DAZL Knockout Pigs as Recipients for Spermatogonial Stem Cell Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetically Modified Pigs
2.2. Spermatogonial Stem Cell Transplantation
2.3. Tissue, Sperm, and Blood Collection
2.4. Testis Morphometry
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
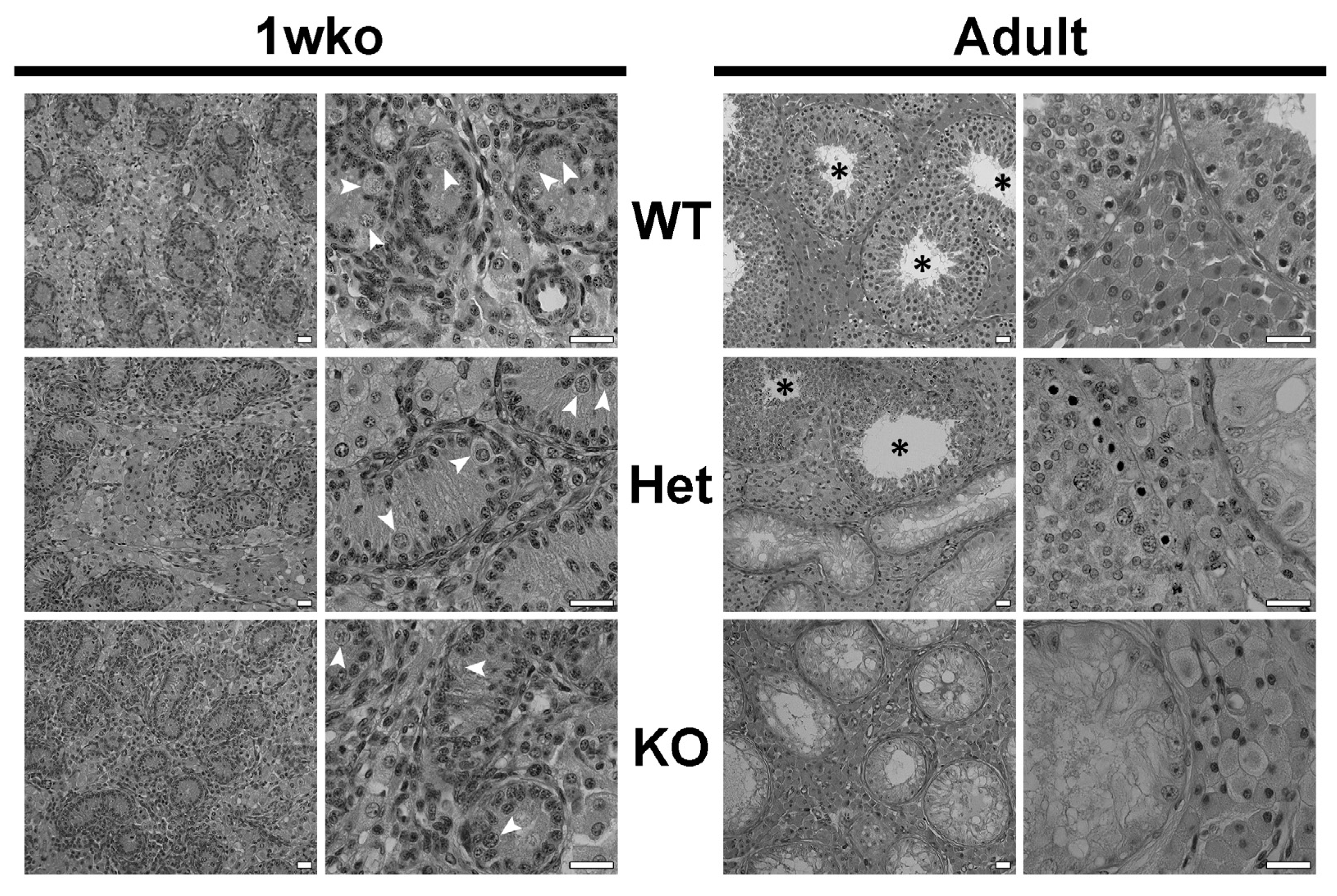
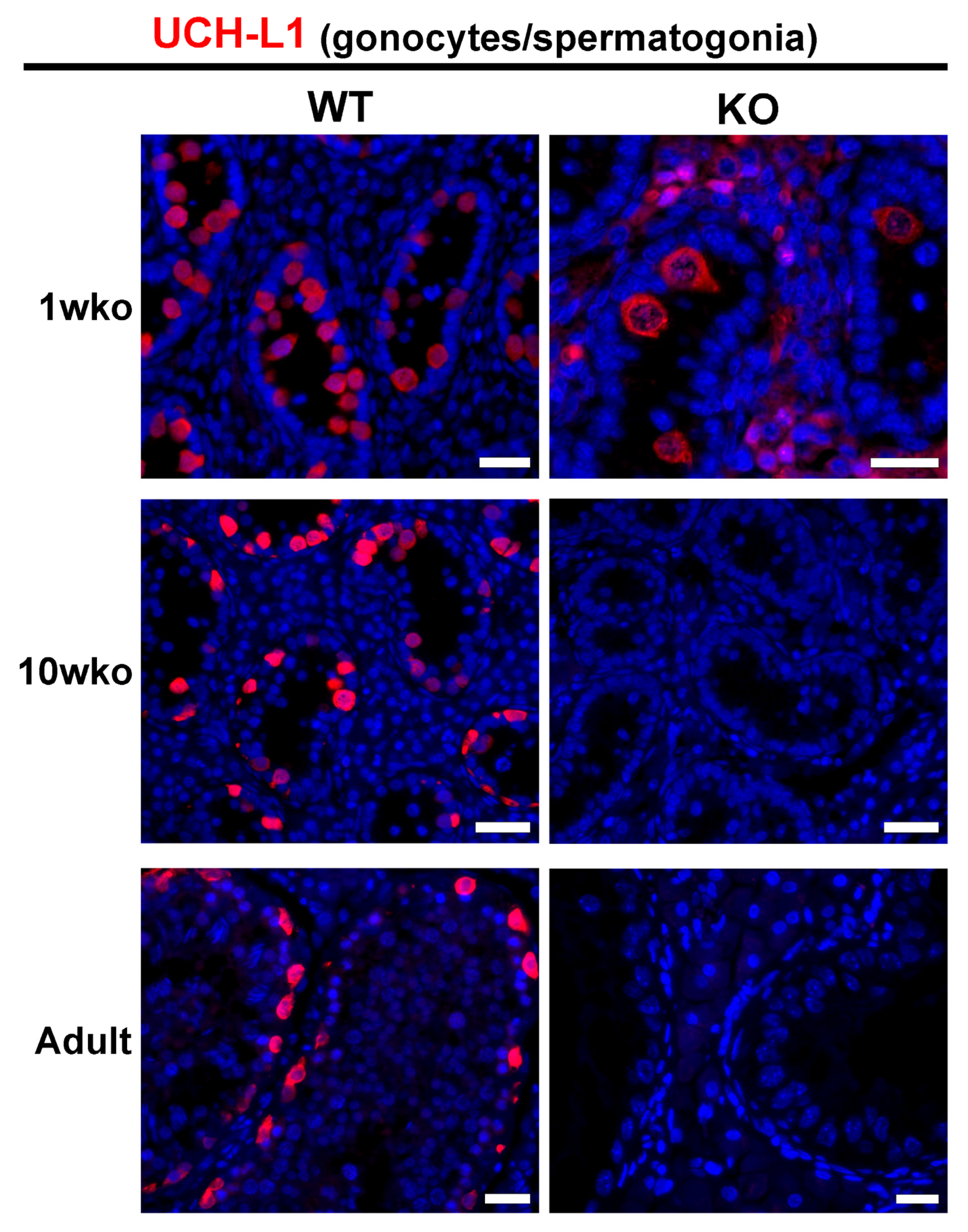
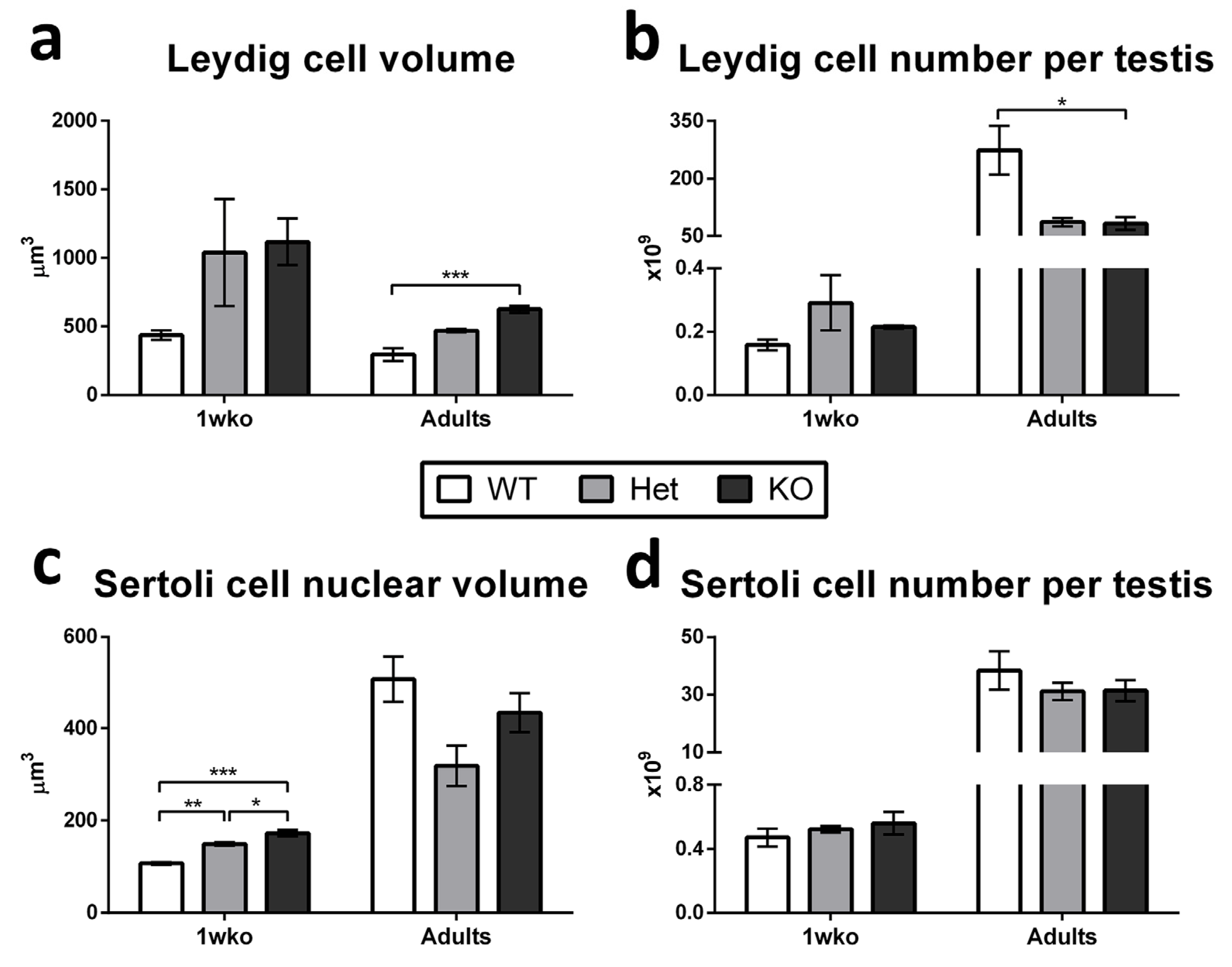
3.1. Composition of the Testicular Parenchyma in DAZL-KO Pigs
3.2. Transplantation Efficiency
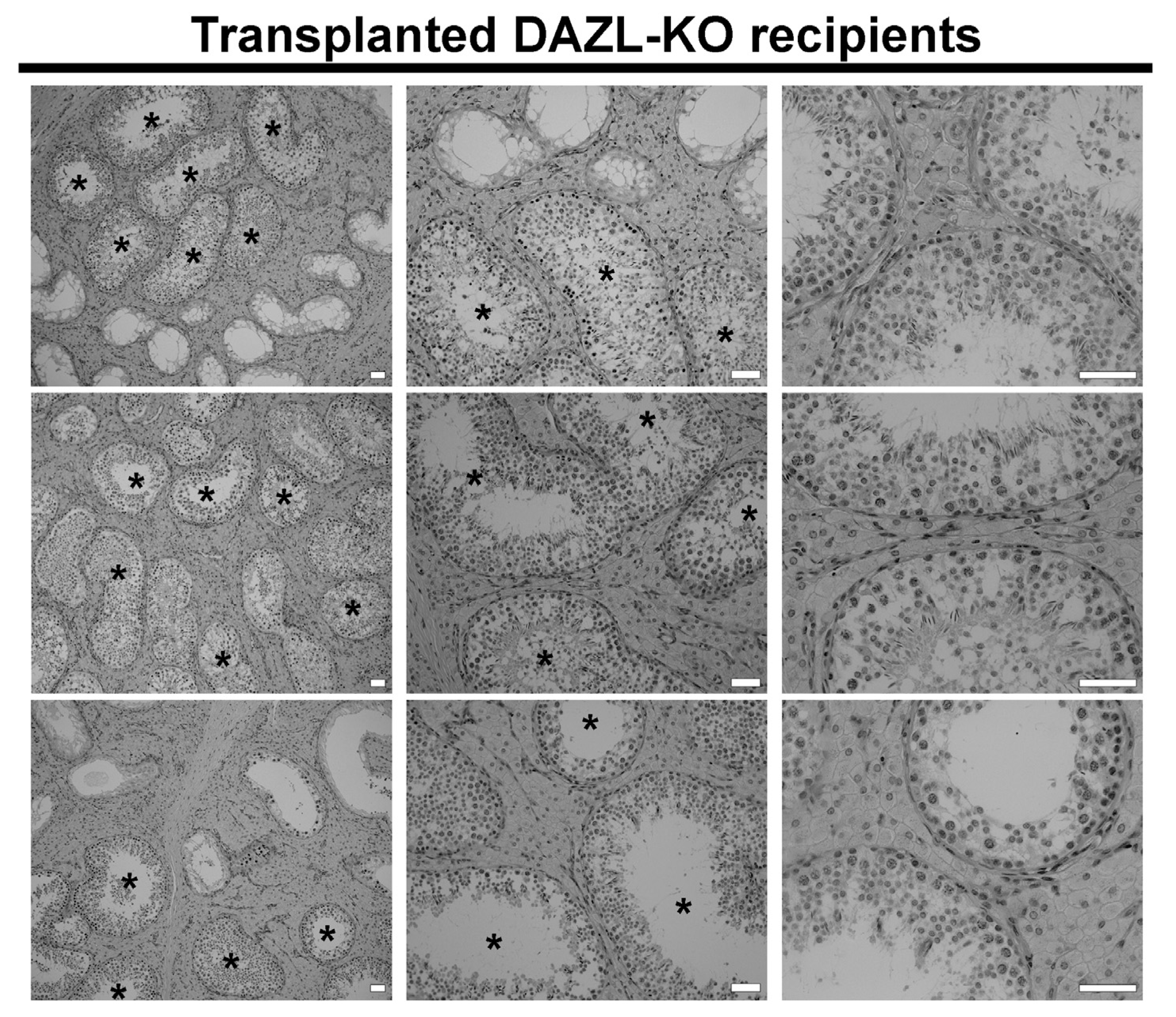
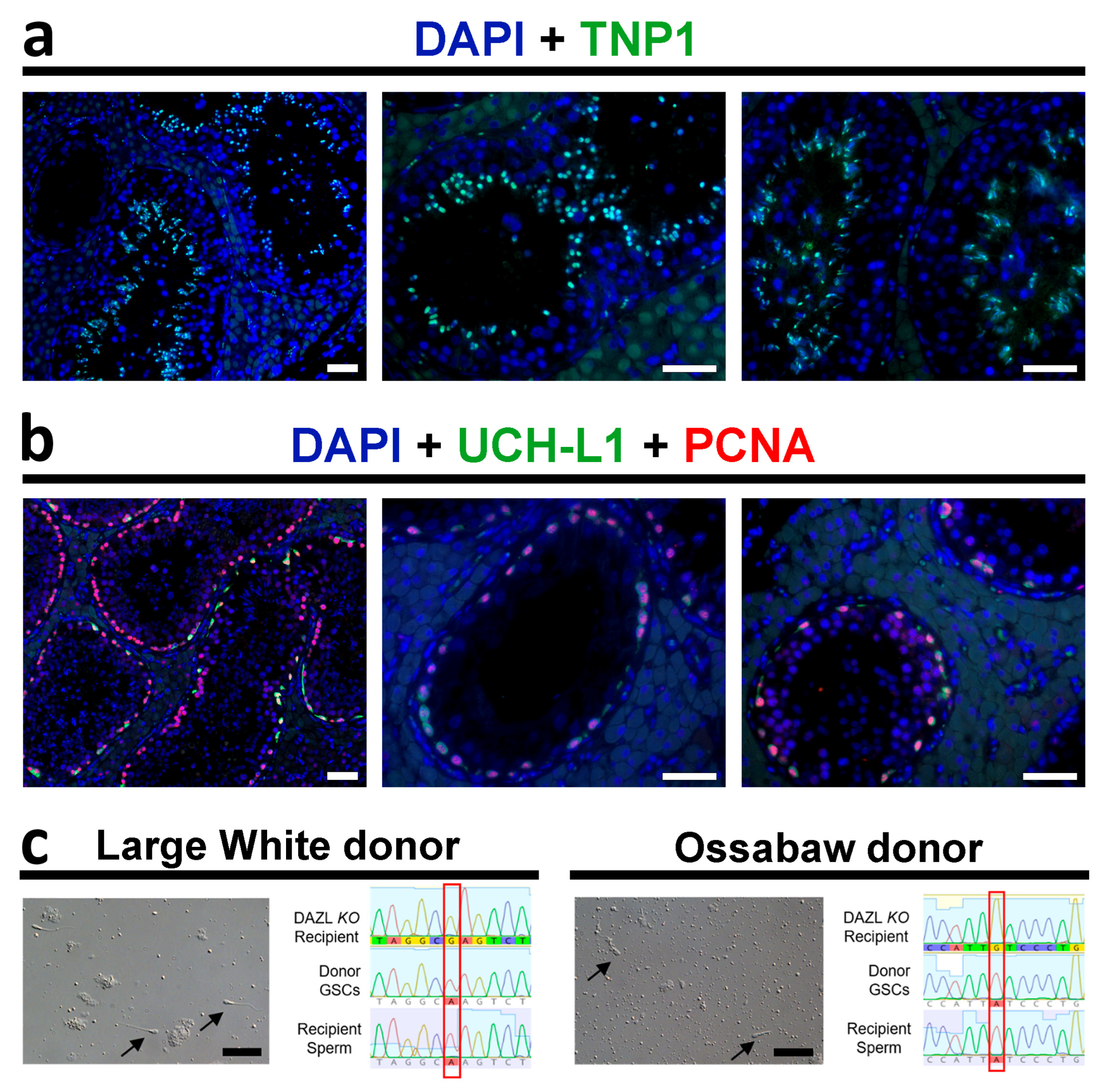
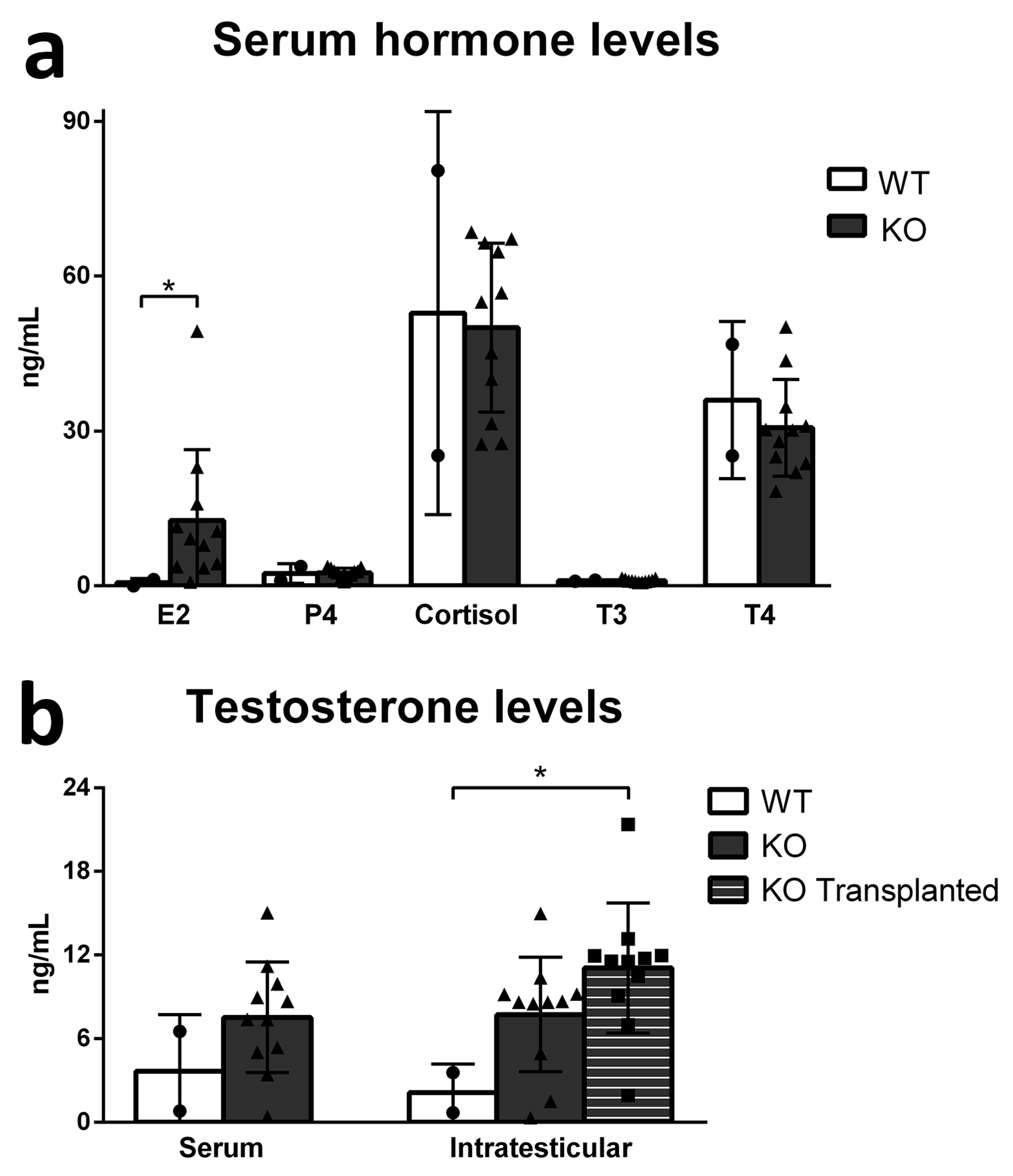
3.3. Functionality of the Recipient Testis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brinster, R.L. Germline stem cell transplantation and transgenesis. Science 2002, 296, 2174–2176. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.R.; Dobrinski, I. Male germ cell transplantation in livestock. Reprod. Fertil. Dev. 2006, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Dobrinski, I.; Travis, A.J. Germ cell transplantation for the propagation of companion animals, non-domestic and endangered species. Reprod. Fertil. Dev. 2007, 19, 732. [Google Scholar] [CrossRef]
- Oatley, J.M. Recent advances for spermatogonial stem cell transplantation in livestock. Reprod. Fertil. Dev. 2018, 30, 44. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Jahnukainen, K.; Mitchell, R.; van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.; et al. Fertility preservation in boys: Recent developments and new insights. Hum. Reprod. Open 2020, 2020, hoaa016. [Google Scholar] [CrossRef] [PubMed]
- Doungkamchan, C.; Orwig, K.E. Recent advances: Fertility preservation and fertility restoration options for males and females. Fac. Rev. 2021, 10, 55. [Google Scholar] [CrossRef]
- Zhao, X.; Wan, W.; Zhang, X.; Wu, Z.; Yang, H. Spermatogonial stem cell transplantation in large animals. Animals 2021, 11, 918. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, R.; Cai, Y.; Xie, W.; Zou, K. Progress in germline stem cell transplantation in mammals and the potential usage. Reprod. Biol. Endocrinol. 2022, 20, 59. [Google Scholar] [CrossRef]
- de Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 21, 776–798. [Google Scholar] [CrossRef]
