Genomic Profiling and Clinical Outcomes of Targeted Therapies in Adult Patients with Soft Tissue Sarcomas
Abstract
:1. Introduction
2. Methods
3. Studies Reporting Sequencing Results Leading to Targeted Therapy Implementation across STS Histotypes Containing Efficacy Data
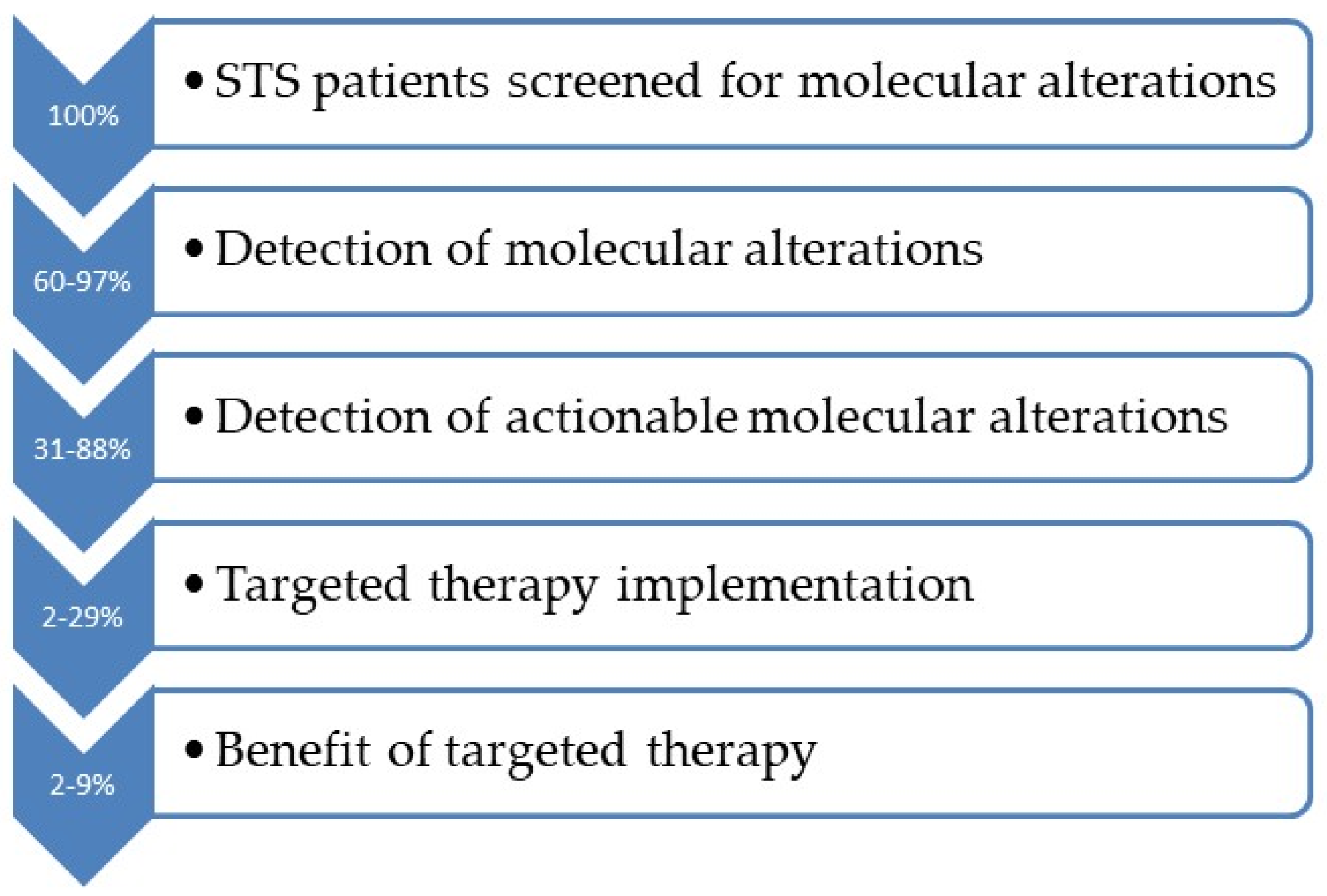
4. Occurrence of Clinically Actionable Genomic Abnormalities in Patients with STS
5. Only a Small Proportion of Patients Receive Matched Therapy
6. Efficacy Results of Targeted Therapies in Soft Tissue Sarcomas Patients
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and Perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.P.; Mytelka, D.S.; Candrilli, S.D.; D’yachkova, Y.; Lorenzo, M.; Kasper, B.; Lopez-Martin, J.A.; Kaye, J.A. Treatment Patterns and Survival among Adult Patients with Advanced Soft Tissue Sarcoma: A Retrospective Medical Record Review in the United Kingdom, Spain, Germany, and France. Sarcoma 2018, 2018, 5467057. [Google Scholar] [CrossRef]
- Italiano, A.; Di Mauro, I.; Rapp, J.; Pierron, G.; Auger, N.; Alberti, L.; Chibon, F.; Escande, F.; Voegeli, A.-C.; Ghnassia, J.-P.; et al. Clinical Effect of Molecular Methods in Sarcoma Diagnosis (GENSARC): A Prospective, Multicentre, Observational Study. Lancet Oncol. 2016, 17, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Ducimetière, F.; Lurkin, A.; Ranchère-Vince, D.; Decouvelaere, A.-V.; Péoc’h, M.; Istier, L.; Chalabreysse, P.; Muller, C.; Alberti, L.; Bringuier, P.-P.; et al. Incidence of Sarcoma Histotypes and Molecular Subtypes in a Prospective Epidemiological Study with Central Pathology Review and Molecular Testing. PLoS ONE 2011, 6, e20294. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A.; Villalobos, V.M. Emerging Role for Precision Therapy Through Next-Generation Sequencing for Sarcomas. JCO Precis. Oncol. 2018, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, C.; Khalifa, E.; Laizet, Y.; Soubeyran, I.; Mathoulin-Pelissier, S.; Chomienne, C.; Italiano, A. Targetable Alterations in Adult Patients With Soft-Tissue Sarcomas: Insights for Personalized Therapy. JAMA Oncol. 2018, 4, 1398–1404. [Google Scholar] [CrossRef]
- Boddu, S.; Walko, C.M.; Bienasz, S.; Bui, M.M.; Henderson-Jackson, E.; Naghavi, A.O.; Mullinax, J.E.; Joyce, D.M.; Binitie, O.; Letson, G.D.; et al. Clinical Utility of Genomic Profiling in the Treatment of Advanced Sarcomas: A Single-Center Experience. JCO Precis. Oncol. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Gusho, C.A.; Weiss, M.C.; Lee, L.; Gitelis, S.; Blank, A.T.; Wang, D.; Batus, M. The Clinical Utility of Next-Generation Sequencing for Bone and Soft Tissue Sarcoma. Acta Oncol. 2022, 61, 38–44. [Google Scholar] [CrossRef]
- Elvin, J.A.; Gay, L.M.; Ort, R.; Shuluk, J.; Long, J.; Shelley, L.; Lee, R.; Chalmers, Z.R.; Frampton, G.M.; Ali, S.M.; et al. Clinical Benefit in Response to Palbociclib Treatment in Refractory Uterine Leiomyosarcomas with a Common CDKN2A Alteration. Oncologist 2017, 22, 416–421. [Google Scholar] [CrossRef]
- Groisberg, R.; Hong, D.S.; Holla, V.; Janku, F.; Piha-Paul, S.; Ravi, V.; Benjamin, R.; Kumar Patel, S.; Somaiah, N.; Conley, A.; et al. Clinical Genomic Profiling to Identify Actionable Alterations for Investigational Therapies in Patients with Diverse Sarcomas. Oncotarget 2017, 8, 39254–39267. [Google Scholar] [CrossRef]
