Carboxylated Poly-L-lysine Potentially Reduces Human Sperm DNA Fragmentation after Freeze-Thawing, and Its Function Is Enhanced by Low-Dose Resveratrol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Cryopreservation Reagents
2.3. Sperm Preparation, Freezing, and Thawing
2.4. SDF Analysis Using the TUNEL Assay
2.5. Evaluation of ROS in Mitochondria and Living Sperm
2.6. Assessment of Lipid Peroxidation
2.7. Assessment of Mitochondrial Membrane Potential
2.8. Statistical Analysis
3. Results
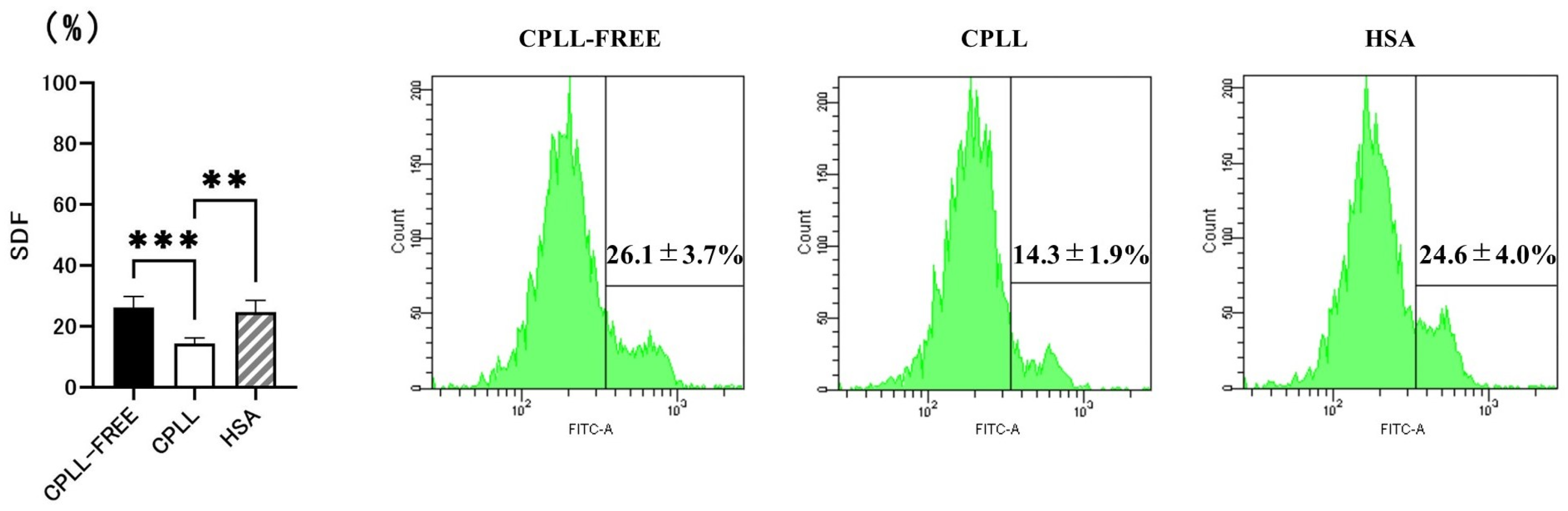
3.1. Effects of CPLL on SDF
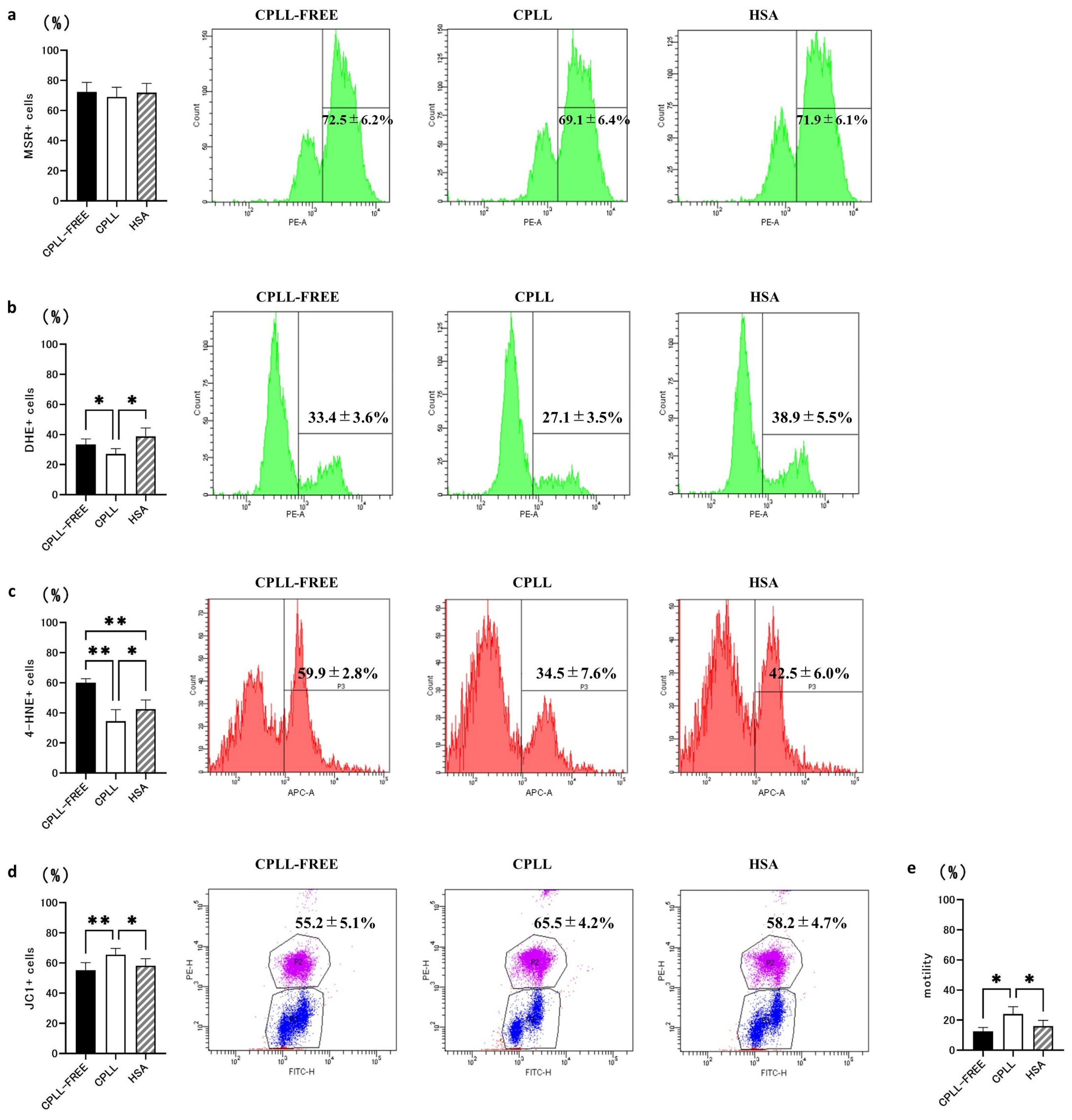
3.2. Effects of CPLL on ROS Levels, LPO, MMP, and Motility Rate
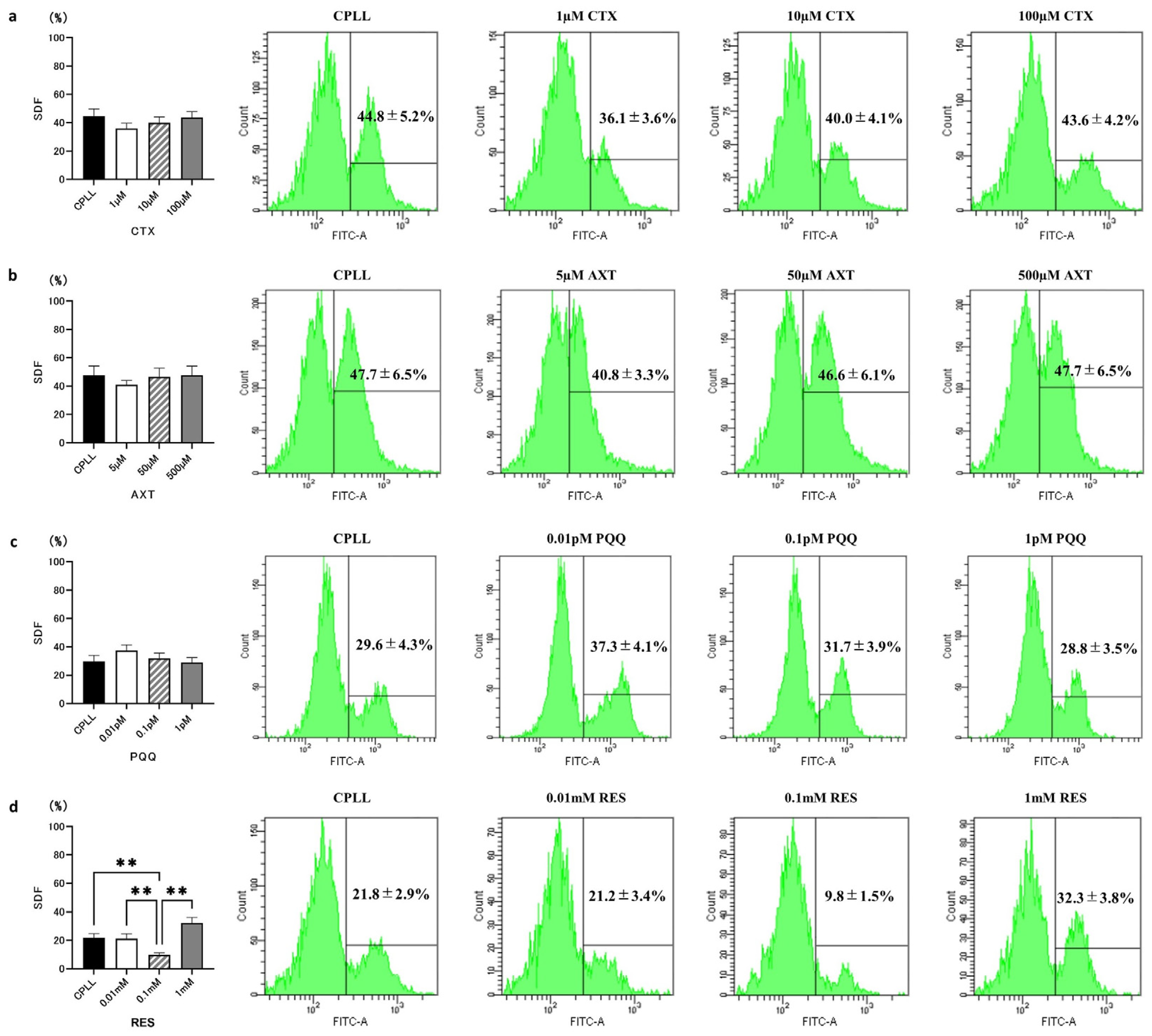
3.3. Effects of Different Antioxidant Compounds on SDF
3.4. Effects of RES on ROS Levels, LPO, MMP, and Motility Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pukazhenthi, B.; Comizzoli, P.; Travis, A.J.; Wildt, D.E. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod. Fertil. Dev. 2006, 18, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Wildt, D.E. Mammalian fertility preservation through cryobiology: Value of classical comparative studies and the need for new preservation options. Reprod. Fertil. Dev. 2013, 26, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H.; Dohle, G.R.; Barratt, C.L. Fertility preservation in men with cancer. Lancet 2014, 384, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, S.; Filimberti, E.; Magini, A.; Krausz, C.; Lombardi, G.; Fino, M.G.; Rastrelli, G.; Maggi, M.; Baldi, E. Semen cryopreservation for men banking for oligospermia, cancers, and other pathologies: Prediction of post-thaw outcome using basal semen quality. Fertil. Steril. 2013, 100, 1555–1563.e3. [Google Scholar] [CrossRef] [PubMed]
- Pedro, P.B.; Zhu, S.E.; Makino, N.; Sakurai, T.; Edashige, K.; Kasai, M. Effects of hypotonic stress on the survival of mouse oocytes and embryos at various stages. Cryobiology 1997, 35, 150–158. [Google Scholar] [CrossRef]
- Graham, E.F.; Crabo, B.G.; Brown, K.I. Effect of some Zwitter ion buffers on the freezing and storage of spermatozoa I. Bull. J. Dairy Sci. 1972, 55, 372–378. [Google Scholar] [CrossRef]