- de Rooij, D. Proliferation and differentiation of spermatogonial stem cells. Reproduction 2001, 121, 347–354. [Google Scholar] [CrossRef]
- Ehmcke, J.; Wistuba, J.; Schlatt, S. Spermatogonial stem cells: Questions, models and perspectives. Hum. Reprod. Update 2006, 12, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.X.; Hofmann, M.C. Regulation of germ line stem cell homeostasis. Anim. Reprod. 2015, 12, 35–45. [Google Scholar] [PubMed]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef]
- Sharma, S.; Wistuba, J.; Pock, T.; Schlatt, S.; Neuhaus, N. Spermatogonial stem cells: Updates from specification to clinical relevance. Hum. Reprod. Update 2019, 25, 275–297. [Google Scholar] [CrossRef]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Avarbock, M.R.; Brinster, R.L. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol. Reprod. 1999, 60, 1429–1436. [Google Scholar] [CrossRef]
- Honaramooz, A.; Megee, S.O.; Dobrinski, I. Germ cell transplantation in pigs. Biol. Reprod. 2002, 66, 21–28. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar] [CrossRef]
- Nakamura, Y.; Jörg, D.J.; Kon, Y.; Simons, B.D.; Yoshida, S. Transient suppression of transplanted spermatogonial stem cell differentiation restores fertility in mice. Cell Stem Cell 2021, 28, 1443–1456.e7. [Google Scholar] [CrossRef]
- Tan, W.; Carlson, D.F.; Lancto, C.A.; Garbe, J.R.; Webster, D.A.; Hackett, P.B.; Fahrenkrug, S.C. Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc. Natl. Acad. Sci. USA 2013, 110, 16526–16531. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Dobrinski, I. Beyond the mouse monopoly: Studying the male germ line in domestic animal models. ILAR J. 2015, 56, 83–98. [Google Scholar] [CrossRef]
- Tang, L.; González, R.; Dobrinski, I. Germline modification of domestic animals. Anim. Reprod. 2015, 12, 93–104. [Google Scholar]
- Whitworth, K.M.; Green, J.A.; Redel, B.K.; Geisert, R.D.; Lee, K.; Telugu, B.P.; Wells, K.D.; Prather, R.S. Improvements in pig agriculture through gene editing. CABI Agric. Biosci. 2022, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, K.R.; Mitchell, R.T. Testicular transplantation for fertility preservation: Clinical potential and current challenges. Reproduction 2019, 158, F1–F14. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.D.; Valli-Pulaski, H.; Colvin, A.; Orwig, K.E. Male fertility preservation and restoration strategies for patients undergoing gonadotoxic therapies. Biol. Reprod. 2022, 107, 382–405. [Google Scholar] [CrossRef]