- Massoth, L.R.; Hung, Y.P.; Ferry, J.A.; Hasserjian, R.P.; Nardi, V.; Nielsen, G.P.; Sadigh, S.; Venkataraman, V.; Selig, M.; Friedmann, A.M.; et al. Histiocytic and Dendritic Cell Sarcomas of Hematopoietic Origin Share Targetable Genomic Alterations Distinct from Follicular Dendritic Cell Sarcoma. Oncologist 2021, 26, e1263–e1272. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Agaram, N.P.; Trabucco, S.E.; Robinson, V.; Ferraro, R.A.; Millis, S.Z.; Krishnan, A.; Lee, J.; Attia, S.; Abida, W.; et al. Clinical Genomic Profiling in the Management of Patients with Soft Tissue and Bone Sarcoma. Nat. Commun. 2022, 13, 3406. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Wang, C.; Jia, D.; Qian, W.; Yin, C.; Wang, D.; Yang, Q.; Li, T.; Zheng, A. Next Generation Sequencing Reveals Pathogenic and Actionable Genetic Alterations of Soft Tissue Sarcoma in Chinese Patients: A Single Center Experience. Technol. Cancer Res. Treat. 2021, 20, 15330338211068964. [Google Scholar] [CrossRef]
- Lopes-Brás, R.; Lopez-Presa, D.; Esperança-Martins, M.; Melo-Alvim, C.; Gallego, L.; Costa, L.; Fernandes, I. Genomic Profiling of Sarcomas: A Promising Weapon in the Therapeutic Arsenal. Int. J. Mol. Sci. 2022, 23, 14227. [Google Scholar] [CrossRef] [PubMed]
- Painter, C.A.; Jain, E.; Tomson, B.N.; Dunphy, M.; Stoddard, R.E.; Thomas, B.S.; Damon, A.L.; Shah, S.; Kim, D.; Gómez Tejeda Zañudo, J.; et al. The Angiosarcoma Project: Enabling Genomic and Clinical Discoveries in a Rare Cancer through Patient-Partnered Research. Nat. Med. 2020, 26, 181–187. [Google Scholar] [CrossRef]
- Morfouace, M.; Horak, P.; Kreutzfeldt, S.; Stevovic, A.; de Rojas, T.; Denisova, E.; Hutter, B.; Bautista, F.; Oliveira, J.; Defachelles, A.-S.; et al. Comprehensive Molecular Profiling of Sarcomas in Adolescent and Young Adult Patients: Results of the EORTC SPECTA-AYA International Proof-of-Concept Study. Eur. J. Cancer 2023, 178, 216–226. [Google Scholar] [CrossRef]
- Horak, P.; Heining, C.; Kreutzfeldt, S.; Hutter, B.; Mock, A.; Hüllein, J.; Fröhlich, M.; Uhrig, S.; Jahn, A.; Rump, A.; et al. Comprehensive Genomic and Transcriptomic Analysis for Guiding Therapeutic Decisions in Patients with Rare Cancers. Cancer Discov. 2021, 11, 2780–2795. [Google Scholar] [CrossRef]
- Brahmi, M.; Tredan, O.; Penel, N.; Chevreau, C.; Perrin, C.; Firmin, N.; Bompas, E.; Bertucci, F.; Attignon, V.; Saintigny, P.; et al. Large versus Limited Molecular Profiling Panel Screening Program in Patients with Metastatic Sarcoma: An Exploratory Subgroup Analysis from the ProfiLER 02 Trial. J. Clin. Oncol. 2023, 41, 11545. [Google Scholar] [CrossRef]
- Arnaud-Coffin, P.; Brahmi, M.; Vanacker, H.; Eberst, L.; Tredan, O.; Attignon, V.; Pissaloux, D.; Sohier, E.; Cassier, P.; Garin, G.; et al. Therapeutic Relevance of Molecular Screening Program in Patients with Metastatic Sarcoma: Analysis from the ProfiLER 01 Trial. Transl. Oncol. 2020, 13, 100870. [Google Scholar] [CrossRef]
- Saijo, K.; Imai, H.; Katayama, H.; Fujishima, F.; Nakamura, K.; Kasahara, Y.; Ouchi, K.; Komine, K.; Shirota, H.; Takahashi, M.; et al. BRAF and MEK Inhibitor Treatment for Metastatic Undifferentiated Sarcoma of the Spermatic Cord with BRAF V600E Mutation. Case Rep. Oncol. 2022, 15, 762–769. [Google Scholar] [CrossRef]