- Prins, G.S.; Weidel, L. A comparative study of buffer systems as cryoprotectants for human spermatozoa. Fertil. Steril. 1986, 46, 147–149. [Google Scholar] [CrossRef]
- Larson, J.M.; McKinney, K.A.; Mixon, B.A.; Burry, K.A.; Wolf, D.P. An intrauterine insemination-ready cryopreservation method compared with sperm recovery after conventional freezing and post-thaw processing. Fertil. Steril. 1997, 68, 143–148. [Google Scholar] [CrossRef]
- Takeuchi, H.; Nishioka, M.; Maezawa, T.; Kitano, Y.; Terada-Yoshikawa, K.; Tachibana, R.; Kato, M.; Hyon, S.H.; Gen, Y.; Tanaka, K.; et al. Carboxylated poly-l-lysine as a macromolecular cryoprotective agent enables the development of defined and Xeno-free human sperm cryopreservation reagents. Cells 2021, 10, 1435. [Google Scholar] [CrossRef]
- Matsumura, K.; Hyon, S.H. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials 2009, 30, 4842–4849. [Google Scholar] [CrossRef]
- Kamoshita, M.; Kato, T.; Fujiwara, K.; Namiki, T.; Matsumura, K.; Hyon, S.H.; Ito, J.; Kashiwazaki, N. Successful vitrification of pronuclear-stage pig embryos with a novel cryoprotective agent, carboxylated ε-poly-L-lysine. PLoS ONE 2017, 12, e0176711. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Ando, T.; Gen, Y.; Hyon, S.H.; Kubota, C. Cryopreservation of bovine somatic cells using antifreeze polyamino-acid (carboxylated poly-l-lysine). Cryobiology 2017, 76, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Bae, J.Y.; Kim, H.H.; Hyon, S.H. Effective vitrification of human induced pluripotent stem cells using carboxylated ε-poly-l-lysine. Cryobiology 2011, 63, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Siddhartha, N.; Reddy, N.S.; Pandurangi, M.; Muthusamy, T.; Vembu, R.; Kasinathan, K. The effect of sperm DNA fragmentation index on the outcome of intrauterine insemination and intracytoplasmic sperm injection. J. Hum. Reprod. Sci. 2019, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Borges, E., Jr.; Zanetti, B.F.; Setti, A.S.; Braga, D.P.A.F.; Provenza, R.R.; Iaconelli, A., Jr. Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non–male factor infertility. Fertil. Steril. 2019, 112, 483–490. [Google Scholar] [CrossRef]
