated Oxidative Stress Leading to Apoptosis in Pancreatic Cancer Cells. PLoS ONE 2011, 6, e20151. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T. Inhibition by Capsaicin of NADH-Quinone Oxidoreductases Is Correlated with the Presence of Energy-Coupling Site 1 in Various Organisms. Arch. Biochem. Biophys. 1990, 281, 305–311. [Google Scholar] [CrossRef]
- Kishi, T.; Morré, D.M.; Morré, D.J. The Plasma Membrane NADH Oxidase of HeLa Cells Has Hydroquinone Oxidase Activity. Biochim. Biophys. Acta (BBA)—Bioenerg. 1999, 1412, 66–77. [Google Scholar] [CrossRef]
- Kanu, N.; Zhang, T.; Burrell, R.A.; Chakraborty, A.; Cronshaw, J.; Dacosta, C.; Grönroos, E.; Pemberton, H.N.; Anderton, E.; Gonzalez, L.; et al. RAD18, WRNIP1 and ATMIN Promote ATM Signalling in Response to Replication Stress. Oncogene 2016, 35, 4009–4019. [Google Scholar] [CrossRef]
- Ito, K.; Nakazato, T.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Segawa, K.; Ikeda, Y.; Kizaki, M. Induction of Apoptosis in Leukemic Cells by Homovanillic Acid Derivative, Capsaicin, through Oxidative Stress: Implication of Phosphorylation of P53 at Ser-15 Residue by Reactive Oxygen Species. Cancer Res. 2004, 64, 1071–1078. [Google Scholar] [CrossRef]
- Amantini, C.; Ballarini, P.; Caprodossi, S.; Nabissi, M.; Morelli, M.B.; Lucciarini, R.; Cardarelli, M.A.; Mammana, G.; Santoni, G. Triggering of Transient Receptor Potential Vanilloid Type 1 (TRPV1) by Capsaicin Induces Fas/CD95-Mediated Apoptosis of Urothelial Cancer Cells in an ATM-Dependent Manner. Carcinogenesis 2009, 30, 1320–1329. [Google Scholar] [CrossRef]
- Kim, S.; Kang, C.; Chan, Y.S.; Sun, W.H.; Young, D.Y.; Won, S.S.; Park, M.Y.; Kim, E.; Kim, M.; Kim, B.M.; et al. TRPV1 Recapitulates Native Capsaicin Receptor in Sensory Neurons in Association with Fas-Associated Factor 1. J. Neurosci. 2006, 26, 2403–2412. [Google Scholar] [CrossRef]
- Menges, C.W.; Altomare, D.A.; Testa, J.R. FAS-Associated Factor 1 (FAF1): Diverse Functions and Implications for Oncogenesis NIH Public Access. Cell Cycle 2009, 8, 2528–2534. [Google Scholar] [CrossRef]
- Santoni, G.; Caprodossi, S.; Farfariello, V.; Liberati, S.; Amantini, C. Role of Death Receptors Belonging to the TNF Family in Capsaicin-Induced Apoptosis of Tumor Cells. In Role of Capsaicin in Oxidative Stress and Cancer; Springer: Dordrecht, The Netherlands, 2013; pp. 19–46. [Google Scholar] [CrossRef]
- Huh, H.C.; Lee, S.Y.; Lee, S.K.; Park, N.H.; Han, I.S. Capsaicin Induces Apoptosis of Cisplatin-Resistant Stomach Cancer Cells by Causing Degradation of Cisplatin-Inducible Aurora-A Protein. Nutr. Cancer 2011, 63, 1095–1103. [Google Scholar] [CrossRef]
- Meral, O.; Alpay, M.; Kismali, G.; Kosova, F.; Cakir, D.U.; Pekcan, M.; Yigit, S.; Sel, T. Capsaicin Inhibits Cell Proliferation by Cytochrome c Release in Gastric Cancer Cells. Tumour Biol. 2014, 35, 6485–6492. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, J.D.; Yu, R.; Kim, B.S.; Shin, M.K.; Han, I.S. Effects of Capsaicin on Induction of C-Jun Proto-Oncogene Expression in Fisher-344 Rats by N-Methyl-N’-Nitro-N-Nitrosoguanidine. Cancer Lett. 1999, 142, 155–160. [Google Scholar] [CrossRef]
- Lu, H.F.; Chen, Y.L.; Yang, J.S.; Yang, Y.Y.; Liu, J.Y.; Hsu, S.C.; Lai, K.C.; Chung, J.G. Antitumor Activity of Capsaicin on Human Colon Cancer Cells in Vitro and Colo 205 Tumor Xenografts in Vivo. J. Agric. Food Chem. 2010, 58, 12999–13005. [Google Scholar] [CrossRef]