- Takashima, S.; Shinohara, T. Culture and transplantation of spermatogonial stem cells. Stem Cell Res. 2018, 29, 46–55. [Google Scholar] [CrossRef]
- Binsila, B.; Selvaraju, S.; Ranjithkumaran, R.; Archana, S.S.; Krishnappa, B.; Ghosh, S.K.; Kumar, H.; Subbarao, R.B.; Arangasamy, A.; Bhatta, R. Current scenario and challenges ahead in application of spermatogonial stem cell technology in livestock. J. Assist. Reprod. Genet. 2021, 38, 3155–3173. [Google Scholar] [CrossRef]
- Ogawa, T.; Dobrinski, I.; Avarbock, M.R.; Brinster, R.L. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat. Med. 2000, 6, 29–34. [Google Scholar] [CrossRef]
- Brinster, C.J.; Ryu, B.-Y.; Avarbock, M.R.; Karagenc, L.; Brinster, R.L.; Orwig, K.E. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol. Reprod. 2003, 69, 412–420. [Google Scholar] [CrossRef]
- Honaramooz, A.; Behboodi, E.; Hausler, C.L.; Blash, S.; Ayres, S.; Azuma, C.; Echelard, Y.; Dobrinski, I. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J. Androl. 2005, 26, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Morimoto, H.; Shinohara, T. Fertility of male germline stem cells following spermatogonial transplantation in infertile mouse models. Biol. Reprod. 2016, 94, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Mitchell, J.M.; Meyer, J.M.; Wu, Z.; Lam, T.N.A.; Phan, T.T.; Zhang, J.; Hill, L.; Tailor, R.C.; Peters, K.A.; et al. Restoration of functional sperm production in irradiated pubertal rhesus monkeys by spermatogonial stem cell transplantation. Andrology 2020, 8, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X.; Li, H.; Cai, G.; Yang, H.; Wu, Z. Intratesticular injection of busulfan for producing recipient male pigs for spermatogonial stem cell transplantation. Livest. Sci. 2021, 245, 104448. [Google Scholar] [CrossRef]
- Schlatt, S.; Foppiani, L.; Rolf, C.; Weinbauer, G.F.; Nieschlag, E. Germ cell transplantation into X-irradiated monkey testes. Hum. Reprod. 2002, 17, 55–62. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, S.; Meistrich, M.L. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J. Cell. Physiol. 2007, 211, 149–158. [Google Scholar] [CrossRef]