- Sivanarayana, T.; Ravi Krishna, C.; Jaya Prakash, G.; Krishna, K.M.; Madan, K.; Sudhakar, G.; Rama Raju, G.A. Sperm DNA fragmentation assay by sperm chromatin dispersion (SCD): Correlation between DNA fragmentation and outcome of intracytoplasmic sperm injection. Reprod. Med. Biol. 2013, 13, 87–94. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular alterations in spermatozoa of a family case living in the land of fires—A first look at possible transgenerational effects of pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Pozzi, E.; Fallara, G.; Belladelli, F.; Corsini, C.; Raffo, M.; Candela, L.; Schifano, N.; d’Arma, A.; Capogrosso, P.; Boeri, L.; et al. Clinical parameters associated with altered sperm DNA fragmentation index among primary infertile men: Findings from a real-life cross-sectional study. Andrology 2023, 11, 1694–1701. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Fernández-Encinas, A.; García-Peiró, A.; Prada, E.; Abad, C.; Amengual, M.J.; Navarro, J.; Benet, J. Human semen cryopreservation: A sperm DNA fragmentation study with alkaline and neutral Comet assay. Andrology 2014, 2, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Zribi, N.; Feki Chakroun, N.; El Euch, H.; Gargouri, J.; Bahloul, A.; Ammar Keskes, L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Founders’ lecture. Human spermatozoa: Fruits of creation, seeds of doubt. Reprod. Fertil. Dev. 2004, 16, 655–664. [Google Scholar] [CrossRef]
- Sikka, S.C. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001, 8, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Bathgate, R. Antioxidant mechanisms and their benefit on post-thaw boar sperm quality. Reprod. Domest. Anim. 2011, 46 (Suppl. S2), 23–25. [Google Scholar] [CrossRef]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef]
- Najafi, L.; Halvaei, I.; Movahedin, M. Canthaxanthin protects human sperm parameters during cryopreservation. Andrologia. 2019, 51, e13389. [Google Scholar] [CrossRef]