- Saller, J.; Walko, C.M.; Millis, S.Z.; Henderson-Jackson, E.; Makanji, R.; Brohl, A.S. Response to Checkpoint Inhibitor Therapy in Advanced Classic Kaposi Sarcoma: A Case Report and Immunogenomic Study. J. Natl. Compr. Cancer Netw. 2018, 16, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; McMahon, C.; Patel, S.; Zinner, R.; Silva, E.G.; Elvin, J.A.; Subbiah, I.M.; Ohaji, C.; Ganeshan, D.M.; Anand, D.; et al. STUMP Un”stumped”: Anti-Tumor Response to Anaplastic Lymphoma Kinase (ALK) Inhibitor Based Targeted Therapy in Uterine Inflammatory Myofibroblastic Tumor with Myxoid Features Harboring DCTN1-ALK Fusion. J. Hematol. Oncol. 2015, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Recine, F.; De Vita, A.; Fausti, V.; Pieri, F.; Bongiovanni, A.; Franchini, E.; Casadei, R.; Falasconi, M.C.; Oboldi, D.; Matteucci, F.; et al. Case Report: Adult NTRK-Rearranged Spindle Cell Neoplasm: Early Tumor Shrinkage in a Case With Bone and Visceral Metastases Treated With Targeted Therapy. Front. Oncol. 2022, 11, 740676. [Google Scholar] [CrossRef]
- Eder, J.P.; Doroshow, D.B.; Do, K.T.; Keedy, V.L.; Sklar, J.S.; Glazer, P.; Bindra, R.; Shapiro, G.I. Clinical Efficacy of Olaparib in IDH1/IDH2-Mutant Mesenchymal Sarcomas. JCO Precis. Oncol. 2021, 5, 466–472. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via Diverse Exon 14 Splicing Alterations Occurs in Multiple Tumor Types and Confers Clinical Sensitivity to MET Inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Taylor, B.S.; Banerji, S.; Ramos, A.H.; Lagos-Quintana, M.; Decarolis, P.L.; Shah, K.; Socci, N.D.; Weir, B.A.; Ho, A.; et al. Subtype-Specific Genomic Alterations Define New Targets for Soft-Tissue Sarcoma Therapy. Nat. Genet. 2010, 42, 715–721. [Google Scholar] [CrossRef]
- Cote, G.M.; He, J.; Choy, E. Next-Generation Sequencing for Patients with Sarcoma: A Single Center Experience. Oncologist 2018, 23, 234–242. [Google Scholar] [CrossRef]
- Xu, L.; Xie, X.; Shi, X.; Zhang, P.; Liu, A.; Wang, J.; Zhang, B. Potential Application of Genomic Profiling for the Diagnosis and Treatment of Patients with Sarcoma. Oncol. Lett. 2021, 21, 353. [Google Scholar] [CrossRef]
- Brohl, A.S.; Solomon, D.A.; Chang, W.; Wang, J.; Song, Y.; Sindiri, S.; Patidar, R.; Hurd, L.; Chen, L.; Shern, J.F.; et al. The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent STAG2 Mutation. PLoS Genet. 2014, 10, e1004475. [Google Scholar] [CrossRef]
- Brohl, A.S.; Kahen, E.; Yoder, S.J.; Teer, J.K.; Reed, D.R. The Genomic Landscape of Malignant Peripheral Nerve Sheath Tumors: Diverse Drivers of Ras Pathway Activation. Sci. Rep. 2017, 7, 14992. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Sheldon, H.; Martincorena, I.; Van Loo, P.; Gundem, G.; Wedge, D.C.; Ramakrishna, M.; Cooke, S.L.; Pillay, N.; et al. Recurrent PTPRB and PLCG1 Mutations in Angiosarcoma. Nat. Genet. 2014, 46, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Kandler, T.; Cortez, E.; Clinton, L.; Hemmerich, A.; Ahmed, O.; Wong, R.; Forns, T.; MacNeill, A.J.; Hamilton, T.D.; Khorasani, M.; et al. A Case Series of Metastatic Malignant Gastrointestinal Neuroectodermal Tumors and Comprehensive Genomic Profiling Analysis of 20 Cases. Curr. Oncol. 2022, 29, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.B.; Jones, R.L.; Huang, P.H. Molecular Profiling in Desmoplastic Small Round Cell Tumours. Int. J. Biochem. Cell Biol. 2023, 157, 106383. [Google Scholar] [CrossRef]