- Herrid, M.; Olejnik, J.; Jackson, M.; Suchowerska, N.; Stockwell, S.; Davey, R.; Hutton, K.; Hope, S.; Hill, J.R. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol. Reprod. 2009, 81, 898–905. [Google Scholar] [CrossRef]
- Ma, W.; An, L.; Wu, Z.; Wang, X.; Guo, M.; Miao, K.; Ma, W.; Tian, J. Efficient and safe recipient preparation for transplantation of mouse spermatogonial stem cells: Pretreating testes with heat shock. Biol. Reprod. 2011, 85, 670–677. [Google Scholar] [CrossRef][Green Version]
- Rasouli, M.H.; Zandi, M.; Sadeghi, A.A.; Emamjomeh-Kashan, N. Spermatogonial stem cell survival in ram lambs following busulfan treatment. Anim. Reprod. 2020, 17, e20200001. [Google Scholar] [CrossRef]
- Rilianawati, R.; Speed, R.; Taggart, M.; Cooke, H. Spermatogenesis in testes of Dazl null mice after transplantation of wild-type germ cells. Reproduction 2003, 126, 599–604. [Google Scholar] [CrossRef]
- Park, K.-E.; Kaucher, A.V.; Powell, A.; Waqas, M.S.; Sandmaier, S.E.S.; Oatley, M.J.; Park, C.-H.; Tibary, A.; Donovan, D.M.; Blomberg, L.A.; et al. Generation of germline ablated male pigs by CRISPR/Cas9 editing of the NANOS2 gene. Sci. Rep. 2017, 7, 40176. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Giassetti, M.I.; Miao, D.; Oatley, M.J.; Robbins, C.; Lopez-Biladeau, B.; Waqas, M.S.; Tibary, A.; Whitelaw, B.; Lillico, S.; et al. Donor-derived spermatogenesis following stem cell transplantation in sterile NANOS2 knockout males. Proc. Natl. Acad. Sci. USA 2020, 117, 24195–24204. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; VanGompel, M.J.W.; Naeem, V.; Chen, Y.; Lee, T.; Angeloni, N.; Wang, Y.; Xu, E.Y. Widespread presence of human BOULE homologs among animals and conservation of their ancient reproductive function. PLoS Genet. 2010, 6, e1001022. [Google Scholar] [CrossRef] [PubMed]
- VanGompel, M.J.W.; Xu, E.Y. The roles of the DAZ family in spermatogenesis. Spermatogenesis 2011, 1, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-F.; Cheng, S.-F.; Wang, L.-Q.; Yin, S.; De Felici, M.; Shen, W. DAZ family proteins, key players for germ cell development. Int. J. Biol. Sci. 2015, 11, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Ruggiu, M.; Speed, R.; Taggart, M.; McKay, S.J.; Kilanowski, F.; Saunders, P.; Dorin, J.; Cooke, H.J. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 1997, 389, 73–77. [Google Scholar] [CrossRef]