- Taylor, K.; Roberts, P.; Sanders, K.; Burton, P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online 2009, 18, 184–189. [Google Scholar] [CrossRef]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.A.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Silva, E.C.; Cajueiro, J.F.; Silva, S.V.; Soares, P.C.; Guerra, M.M. Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology 2012, 77, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, D. Effects of astaxanthin on miniature pig sperm cryopreservation. BioMed. Res. Int. 2018, 2018, 6784591. [Google Scholar] [CrossRef]
- Branco, C.S.; Garcez, M.E.; Pasqualotto, F.F.; Erdtman, B.; Salvador, M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 2010, 60, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L.; Barratt, C.L.; Mortimer, D.; Jouannet, P. “How to count sperm properly”: Checklist for acceptability of studies based on human semen analysis. Hum. Reprod. 2016, 31, 227–232. [Google Scholar] [CrossRef]
- Sharma, R.; Ahmad, G.; Esteves, S.C.; Agarwal, A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: Protocol, reference values, and quality control. J. Assist. Reprod. Genet. 2016, 33, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Bioenergetics 2018, 9, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Soufir, J.C.; Martin, M.; David, G. Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res. 1989, 24, 127–134. [Google Scholar] [CrossRef]
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef]
- Plaza Davila, M.; Martin Muñoz, P.; Tapia, J.A.; Ortega Ferrusola, C.; Balao Da Silva, C.; Peña, F.J. Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Bae, J.Y.; Hyon, S.H. Polyampholytes as cryoprotective agents for mammalian cell cryopreservation. Cell. Transplant. 2010, 19, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The role of resveratrol in mammalian reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, V.; Zullo, G.; Salzano, A.; De Canditiis, C.; Cammarano, A.; De Luise, L.; Puzio, M.V.; Neglia, G.; Gasparrini, B. Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology 2017, 88, 1–8. [Google Scholar] [CrossRef]