- de Alava, E.; Gerald, W.L. Molecular Biology of the Ewing’s Sarcoma/Primitive Neuroectodermal Tumor Family. J. Clin. Oncol. 2000, 18, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Garbutt, C.C.; Hornicek, F.; Guo, Z.; Duan, Z. Advances in Chromosomal Translocations and Fusion Genes in Sarcomas and Potential Therapeutic Applications. Cancer Treat. Rev. 2018, 63, 61–70. [Google Scholar] [CrossRef]
- Ladanyi, M.; Antonescu, C.R.; Leung, D.H.; Woodruff, J.M.; Kawai, A.; Healey, J.H.; Brennan, M.F.; Bridge, J.A.; Neff, J.R.; Barr, F.G.; et al. Impact of SYT-SSX Fusion Type on the Clinical Behavior of Synovial Sarcoma: A Multi-Institutional Retrospective Study of 243 Patients. Cancer Res. 2002, 62, 135–140. [Google Scholar]
- Williamson, D.; Missiaglia, E.; de Reyniès, A.; Pierron, G.; Thuille, B.; Palenzuela, G.; Thway, K.; Orbach, D.; Laé, M.; Fréneaux, P.; et al. Fusion Gene-Negative Alveolar Rhabdomyosarcoma Is Clinically and Molecularly Indistinguishable from Embryonal Rhabdomyosarcoma. J. Clin. Oncol. 2010, 28, 2151–2158. [Google Scholar] [CrossRef]
- Trautmann, M.; Cyra, M.; Isfort, I.; Jeiler, B.; Krüger, A.; Grünewald, I.; Steinestel, K.; Altvater, B.; Rossig, C.; Hafner, S.; et al. Phosphatidylinositol-3-Kinase (PI3K)/Akt Signaling Is Functionally Essential in Myxoid Liposarcoma. Mol. Cancer Ther. 2019, 18, 834–844. [Google Scholar] [CrossRef]
- Weiss, M.C.; Blank, A.; Gitelis, S.; Fidler, M.J.; Batus, M. Clinical Benefit of next Generation Sequencing in Soft Tissue and Bone Sarcoma: Rush University Medical Center’s Experience. J. Clin. Oncol. 2019, 37, e22552. [Google Scholar] [CrossRef]
- Gounder, M.; Schöffski, P.; Jones, R.L.; Agulnik, M.; Cote, G.M.; Villalobos, V.M.; Attia, S.; Chugh, R.; Chen, T.W.-W.; Jahan, T.; et al. Tazemetostat in Advanced Epithelioid Sarcoma with Loss of INI1/SMARCB1: An International, Open-Label, Phase 2 Basket Study. Lancet Oncol. 2020, 21, 1423–1432. [Google Scholar] [CrossRef]
- Italiano, A.; Dinart, D.; Soubeyran, I.; Bellera, C.; Espérou, H.; Delmas, C.; Mercier, N.; Albert, S.; Poignie, L.; Boland, A.; et al. Molecular Profiling of Advanced Soft-Tissue Sarcomas: The MULTISARC Randomized Trial. BMC Cancer 2021, 21, 1180. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly Targeted Therapy Based on Tumour Molecular Profiling versus Conventional Therapy for Advanced Cancer (SHIVA): A Multicentre, Open-Label, Proof-of-Concept, Randomised, Controlled Phase 2 Trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.H.; Fu, S.; Hong, D.S.; Karp, D.D.; Piha-Paul, S.; Subbiah, V.; Janku, F.; Naing, A.; Yap, T.A.; Rodon, J.; et al. Challenges and Opportunities Associated with the MD Anderson IMPACT2 Randomized Study in Precision Oncology. NPJ Precis. Oncol. 2022, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef]
- Butrynski, J.E.; D’Adamo, D.R.; Hornick, J.L.; Dal Cin, P.; Antonescu, C.R.; Jhanwar, S.C.; Ladanyi, M.; Capelletti, M.; Rodig, S.J.; Ramaiya, N.; et al. Crizotinib in ALK-Rearranged Inflammatory Myofibroblastic Tumor. N. Engl. J. Med. 2010, 363, 1727–1733. [Google Scholar] [CrossRef]