- Lin, Y.; Page, D.C. Dazl deficiency leads to embryonic arrest of germ cell development in XY C57BL/6 mice. Dev. Biol. 2005, 288, 309–316. [Google Scholar] [CrossRef]
- Li, H.; Liang, Z.; Yang, J.; Wang, D.; Wang, H.; Zhu, M.; Geng, B.; Xu, E.Y. DAZL is a master translational regulator of murine spermatogenesis. Natl. Sci. Rev. 2019, 6, 455–468. [Google Scholar] [CrossRef]
- Mikedis, M.M.; Fan, Y.; Nicholls, P.K.; Endo, T.; Jackson, E.K.; Cobb, S.A.; de Rooij, D.G.; Page, D.C. DAZL mediates a broad translational program regulating expansion and differentiation of spermatogonial progenitors. Elife 2020, 9, e56523. [Google Scholar] [CrossRef]
- Saunders, P.; Turner, J.; Ruggiu, M.; Taggart, M.; Burgoyne, P.; Elliott, D.; Cooke, H. Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction 2003, 126, 589–597. [Google Scholar] [CrossRef]
- Reynolds, N.; Collier, B.; Maratou, K.; Bingham, V.; Speed, R.M.; Taggart, M.; Semple, C.A.; Gray, N.K.; Cooke, H.J. Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum. Mol. Genet. 2005, 14, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Kee, K.; Angeles, V.T.; Flores, M.; Nguyen, H.N.; Reijo Pera, R.A. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 2009, 462, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.E.; Hu, Y.-C.; Lin, Y.; Page, D.C. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7443–7448. [Google Scholar] [CrossRef]
- Nicholls, P.K.; Schorle, H.; Naqvi, S.; Hu, Y.-C.; Fan, Y.; Carmell, M.A.; Dobrinski, I.; Watson, A.L.; Carlson, D.F.; Fahrenkrug, S.C.; et al. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 2019, 116, 25677–25687. [Google Scholar] [CrossRef]
- Reijo, R.; Lee, T.-Y.; Salo, P.; Alagappan, R.; Brown, L.G.; Rosenberg, M.; Rozen, S.; Jaffe, T.; Straus, D.; Hovatta, O.; et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA–binding protein gene. Nat. Genet. 1995, 10, 383–393. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, C.W.; Sun, H.S.; Tsai, S.J.; Hsu, C.C.; Teng, Y.N.; Lin, J.S.; Kuo, P.L. Expression patterns and transcript concentrations of the autosomal DAZL gene in testes of azoospermic men. Mol. Hum. Reprod. 2001, 7, 1015–1022. [Google Scholar] [CrossRef]