- Stojanović, S.; Sprinz, H.; Brede, O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch. Biochem. Biophys. 2001, 391, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Kuno, K.; Horio, Y. Cellular effects of resveratrol in health and disease: Roles of SIRT1. J. Jpn. Biochem. Soc. 2021, 93, 100–108. [Google Scholar]
- Shabani Nashtaei, M.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5′ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef]
- Meamar, M.; Zribi, N.; Cambi, M.; Tamburrino, L.; Marchiani, S.; Filimberti, E.; Fino, M.G.; Biggeri, A.; Menezo, Y.; Forti, G.; et al. Sperm DNA fragmentation induced by cryopreservation: New insights and effect of a natural extract from Opuntia ficus-indica. Fertil. Steril. 2012, 98, 326–333. [Google Scholar] [CrossRef]
- Bucak, M.N.; Ataman, M.B.; Başpınar, N.; Uysal, O.; Taşpınar, M.; Bilgili, A.; Öztürk, C.; Güngör, Ş.; İnanç, M.E.; Akal, E. Lycopene and resveratrol improve post-thaw bull sperm parameters: Sperm motility, mitochondrial activity and DNA integrity. Andrologia 2015, 47, 545–552. [Google Scholar] [CrossRef]
- Fujikawa, T.; Imamura, S.; Tokumaru, M.; Ando, T.; Gen, Y.; Hyon, S.H.; Kubota, C. Cryoprotective effect of antifreeze polyamino-acid (Carboxylated Poly-l-lysine) on bovine sperm: A technical note. Cryobiology 2018, 82, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Awan, M.A.; Arshad, J.; Rakha, B.A.; Ansari, M.S.; Iqbal, S. Effect of synergism between carboxylated poly-l-lysine and glycerol on freezability of Nili-Ravi buffalo (Bubalus bubalis) Semen. Biopreserv. Biobank. 2020, 18, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Esmeryan, K.D.; Lazarov, Y.; Stamenov, G.S.; Chaushev, T.A. When condensed matter physics meets biology: Does superhydrophobicity benefiting the cryopreservation of human spermatozoa? Cryobiology 2019, 92, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Esmeryan, K.D.; Fedchenko, Y.I.; Gyoshev, S.D.; Lazarov, Y.; Chaushev, T.A.; Grakov, T. On the development of ultradurable extremely water-repellent and oleophobic soot-based fabrics with direct relevance to sperm cryopreservation. ACS Appl. Bio. Mater. 2022, 5, 3519–3529. [Google Scholar] [CrossRef] [PubMed]