- McArthur, G.A.; Demetri, G.D.; van Oosterom, A.; Heinrich, M.C.; Debiec-Rychter, M.; Corless, C.L.; Nikolova, Z.; Dimitrijevic, S.; Fletcher, J.A. Molecular and Clinical Analysis of Locally Advanced Dermatofibrosarcoma Protuberans Treated with Imatinib: Imatinib Target Exploration Consortium Study B2225. J. Clin. Oncol. 2005, 23, 866–873. [Google Scholar] [CrossRef]
- Dickson, M.A.; Schwartz, G.K.; Keohan, M.L.; D’Angelo, S.P.; Gounder, M.M.; Chi, P.; Antonescu, C.R.; Landa, J.; Qin, L.-X.; Crago, A.M.; et al. Progression-Free Survival Among Patients With Well-Differentiated or Dedifferentiated Liposarcoma Treated With CDK4 Inhibitor Palbociclib: A Phase 2 Clinical Trial. JAMA Oncol. 2016, 2, 937–940. [Google Scholar] [CrossRef]
- Schöffski, P.; Kubickova, M.; Wozniak, A.; Blay, J.Y.; Strauss, S.J.; Stacchiotti, S.; Switaj, T.; Bücklein, V.; Leahy, M.G.; Italiano, A.; et al. Long-term efficacy update of crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumour from EORTC trial 90101 CREATE. Eur. J. Cancer 2021, 156, 12–23. [Google Scholar] [CrossRef]
- Schöffski, P.; Wozniak, A.; Kasper, B.; Aamdal, S.; Leahy, M.G.; Rutkowski, P.; Bauer, S.; Gelderblom, H.; Italiano, A.; Lindner, L.H.; et al. Activity and safety of crizotinib in patients with alveolar soft part sarcoma with rearrangement of TFE3: European Organization for Research and Treatment of Cancer (EORTC) phase II trial 90101 ‘CREATE’. Ann. Oncol. 2018, 29, 758–765. [Google Scholar] [CrossRef]
- Schöffski, P.; Wozniak, A.; Stacchiotti, S.; Rutkowski, P.; Blay, J.Y.; Lindner, L.H.; Strauss, S.J.; Anthoney, A.; Duffaud, F.; Richter, S.; et al. Activity and safety of crizotinib in patients with advanced clear-cell sarcoma with MET alterations: European Organization for Research and Treatment of Cancer phase II trial 90101 ‘CREATE’. Ann. Oncol. 2019, 30, 344. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Antonescu, C.R.; Bjerkehagen, B.; Bovée, J.V.M.G.; Boye, K.; Chacón, M.; Dei Tos, A.P.; Desai, J.; Fletcher, J.A.; Gelderblom, H.; et al. Diagnosis and Management of Tropomyosin Receptor Kinase (TRK) Fusion Sarcomas: Expert Recommendations from the World Sarcoma Network. Ann. Oncol. 2020, 31, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, E.; Jonsson, P.; Seier, K.; Qin, L.-X.; Chi, P.; Dickson, M.; Gounder, M.; Kelly, C.; Keohan, M.L.; Nacev, B.; et al. Clinical Outcome of Leiomyosarcomas With Somatic Alteration in Homologous Recombination Pathway Genes. JCO Precis. Oncol. 2020, 4, 1350–1360. [Google Scholar] [CrossRef]
- Ingham, M.; Allred, J.B.; Chen, L.; Das, B.; Kochupurakkal, B.; Gano, K.; George, S.; Attia, S.; Burgess, M.A.; Seetharam, M.; et al. Phase II Study of Olaparib and Temozolomide for Advanced Uterine Leiomyosarcoma (NCI Protocol 10250). J. Clin. Oncol. 2023, 41, 4154–4163. [Google Scholar] [CrossRef]
- Al Baghdadi, T.; Halabi, S.; Garrett-Mayer, E.; Mangat, P.K.; Ahn, E.R.; Sahai, V.; Alvarez, R.H.; Kim, E.S.; Yost, K.J.; Rygiel, A.L.; et al. Palbociclib in Patients With Pancreatic and Biliary Cancer With CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Ahn, E.R.; Mangat, P.K.; Garrett-Mayer, E.; Halabi, S.; Dib, E.G.; Haggstrom, D.E.; Alguire, K.B.; Calfa, C.J.; Cannon, T.L.; Crilley, P.A.; et al. Palbociclib in Patients With Non-Small-Cell Lung Cancer With CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis. Oncol. 2020, 4, 757–766. [Google Scholar] [CrossRef]