- Tung, J.Y.; Rosen, M.P.; Nelson, L.M.; Turek, P.J.; Witte, J.S.; Cramer, D.W.; Cedars, M.I.; Reijo-Pera, R.A. Novel missense mutations of the Deleted-in-AZoospermia-Like (DAZL) gene in infertile women and men. Reprod. Biol. Endocrinol. 2006, 4, 40. [Google Scholar] [CrossRef]
- Nailwal, M.; Chauhan, J.B. In silico analysis of non-synonymous single nucleotide polymorphisms in human DAZL gene associated with male infertility. Syst. Biol. Reprod. Med. 2017, 63, 248–258. [Google Scholar] [CrossRef]
- Schrans-Stassen, B.H.G.J.; Saunders, P.T.K.; Cooke, H.J.; de Rooij, D.G. Nature of the spermatogenic arrest in Dazl−/− mice. Biol. Reprod. 2001, 65, 771–776. [Google Scholar] [CrossRef]
- Maratou, K.; Forster, T.; Costa, Y.; Taggart, M.; Speed, R.M.; Ireland, J.; Teague, P.; Roy, D.; Cooke, H.J. Expression profiling of the developing testis in wild-type and Dazl knockout mice. Mol. Reprod. Dev. 2004, 67, 26–54. [Google Scholar] [CrossRef]
- McLean, Z.L.; Appleby, S.J.; Wei, J.; Snell, R.G.; Oback, B. Testes of DAZL null neonatal sheep lack prospermatogonia but maintain normal somatic cell morphology and marker expression. Mol. Reprod. Dev. 2021, 88, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Tang, L.; Bondareva, A.; Honaramooz, A.; Tanco, V.; Dores, C.; Megee, S.; Modelski, M.; Rodriguez-Sosa, J.R.; Paczkowski, M.; et al. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol. Reprod. 2013, 88, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bondareva, A.; González, R.; Rodriguez-Sosa, J.R.; Carlson, D.F.; Webster, D.; Fahrenkrug, S.; Dobrinski, I. TALEN-mediated gene targeting in porcine spermatogonia. Mol. Reprod. Dev. 2018, 85, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Avelar, G.F.; Oliveira, C.F.A.; Soares, J.M.; Silva, I.J.; Dobrinski, I.; Hess, R.A.; França, L.R. Postnatal somatic cell proliferation and seminiferous tubule maturation in pigs: A non-random event. Theriogenology 2010, 74, 11–23. [Google Scholar] [CrossRef]
- Webster, D.; Bondareva, A.; Solin, S.; Goldsmith, T.; Su, L.; Lara, N.L.M.; Carlson, D.F.; Dobrinski, I. Targeted gene editing in porcine spermatogonia. Front. Genet. 2021, 11, 1861. [Google Scholar] [CrossRef]
- Shinohara, T.; Orwig, K.E.; Avarbock, M.R.; Brinster, R.L. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc. Natl. Acad. Sci. USA 2001, 98, 6186–6191. [Google Scholar] [CrossRef]