| Reagents | Components | Statistical Analysis | |
|---|---|---|---|
| Components of cryopreservation reagents containing CPLL | CPLL | 0.3% w/v CPLL + 7% v/v glycerol + 0.1 mM raffinose | Tukey’s multiple comparing test (n = 10) |
| CPLL-FREE | 7% v/v glycerol + 0.1 mM raffinose | ||
| HSA | 5% v/v HSA + 7% v/v glycerol + 0.1 mM raffinose | ||
| Antioxidant compounds added to cryopreservation reagents | CTX | 0.3% w/v CPLL + 7% v/v glycerol + 0.1 mM raffinose + CTX (1 µM, 10 µM, 100 µM) | |
| AXT | 0.3% w/v CPLL + 7% v/v glycerol + 0.1 mM raffinose + AXT (5 µM, 50 µM, 500 µM) | ||
| PQQ | 0.3% w/v CPLL + 7% v/v glycerol + 0.1 mM raffinose + PQQ (0.01 pM, 0.1 pM, 1 pM) | ||
| RES | 0.3% w/v CPLL + 7% v/v glycerol + 0.1 mM raffinose + RES (0.01 mM, 0.1 mM, 1 mM) | ||
| Composition of RES and CPLL cryopreservation reagents | CPLL | 0.3% w/v CPLL + 7% v/v glycerol + 0.1 mM raffinose | Paired-sample t-test (n = 10) |
| RES | 0.3% w/v CPLL + 7% v/v glycerol + 0.1 mM raffinose + 0.1 mM RES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, R.; Takeuchi, H.; Yoshikawa-Terada, K.; Maezawa, T.; Nishioka, M.; Takayama, E.; Tanaka, H.; Tanaka, K.; Hyon, S.-h.; Gen, Y.; et al. Carboxylated Poly-L-lysine Potentially Reduces Human Sperm DNA Fragmentation after Freeze-Thawing, and Its Function Is Enhanced by Low-Dose Resveratrol. Cells 2023, 12, 2585. https://doi.org/10.3390/cells12222585
Tachibana R, Takeuchi H, Yoshikawa-Terada K, Maezawa T, Nishioka M, Takayama E, Tanaka H, Tanaka K, Hyon S-h, Gen Y, et al. Carboxylated Poly-L-lysine Potentially Reduces Human Sperm DNA Fragmentation after Freeze-Thawing, and Its Function Is Enhanced by Low-Dose Resveratrol. Cells. 2023; 12(22):2585. https://doi.org/10.3390/cells12222585
Chicago/Turabian StyleTachibana, Ryota, Hiroki Takeuchi, Kento Yoshikawa-Terada, Tadashi Maezawa, Mikiko Nishioka, Erina Takayama, Hiroaki Tanaka, Kayo Tanaka, Suong-hyu Hyon, Yuki Gen, and et al. 2023. "Carboxylated Poly-L-lysine Potentially Reduces Human Sperm DNA Fragmentation after Freeze-Thawing, and Its Function Is Enhanced by Low-Dose Resveratrol" Cells 12, no. 22: 2585. https://doi.org/10.3390/cells12222585