- Kobayashi, H.; Zhang, L.; Okajima, K.; Ishibashi, Y.; Hirai, T.; Tsuda, Y.; Ikegami, M.; Kage, H.; Shinozaki-Ushiku, A.; Oda, K.; et al. BRAF Mutations and Concurrent Alterations in Patients with Soft Tissue Sarcoma. Genes Chromosomes Cancer 2023, 62, 648–654. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Gray, R.; Chen, A.; Li, S.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Mitchell, E.P.; Iafrate, A.J.; Sklar, J.; et al. The Molecular Analysis for Therapy Choice (NCI-MATCH) Trial: Lessons for Genomic Trial Design. J. Natl. Cancer Inst. 2020, 112, 1021–1029. [Google Scholar] [CrossRef]
- Song, I.-W.; Vo, H.H.; Chen, Y.-S.; Baysal, M.A.; Kahle, M.; Johnson, A.; Tsimberidou, A.M. Precision Oncology: Evolving Clinical Trials across Tumor Types. Cancers 2023, 15, 1967. [Google Scholar] [CrossRef]
- Davis, J.L.; Al-Ibraheemi, A.; Rudzinski, E.R.; Surrey, L.F. Mesenchymal Neoplasms with NTRK and Other Kinase Gene Alterations. Histopathology 2022, 80, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Owosho, A.A.; Sung, Y.-S.; Zhang, L.; Fujisawa, Y.; Lee, J.-C.; Wexler, L.; Argani, P.; Swanson, D.; Dickson, B.C.; et al. BCOR-CCNB3 Fusion Positive Sarcomas: A Clinicopathologic and Molecular Analysis of 36 Cases with Comparison to Morphologic Spectrum and Clinical Behavior of Other Round Cell Sarcomas. Am. J. Surg. Pathol. 2018, 42, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Shukla, N.; Ameur, N.; Ito, T.; Ng, C.K.Y.; Wang, L.; Lim, D.; Marchetti, A.; Viale, A.; Pirun, M.; et al. Next-Generation Sequencing Approaches for the Identification of Pathognomonic Fusion Transcripts in Sarcomas: The Experience of the Italian ACC Sarcoma Working Group. Front. Oncol. 2020, 10, 489. [Google Scholar] [CrossRef]
- Kohsaka, S.; Shukla, N.; Ameur, N.; Ito, T.; Ng, C.K.Y.; Wang, L.; Lim, D.; Marchetti, A.; Viale, A.; Pirun, M.; et al. A Recurrent Neomorphic Mutation in MYOD1 Defines a Clinically Aggressive Subset of Embryonal Rhabdomyosarcoma Associated with PI3K-AKT Pathway Mutations. Nat. Genet. 2014, 46, 595–600. [Google Scholar] [CrossRef]
- Chibon, F.; Lagarde, P.; Salas, S.; Pérot, G.; Brouste, V.; Tirode, F.; Lucchesi, C.; de Reynies, A.; Kauffmann, A.; Bui, B.; et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat. Med. 2010, 16, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Dumbrava, E.E.; Johnson, M.L.; Tolcher, A.W.; Shapiro, G.; Thompson, J.A.; El-Khoueiry, A.B.; Vandross, A.L.; Kummar, S.; Parikh, A.R.; Munster, P.N.; et al. First-in-human study of PC14586, a small molecule structural corrector of Y220C mutant p53, in patients with advanced solid tumors harboring a TP53 Y220C mutation. J. Clin. Oncol. 2022, 40, 3003. [Google Scholar] [CrossRef]
| First Author, Year | Histology | Gene Alteration(s) | Treatment | Outcome |
|---|---|---|---|---|
| Groisberg, 2017 [10] | Myxoid LPS | AKT1 E17K | AKT inhibitor | SD stopped for toxicity |
| Arnaud-Coffin, 2020 [19] | LMS | AKT2 ampl. | everolimus | PD, PFS 2.6 m., OS 10.9 m. |
| Arnaud-Coffin, 2020 [19] | UPS | AKT2 del. | everolimus | PD, PFS 1.4 m., OS 4.1 m. |
| Groisberg, 2017 [10] | Pleomorphic Sa | ALK fus. | ceritinib | PD after 4 cycles |
| Subbiah et al., 2015 [22] | uIMT | ALK fus. | crizotinib/pazopanib | PR > 6 m. |
| Groisberg, 2017 [10] | Spindle cell Sa | BRAF fusion | sorafenib/bev/temsirol. | SD for 11 cycles |