- Mikkola, M.; Sironen, A.; Kopp, C.; Taponen, J.; Sukura, A.; Vilkki, J.; Katila, T.; Andersson, M. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod. Domest. Anim. 2006, 41, 124–128. [Google Scholar] [CrossRef]
- Mutoji, K.N.; Hermann, B.P. Defining the phenotype and function of mammalian spermatogonial stem cells. In The Biology of Mammalian Spermatogonia; Oatley, J., Griswold, M., Eds.; Springer: New York, NY, USA, 2017; pp. 67–90. [Google Scholar]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef]
- Potter, S.J.; DeFalco, T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 2017, 153, R151–R162. [Google Scholar] [CrossRef]
- Van Saen, D.; Goossens, E. Testicular Tissue Transplantation. In Female and Male Fertility Preservation; Grynberg, M., Patrizio, P., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 529–554. [Google Scholar]
- França, L.R.; Auharek, S.A.; Hess, R.A.; Dufour, J.M.; Hinton, B.T. Blood-tissue barriers. In Biology and Regulation of Blood Tissue Barriers; Cheng, C.Y., Ed.; Landes Bioscience and Springer Science: Berlin/Heidelberg, Germany, 2012; pp. 237–259. [Google Scholar]
- Shetty, G.; Wu, Z.; Lam, T.N.A.; Phan, T.T.; Orwig, K.E.; Meistrich, M.L. Effect of hormone modulations on donor-derived spermatogenesis or colonization after syngeneic and xenotransplantation in mice. Andrology 2019, 7, 257–265. [Google Scholar] [CrossRef]
| Recipient ID | Donor Breed | Donor Genetics | GCs Injected (million) | Peak Sperm Concentration/mL | Sperm Genotype |
|---|---|---|---|---|---|
| 2908 | Yorkshire | WT | 50 | 6 × 106 | Donor |
| 2906 | Yorkshire | Edited | 50 | 4.2 × 106 | Donor |
| 3412 | Yorkshire | Pooled edited | 137.75 | Low | Donor |
| 2615 | Cross | WT | 122 | Low | Donor |
| 3327 | Cross | WT | 70 | Low | Donor |
| 3346 | Cross | WT | 50 | 2.5 × 105 | Donor |
| 3348 | Cross | WT | 70 | 5 × 104 | Donor |
| 3661 | Cross | Pooled WT | 68 | 5.4 × 106 | ND |
| 2616 | Cross | Edited | 93 | Low | Donor |
| 3234 | Cross | Edited | 50 | 5 × 104 | ND |
| 3238 | Cross | Edited | 50 | 4 × 105 | Donor |
| 3249 | Ossabaw | WT | 26.46 | 0 | ND |
| 2626 | Ossabaw | Pooled WT | 34.2 | Low | Donor |
| 2988 | Ossabaw | Pooled WT | 50 | 7.6 × 106 | Donor |
| 3719 | Ossabaw | Edited | 39.25 | 7.3 × 106 | Donor |
| 3310 | Ossabaw | Pooled edited | 70 | 0 | ND |
| 5043 | Ossabaw | Pooled edited | 46.4 | 1.7 × 105 | ND |
| 5047 | Ossabaw | Pooled edited | 46.4 | 3 × 106 | Donor |