- Bai, H.; Li, H.; Zhang, W.; Matkowskyj, K.A.; Liao, J.; Srivastava, S.K.; Yang, G.Y. Inhibition of Chronic Pancreatitis and Pancreatic Intraepithelial Neoplasia (PanIN) by Capsaicin in LSL-KrasG12D/Pdx1-Cre Mice. Carcinogenesis 2011, 32, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Basu, S. Tumor Macrophages as a Target for Capsaicin Mediated Immunotherapy. Cancer Lett. 2012, 324, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hsieh, P.-F.; Liu, P.-F.; Chou, J.-C.; Liao, J.-W.; Hsieh, M.-K.; Chueh, P.J. Capsaicin Exerts Therapeutic Effects by Targeting TNOX-SIRT1 Axis and Augmenting ROS-Dependent Autophagy in Melanoma Cancer Cells. Am. J. Cancer Res. 2021, 11, 4199. [Google Scholar]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In Vitro and in Vivo Induction of Apoptosis by Capsaicin in Pancreatic Cancer Cells Is Mediated through ROS Generation and Mitochondrial Death Pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Capsaicin Provokes Apoptosis and Restricts Benzo(a)Pyrene Induced Lung Tumorigenesis in Swiss Albino Mice. Int. Immunopharmacol. 2013, 17, 254–259. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Lysosomal Abnormalities during Benzo(a)Pyrene-Induced Experimental Lung Carcinogenesis—Defensive Role of Capsaicin. Fundam. Clin. Pharmacol. 2009, 23, 97–103. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Effect of Capsaicin on Glucose Metabolism Studied in Experimental Lung Carcinogenesis. Nat. Prod. Res. 2009, 23, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Jagan, S.; Kamaraj, S.; Ramakrishnan, G.; Titto, A.A.; Devaki, T. Beneficial Influence of Capsaicin on Lipid Peroxidation, Membrane-Bound Enzymes and Glycoprotein Profile during Experimental Lung Carcinogenesis. J. Pharm. Pharmacol. 2008, 60, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Asokkumar, S.; Naveenkumar, C.; Raghunandhakumar, S.; Devaki, T. Capsaicin Inhibits Benzo(a)Pyrene-Induced Lung Carcinogenesis in an in Vivo Mouse Model. Inflamm. Res. 2012, 61, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Yoshitani, S.I.; Tanaka, T.; Kohno, H.; Takashima, S. Chemoprevention of Azoxymethane-Induced Rat Colon Carcinogenesis by Dietary Capsaicin and Rotenone. Int. J. Oncol. 2001, 19, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Xie, Y.; Gao, Y.; McClements, D.J.; Zhang, L.; Zou, L.; Liu, W. Capsaicin Encapsulated in W/O/W Double Emulsions Fabricated via Ethanol-Induced Pectin Gelling: Improvement of Bioaccessibility and Reduction of Irritation. Int. J. Biol. Macromol. 2023, 235, 123899. [Google Scholar] [CrossRef]
- Surh, Y.J.; Sup Lee, S. Capsaicin, a Double-Edged Sword: Toxicity, Metabolism, and Chemopreventive Potential. Life Sci. 1995, 56, 1845–1855. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Orsulic, S.; Huber, O.; Aberle, H.; Arnold, S.; Kemler, R. E-Cadherin Binding Prevents Beta-Catenin Nuclear Localization and Beta-Catenin/LEF-1-Mediated Transactivation. J. Cell Sci. 1999, 112, 1237–1245. [Google Scholar] [CrossRef]
- Spadaro, D.; Tapia, R.; Jond, L.; Sudol, M.; Fanning, A.S.; Citi, S. ZO Proteins Redundantly Regulate the Transcription Factor DbpA/ZONAB. J. Biol. Chem. 2014, 289, 22500–22511. [Google Scholar] [CrossRef]
- Ozawa, M. The N-Cadherin Cytoplasmic Domain Confers Anchorage-Independent Growth and the Loss of Contact Inhibition. Sci. Rep. 2015, 5, 15368. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Reginato, M.J.; Mills, K.R.; Paulus, J.K.; Lynch, D.K.; Sgroi, D.C.; Debnath, J.; Muthuswamy, S.K.; Brugge, J.S. Integrins and EGFR Coordinately Regulate the Pro-Apoptotic Protein Bim to Prevent Anoikis. Nat. Cell Biol. 2003, 5, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and Oncogene Rescue of Metabolic Defects Caused by Loss of Matrix Attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Weigel, K.J.; Jakimenko, A.; Conti, B.A.; Chapman, S.E.; Kaliney, W.J.; Leevy, W.M.; Champion, M.M.; Schafer, Z.T. CAF-Secreted IGFBPs Regulate Breast Cancer Cell Anoikis. Mol. Cancer Res. 2014, 12, 855–866. [Google Scholar] [CrossRef]