| Gounder, 2022 [12] | Sa NOS | BRAF V600E mut. | vemurafenib + trametinib | rapid response |
| Groisberg, 2017 [10] | Brain GLIOSa | BRAF V600E mut. | vemurafenib | 86% decrease, DOR 16 m. |
| Jin, 2021 [13] | CCS | BRAF V600E mut. | vemurafenib | PFS 21 m. |
| Lucchesi, 2018 [6] | UPS | BRAF V600E mut. | BRAF inhibitor | PR, PFS 7.1 m. |
| Massoth, 2021 [11] | HS | BRAF V600E mut. | dabrafenib + trametinib | rapid response, PFS 2 m. |
| Saijo et al., 2022 [20] | Sperm. cord Sa | BRAF V600E mut. | dabrafenib + trametinib | PFS 6.5 m. |
| Boddu, 2018 [7] | CCS | BRAF V600M mut. | vemurafenib | PD |
| Morfouace, 2023 [16] | eRMS | BRCA1, BRCA2 loss | olaparib + trabectedin | PD at 2 m. |
| Jin, 2021 [13] | LPS | CDK4 ampl. | palbociclib | PFS 4 m. |
| Gusho, 2022 [8] | LPS | CDK4, MDM2 | palbociclib | PD |
| Gusho, 2022 [8] | LPS | CDK4, MDM2 | palbociclib | SD then PD |
| Elvin, 2017 [9] | uLMS | CDKN2A mut. | palbociclib | PFS 8 m. |
| Gusho, 2022 [8] | Phyllodes t. | CDKN2A/B | palbociclib | PD |
| Gusho, 2022 [8] | Phyllodes t. | CDKN2A, MTAP | palbociclib | SD for 5 m.; PD at restart |
| Gusho, 2022 [8] | UPS | CDKN2A/B | palbociclib | PD |
| Gusho, 2022 [8] | PNST | CDKN2A/B | palbociclib | PD |
| Boddu, 2018 [7] | Soft parts GCT | CDKN2A/B loss | palbociclib | SD at 2 m. |
| Boddu, 2018 [7] | LMS | CDKN2A/B loss | palbociclib + fulvestrant | PD |
| Gusho, 2022 [8] | UPS | CDKN2A/B, TP53 | palbociclib > pazopanib | PD on both drugs |
| Jin, 2021 [13] | FS | COL1A1-PDGFB fus. | imatinib | PFS 10 m. |
| Arnaud-Coffin, 2020 [19] | MPNST | ERBB2 mut. | lapatinib | SD, PFS 1.9 m., OS 3.8 m. |
| Gounder, 2022 [12] | IMT | ETV6-NTRK3 fus. | larotrectinib | durable CR |
| Recine, 2022 [23] | Spindle-cell n. | TPM4-NTRK1 fus. | larotrectinib | PR, PFS 2 y. |
| Horak, 2021 [17] | LMS | FGF2 fus. | pazopanib | PD, PFS 6 m. |
| Boddu, 2018 [7] | LMS | FGFR1 amp. | pazopanib | PD |
| Boddu, 2018 [7] | UPS | FGFR1 ampl. | pazopanib | PD |
| Lucchesi, 2018 [6] | RMS | FGFR4 mut. | FGFR inhibitor | PD |
| Arnaud-Coffin, 2020 [19] | AS | FLT4 ampl. | pazopanib | PR, PFS 3.1 m., OS 10.7 m. |
| Lucchesi, 2018 [6] | DDLPS | FRS2 ampl. | FGFR inhibitor | SD at 5.7 m. |
| Lucchesi, 2018 [6] | DDLPS | FRS2 ampl. | FGFR inhibitor | SD at 6 m. |
| Brahmi, 2023 [18] | MPNST | high TMB | durva + treme | CR |
| Gounder, 2022 [12] | UPS | high TMB | pembrolizumab | near CR |
| Painter et al., 2020 [15] | AS | high TMB | ICI | PFS 32.9 m. |
| Painter et al., 2020 [15] | AS | high TMB | ICI | PFS 44.3 m. |
| Boddu, 2018 [7] | UPS | IDH1 R132C | IDH1 inhibitor | PD |
| Eder, 2021 [24] | lung EHE | IDH2 mut. | olaparib | SD 11 m. |
| Lucchesi, 2018 [6] | LMS | IGF1R ampl. | mTOR inhibitor | PR |
| Boddu, 2018 [7] | Kaposi Sa | intermediate TMB | pembrolizumab | PR |
| Gounder, 2022 [12] | PEComa | intermediate TMB | nivolumab + ipilimumab | CR |
| Saller et al., 2018 [21] | Kaposi Sa | intermediate TMB | pembrolizumab | PFS 10.5 m. |
| Lucchesi, 2018 [6] | DDLPS | KRAS mut. | MAPK inhibitor | SD at 12.6 m. |
| Jin, 2021 [13] | Myofibrobl. Sa | MAP2K1 K57N | trametinib | PFS 3 m. |
| Groisberg, 2017 [10] | DDLPS | MDM2 ampl. | MDM2 inhibitor | PR x3 cycles |
| Groisberg, 2017 [10] | WDLPS | MDM2 ampl. | MDM2 inhibitor | SD x8 cycles |