| 1 wko | Adults | |||||
|---|---|---|---|---|---|---|
| WT | Het | KO | WT | Het | KO | |
| Seminiferous tubules | 51.6 ± 4.7 | 36.2 ± 0.9 | 39.3 ± 2.5 | 83.4 ± 3.5 a | 66.1 ± 4.6 b | 58.4 ± 2.6 b |
| Seminiferous epithelium | 27.6 ± 2.4 | 25.5 ± 1.1 | 26.2 ± 1.9 | 59.9 ± 2.9 a | 46.4 ± 1.3 b | 33.1 ± 2.3 c |
| Germ cells | 6.8 ± 0.7 a | 0.8 ± 0.1 b | 0.2 ± 0.1 b | - | - | - |
| Tubular lumen | - | - | - | 17.5 ± 1.2 | 11.3 ± 4.2 | 16.1 ± 1.5 |
| Tunica propria | 17.2 ± 1.9 | 9.9 ± 0.1 | 12.9 ± 0.7 | 6.0 ± 0.5 a | 8.4 ± 0.8 ab | 9.2 ± 0.9 b |
| Intertubular compartment | 48.3 ± 4.7 | 63.8 ± 0.9 | 60.7 ± 2.5 | 16.6 ± 3.5 a | 33.9 ± 4.6 b | 41.6 ± 2.6 b |
| Leydig cells | 19.6 ± 2.9 | 30.7 ± 0.9 | 24.3 ± 1.0 | 9.2 ± 1.6 a | 16.9 ± 2.8 ab | 22.8 ± 1.4 b |
| Connective tissue | 14.4 ± 0.4 a | 21.7 ± 0.3 b | 24.2 ± 1.5 b | 4.0 ± 0.9 a | 10.2 ± 1.4 b | 11.6 ± 1.4 b |
| Blood vessels | 14.3 ± 1.7 | 11.4 ± 0.3 | 12.2 ± 2.9 | 3.4 ± 1.2 | 6.8 ± 0.4 | 7.2 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, N.L.M.; Goldsmith, T.; Rodriguez-Villamil, P.; Ongaratto, F.; Solin, S.; Webster, D.; Ganbaatar, U.; Hodgson, S.; Corbière, S.M.A.S.; Bondareva, A.; et al. DAZL Knockout Pigs as Recipients for Spermatogonial Stem Cell Transplantation. Cells 2023, 12, 2582. https://doi.org/10.3390/cells12212582
Lara NLM, Goldsmith T, Rodriguez-Villamil P, Ongaratto F, Solin S, Webster D, Ganbaatar U, Hodgson S, Corbière SMAS, Bondareva A, et al. DAZL Knockout Pigs as Recipients for Spermatogonial Stem Cell Transplantation. Cells. 2023; 12(21):2582. https://doi.org/10.3390/cells12212582
Chicago/Turabian StyleLara, Nathalia L. M., Taylor Goldsmith, Paula Rodriguez-Villamil, Felipe Ongaratto, Staci Solin, Dennis Webster, Uyanga Ganbaatar, Shane Hodgson, Stanislas M. A. S. Corbière, Alla Bondareva, and et al. 2023. "DAZL Knockout Pigs as Recipients for Spermatogonial Stem Cell Transplantation" Cells 12, no. 21: 2582. https://doi.org/10.3390/cells12212582
APA StyleLara, N. L. M., Goldsmith, T., Rodriguez-Villamil, P., Ongaratto, F., Solin, S., Webster, D., Ganbaatar, U., Hodgson, S., Corbière, S. M. A. S., Bondareva, A., Carlson, D. F., & Dobrinski, I. (2023). DAZL Knockout Pigs as Recipients for Spermatogonial Stem Cell Transplantation. Cells, 12(21), 2582. https://doi.org/10.3390/cells12212582