- Buchheit, C.L.; Angarola, B.L.; Steiner, A.; Weigel, K.J.; Schafer, Z.T. Anoikis Evasion in Inflammatory Breast Cancer Cells Is Mediated by Bim-EL Sequestration. Cell Death Differ. 2015, 22, 1275–1286. [Google Scholar] [CrossRef]
- Luciano, F.; Jacquel, A.; Colosetti, P.; Herrant, M.; Cagnol, S.; Pages, G.; Auberger, P. Phosphorylation of Bim-EL by Erk1/2 on Serine 69 Promotes Its Degradation via the Proteasome Pathway and Regulates Its Proapoptotic Function. Oncogene 2003, 22, 6785–6793. [Google Scholar] [CrossRef]
- Nagy, I.; Friston, D.; Valente, J.S.; Perez, J.V.T.; Andreou, A.P. Pharmacology of the Capsaicin Receptor, Transient Receptor Potential Vanilloid Type-1 Ion Channel. Prog. Drug Res. 2014, 68, 39–76. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in Tumour Metastasis: New Roles for Known Actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef]
- Davis, F.M.; Azimi, I.; Faville, R.A.; Peters, A.A.; Jalink, K.; Putney, J.W.; Goodhill, G.J.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Induction of Epithelial-Mesenchymal Transition (EMT) in Breast Cancer Cells Is Calcium Signal Dependent. Oncogene 2014, 33, 2307–2316. [Google Scholar] [CrossRef]
- Archer, V.E.; Jones, D.W. Capsaicin Pepper, Cancer and Ethnicity. Med. Hypotheses 2002, 59, 450–457. [Google Scholar] [CrossRef] [PubMed]
- López-Carrillo, L.; Camargo, M.C.; Schneider, B.G.; Sicinschi, L.A.; Hernández-Ramírez, R.U.; Correa, P.; Cebrian, M.E. Capsaicin Consumption, Helicobacter Pylori CagA Status and IL1B-31C>T Genotypes: A Host and Environment Interaction in Gastric Cancer. Food Chem. Toxicol. 2012, 50, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Pabalan, N.; Jarjanazi, H.; Ozcelik, H. The Impact of Capsaicin Intake on Risk of Developing Gastric Cancers: A Meta-Analysis. J. Gastrointest. Cancer 2014, 45, 334–341. [Google Scholar] [CrossRef]
- Özkan, A.; Bindak, R.; Erkmen, O. Aflatoxin B1 and Aflatoxins in Ground Red Chilli Pepper after Drying. Food Addit. Contam. Part B 2015, 8, 227–233. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Wiessler, M.; Hecker, E.; Bhide, S.v. Tumour-Promoting Effect of Chilli Extract in BALB/c Mice. Int. J. Cancer 1986, 38, 689–695. [Google Scholar] [CrossRef]
- Kim, J.P.; Park, J.G.; Lee, M.D.; Han, M.D.; Park, S.T.; Lee, B.H.; Jung, S.E. Co-Carcinogenic Effects of Several Korean Foods on Gastric Cancer Induced by N-Methyl-N’-Nitro-N-Nitrosoguanidine in Rats. Jpn. J. Surg. 1985, 15, 427–437. [Google Scholar] [CrossRef]
- Akagi, A.; Sano, N.; Uehara, H.; Minami, T.; Otsuka, H.; Izumi, K. Non-Carcinogenicity of Capsaicinoids in B6C3F1 Mice. Food Chem. Toxicol. 1998, 36, 1065–1071. [Google Scholar] [CrossRef]
- Caprodossi, S.; Amantini, C.; Nabissi, M.; Morelli, M.B.; Farfariello, V.; Santoni, M.; Gismondi, A.; Santoni, G. Capsaicin Promotes a More Aggressive Gene Expression Phenotype and Invasiveness in Null-TRPV1 Urothelial Cancer Cells. Carcinogenesis 2011, 32, 686–694. [Google Scholar] [CrossRef]