| Groisberg, 2017 [10] | WDLPS | MDM2 ampl. | MDM2 inhibitor | CR (with resections) |
| Groisberg, 2017 [10] | WDLPS | MDM2 ampl. | MDM2/MDMX inhibitor | SD for 2 cycles, toxicity |
| Groisberg, 2017 [10] | WDLPS | MDM2 ampl. | MDM2 inhibitor | SD for 23 m. |
| Frampton, 2015 [25] | HS | METex14 alter. | crizotinib | PFS 11 m., response > 60% |
| Massoth, 2021 [11] | HS | MTOR mut. | temsirolimus/sirolimus | PFS 9 m. |
| Gusho, 2022 [8] | AS | MYC, CUX1 | palbociclib | SD, then PD |
| Gusho, 2022 [8] | AS | MYC, TP53, GNA11 | palbociclib > pazopanib | PD on both drugs |
| Horak, 2021 [17] | LMS | PDGFRA ampl. | pazopanib | PD, PFS 3.8 m. |
| Lopes-Brás, 2022 [14] | LPS | PDGFRA del. | imatinib | PR then PD, OS 2 m. |
| Horak, 2021 [17] | STS other | PDGFRA/KIT ampl. | pazopanib | SD, PFS 6 m. |
| Lopes-Brás, 2022 [14] | RMS NOS | PIK3CA N345I | everolimus | PR then PD, OS 4 m. |
| Groisberg, 2017 [10] | LMS | PTEN alter. | PI3K inhibitor | PD death after 3 d. |
| Horak, 2021 [17] | STS other | PTPRB mut. | pazopanib | SD, PFS 5.4 m. |
| Horak, 2021 [17] | LMS | RAD18 and BAP1 del. | olaparib + trabectedin | PFS 3 m. |
| Lucchesi, 2018 [6] | LMS | RICTOR ampl. | mTOR inhibitor | PD |
| Groisberg, 2017 [10] | DDLPS | ROS1 ampl. | ceritinib | SD for 5 m. |
| Groisberg, 2017 [10] | LMS | ROS1 D1538V | pazopanib + crizotinib | SD for 6 m. |
| Groisberg, 2017 [10] | LMS | ROS1 D1538V | pazopanib + crizotinib | PD death prior to restaging |
| Boddu, 2018 [7] | AS | ROS1 S884F | ALK/ROS/NTRK inh. | PD |
| Gounder, 2022 [12] | Sa NOS | SMARCB1 del. | tazemetostat | durable PR |
| Histotype | Gene(s) | Type of Alteration |
|---|---|---|
| Alveolar RMS | PAX3-FOXO1 | fusion |
| Alveolar RMS | PAX7-FOXO1 | fusion |
| ASPS | TFE3-ASPSCR1 | fusion |
| Desmoid tumor | Beta-catenin | mutation |
| DFSP | COL1A1-PDGFB | fusion |
| DSRCT | EWSR1-WT1 | fusion |
| EMC | EWSR1-NR4A3 | fusion |
| ESS | JAZF1-SUZ12 | fusion |
| ESS | MEAF6-PHF1 | fusion |
| Ewing/PNET | EWSR1-FLI1 | fusion |
| Ewing/PNET | EWSR1-ERG | fusion |
| Ewing/PNET | EWSR1-FEV | fusion |
| Ewing-like | CIC-DUX4 | fusion |
| Ewing-like | CIC-FOXO4 | fusion |
| GIST | KIT | mutation |
| GIST | PDGFRa | mutation |
| IMT | ALK | fusions |
| IMT | ROS1 | fusions |
| Myxoid LPS | FUS-DDIT3 | fusion |
| Myxoid LPS | EWSR1-DDIT3 | fusion |
| NTRK-rearranged sarcoma | NTRK1, NTRK2, NTRK3 | fusions |
| SS | SYT-SSX1 | fusion |
| SS | SYT-SSX2 | fusion |
| WDLPS/DDLPS | MDM2 | amplification |
| WDLPS/DDLPS | CDK4 | amplification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkali, S.; Georgaki, E.; Mandrakis, G.; Valverde, C.; Theocharis, S. Genomic Profiling and Clinical Outcomes of Targeted Therapies in Adult Patients with Soft Tissue Sarcomas. Cells 2023, 12, 2632. https://doi.org/10.3390/cells12222632
Kokkali S, Georgaki E, Mandrakis G, Valverde C, Theocharis S. Genomic Profiling and Clinical Outcomes of Targeted Therapies in Adult Patients with Soft Tissue Sarcomas. Cells. 2023; 12(22):2632. https://doi.org/10.3390/cells12222632
Chicago/Turabian StyleKokkali, Stefania, Eleni Georgaki, Georgios Mandrakis, Claudia Valverde, and Stamatios Theocharis. 2023. "Genomic Profiling and Clinical Outcomes of Targeted Therapies in Adult Patients with Soft Tissue Sarcomas" Cells 12, no. 22: 2632. https://doi.org/10.3390/cells12222632