- Metalli, D.; Lovat, F.; Tripodi, F.; Genua, M.; Xu, S.Q.; Spinelli, M.; Alberghina, L.; Vanoni, M.; Baffa, R.; Gomella, L.G.; et al. The Insulin-like Growth Factor Receptor I Promotes Motility and Invasion of Bladder Cancer Cells through Akt- and Mitogen-Activated Protein Kinase-Dependent Activation of Paxillin. Am. J. Pathol. 2010, 176, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, Z.; Wang, Y.; Zhu, G.; Wang, X. Capsaicin Induces Cycle Arrest by Inhibiting Cyclin-Dependent-Kinase in Bladder Carcinoma Cells. Int. J. Urol. 2012, 19, 662–668. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L.; et al. Capsaicin Enhances Anti-Proliferation Efficacy of Pirarubicin via Activating TRPV1 and Inhibiting PCNA Nuclear Translocation in 5637 Cells. Mol. Med. Rep. 2016, 13, 881–887. [Google Scholar] [CrossRef]
- Su, Y.-C.; Lin, Y.; Zeng, Z.-M.; Shao, K.-N.; Chueh, P.J. Chemotherapeutic Agents Enhance Cell Migration and Epithelial-to-Mesenchymal Transition through Transient up-Regulation of TNOX (ENOX2) Protein. Biochim. Biophys. Acta 2012, 1820, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Dornfeld, K.; Bjork, J.; Folkert, G.; Skildum, A.; Wallace, K.B. Mitochondrial Activities Play a Pivotal Role in Regulating Cell Cycle in Response to Doxorubicin. Cell Cycle 2021, 20, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Unten, Y.; Murai, M.; Koshitaka, T.; Kitao, K.; Shirai, O.; Masuya, T.; Miyoshi, H. Comprehensive Understanding of Multiple Actions of Anticancer Drug Tamoxifen in Isolated Mitochondria. Biochim. Biophys. Acta (BBA)—Bioenerg. 2022, 1863, 148520. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.C.; Wang, H.M.; Lin, Y.Y.; Chang, T.K.; Hsin, Y.H.; Chueh, P.J. Stress-Induced down-Regulation of Tumor-Associated NADH Oxidase during Apoptosis in Transformed Cells. FEBS Lett. 2008, 582, 3445–3450. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a Component of Red Peppers, Inhibits the Growth of Androgen-Independent, P53 Mutant Prostate Cancer Cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef]
- Shim, Y.; Song, J.M. Quantum Dot Nanoprobe-Based High-Content Monitoring of Notch Pathway Inhibition of Breast Cancer Stem Cell by Capsaicin. Mol. Cell Probes 2015, 29, 376–381. [Google Scholar] [CrossRef]
- Lee, S.H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin Represses Transcriptional Activity of β-Catenin in Human Colorectal Cancer Cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef]
- Perucka, I.; Materska, M. Phenylalanine Ammonia-Lyase and Antioxidant Activities of Lipophilic Fraction of Fresh Pepper Fruits Capsicum annum L. Innov. Food Sci. Emerg. Technol. 2001, 2, 189–192. [Google Scholar] [CrossRef]
- Seon, H.O.; Young, S.K.; Sung, C.L.; Yi, F.H.; In, Y.C.; Ho, J.Y. Dihydrocapsaicin (DHC), a Saturated Structural Analog of Capsaicin, Induces Autophagy in Human Cancer Cells in a Catalase-Regulated Manner. Autophagy 2008, 4, 1009–1019. [Google Scholar] [CrossRef]
- Oh, S.H.; Lim, S.C. Endoplasmic Reticulum Stress-Mediated Autophagy/Apoptosis Induced by Capsaicin (8-Methyl-N-Vanillyl-6-Nonenamide) and Dihydrocapsaicin Is Regulated by the Extent of c-Jun NH2-Terminal Kinase/Extracellular Signal-Regulated Kinase Activation in WI38 Lung Epithelial Fibroblast Cells. J. Pharmacol. Exp. Ther. 2009, 329, 112–122. [Google Scholar] [CrossRef]
- Choi, C.H.; Jung, Y.K.; Oh, S.H. Selective Induction of Catalase-Mediated Autophagy by Dihydrocapsaicin in Lung Cell Lines. Free Radic. Biol. Med. 2010, 49, 245–257. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 Conjugation System in Mammalian Autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef]
- Liu, B.; Wen, X.; Cheng, Y. Survival or Death: Disequilibrating the Oncogenic and Tumor Suppressive Autophagy in Cancer. Cell Death Dis. 2013, 4, e892. [Google Scholar] [CrossRef]
- Tran, S.; Fairlie, W.D.; Lee, E.F. Beclin1: Protein Structure, Function and Regulation. Cells 2021, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin-Proteasome System upon Ubiquitinated Protein Degradation. Cell. Mol. Biol. Lett. 2016, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Dong, F.X.; Chai, X.; Zhu, S.; Zhang, B.L.; Gao, D.S. Role of Autophagy in Capsaicin-Induced Apoptosis in U251 Glioma Cells. Cell. Mol. Neurobiol. 2016, 36, 737–743. [Google Scholar] [CrossRef]
- Granato, M.; Gilardini Montani, M.S.; Filardi, M.; Faggioni, A.; Cirone, M. Capsaicin Triggers Immunogenic PEL Cell Death, Stimulates DCs and Reverts PEL-Induced Immune Suppression. Oncotarget 2015, 6, 29543–29554. [Google Scholar] [CrossRef] [PubMed]
- Journae, P. Irreversible Inactivation of Catalase by 3-Amino-l,2,4-Triazole. Biochem. Pharmacol. 1986, 35, 3642. [Google Scholar]
- Sun, Y.; Yin, E.; Tan, Y.; Yang, T.; Song, D.; Jin, S.; Guo, Z.; Wang, X. Immunogenicity and Cytotoxicity of a Platinum(Iv) Complex Derived from Capsaicin. Dalton Trans. 2021, 50, 3516–3522. [Google Scholar] [CrossRef]
- He, Y.; Tian, Z. NK Cell Education via Nonclassical MHC and Non-MHC Ligands. Cell Mol. Immunol. 2017, 14, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Raulet, D.H.; Guerra, N. Oncogenic Stress Sensed by the Immune System: Role of Natural Killer Cell Receptors. Nat. Rev. Immunol. 2009, 9, 568–580. [Google Scholar] [CrossRef]
- Basu, S.; Srivastava, P. Immunological Role of Neuronal Receptor Vanilloid Receptor 1 Expressed on Dendritic Cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5120–5125. [Google Scholar] [CrossRef]
- Cirone, M.; Lucania, G.; Aleandri, S.; Borgia, G.; Trivedi, P.; Cuomo, L.; Frati, L.; Faggioni, A. Suppression of Dendritic Cell Differentiation through Cytokines Released by Primary Effusion Lymphoma Cells. Immunol. Lett. 2008, 120, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic Cell Death and DAMPs in Cancer Therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Manzi, L.; Velotti, F. Capsaicin as an Inducer of Damage-Associated Molecular Patterns (DAMPs) of Immunogenic Cell Death (ICD) in Human Bladder Cancer Cells. Cell Stress Chaperones 2013, 18, 801–808. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; D’Eliseo, D.; Cirone, M.; di Renzo, L.; Faggioni, A.; Santoni, A.; Velotti, F. Capsaicin-Mediated Apoptosis of Human Bladder Cancer Cells Activates Dendritic Cells via CD91. Nutrition 2015, 31, 578–581. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- WHOCC ATC/DDD Index 2023. Available online: https://www.whocc.no/atc_ddd_index/?code=L&showdescription=no (accessed on 22 May 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luján-Méndez, F.; Roldán-Padrón, O.; Castro-Ruíz, J.E.; López-Martínez, J.; García-Gasca, T. Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale. Cells 2023, 12, 2573. https://doi.org/10.3390/cells12212573
Luján-Méndez F, Roldán-Padrón O, Castro-Ruíz JE, López-Martínez J, García-Gasca T. Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale. Cells. 2023; 12(21):2573. https://doi.org/10.3390/cells12212573
Chicago/Turabian StyleLuján-Méndez, Francisco, Octavio Roldán-Padrón, J. Eduardo Castro-Ruíz, Josué López-Martínez, and Teresa García-Gasca. 2023. "Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale" Cells 12, no. 21: 2573. https://doi.org/10.3390/cells12212573
APA StyleLuján-Méndez, F., Roldán-Padrón, O., Castro-Ruíz, J. E., López-Martínez, J., & García-Gasca, T. (2023). Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale. Cells, 12(21), 2573. https://doi.org/10.3390/cells12212573