Chronological Changes in Gonadotropin-Releasing Hormone 1, Gonadotropins, and Sex Steroid Hormones along the Brain–Pituitary–Gonadal Axis during Gonadal Sex Differentiation and Development in the Longtooth Grouper, Epinephelus bruneus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Sampling Procedures
2.3. Histological Procedures of Gonads and Pituitary Glands and Immunohistochemistry (ICH)
2.4. Total RNA Extraction and cDNA Synthesis
2.5. Procedures for Making Standard Plasmids
2.5.1. Electrophoresis with 1.5% Agarose Gel
2.5.2. Gel Extraction
2.5.3. Ligation and Transformation
2.5.4. PCR Cloning
2.5.5. Liquid Culture of Colon Bacillus
2.5.6. Plasmid Extraction and Sequencing Analysis
2.6. Quantitative Real-Time PCR Analysis for gnrh1, fshβ, and lhβ
2.7. Quantification of Serum E2 and T Concentrations
2.8. Statistical Analysis
3. Results
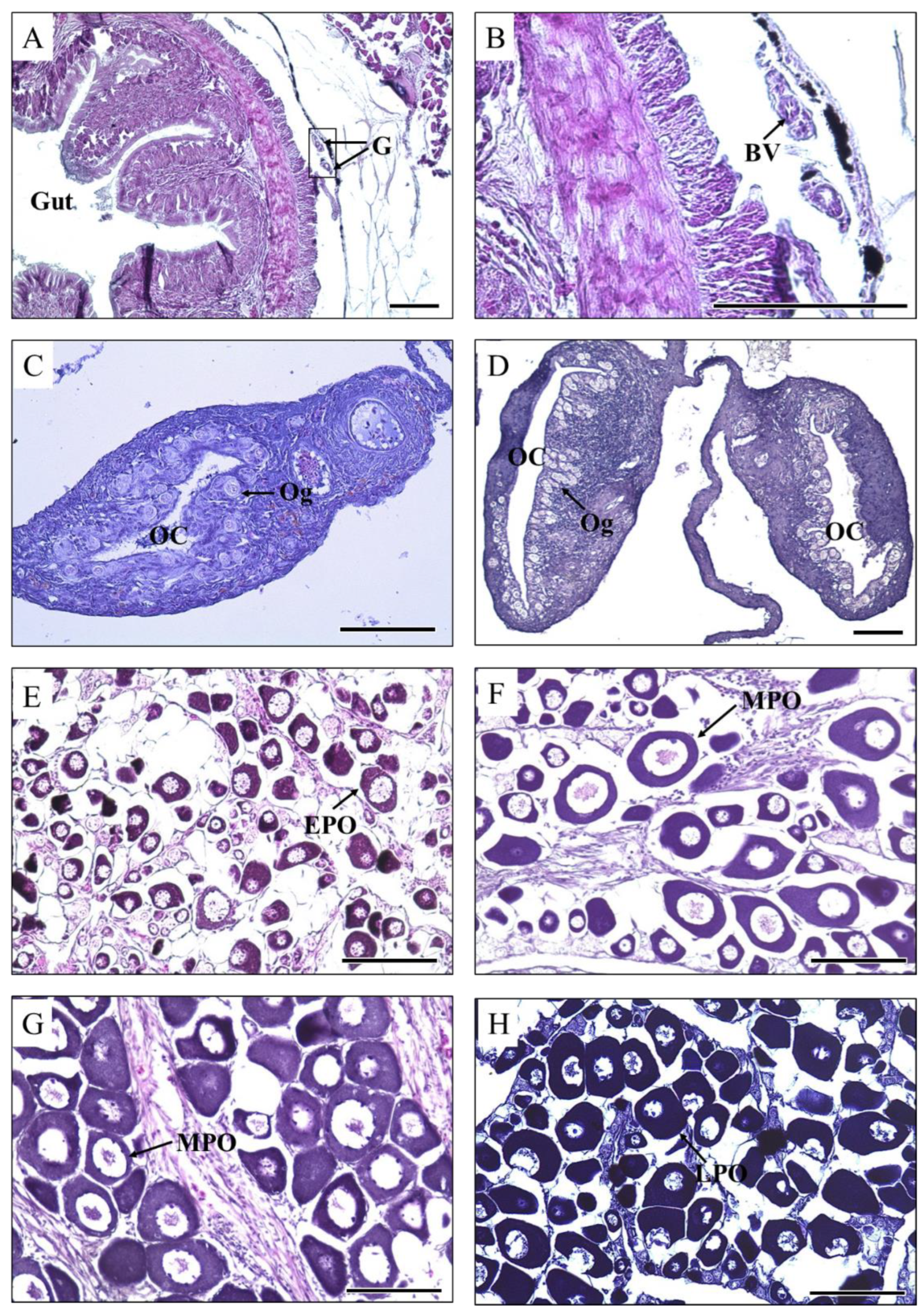
3.1. Gonadal Development
3.2. General Pituitary Structures and Fshβ and Lhβ Immunoreactivity in the Pituitary Gland
3.3. Gene Expression of gnrh1 in the Brain and fshβ and lhβ in the Pituitary Gland
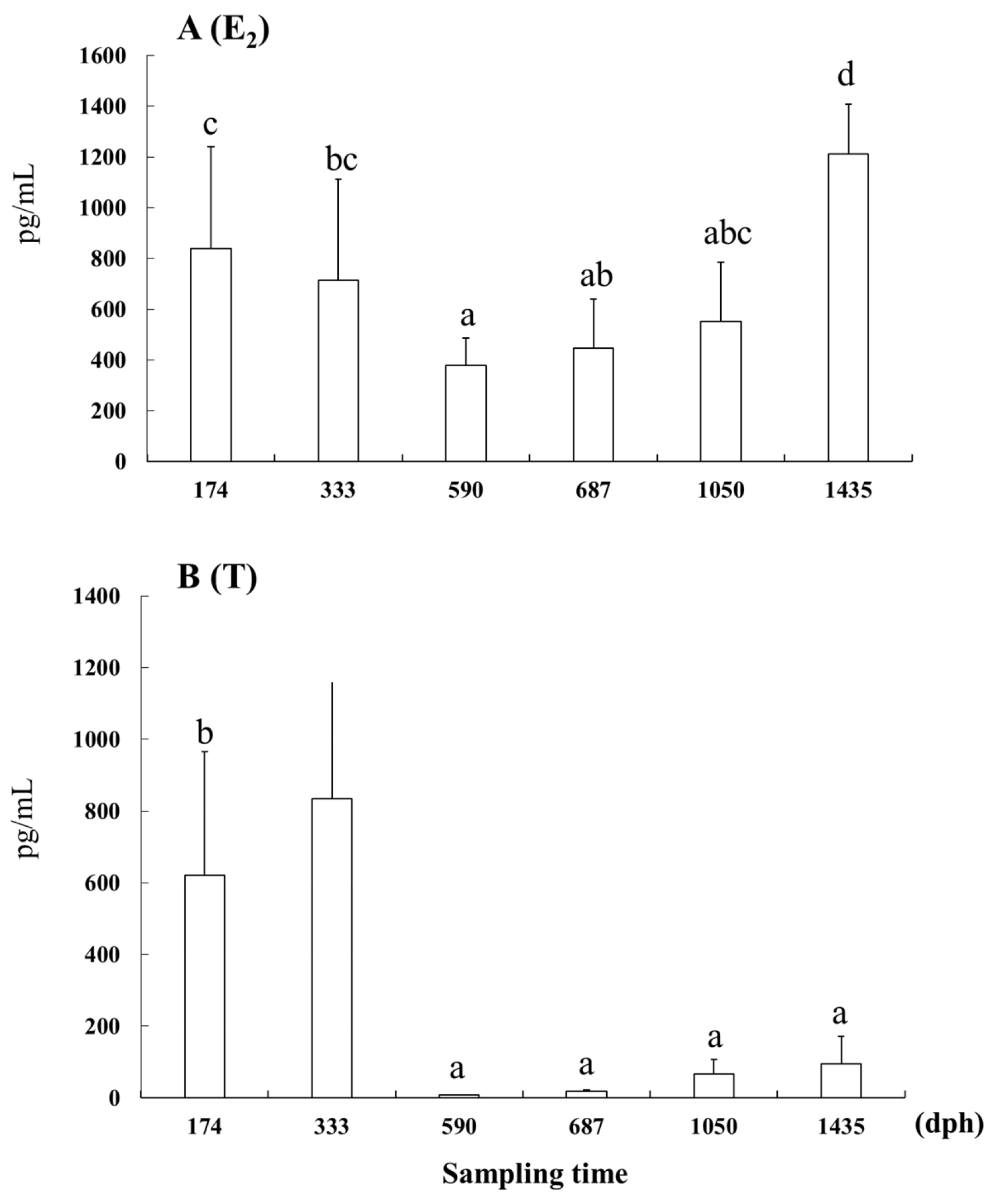
3.4. E2 and T Concentrations in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tupper, M.; Sheriff, N. Capture-based aquaculture of groupers. In Capture-Based Aquaculture: Global Overview; Lovatelli, A., Holthus, P.F., Eds.; FAO Fisheries Technical Paper 508; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; pp. 217–253. Available online: https://hdl.handle.net/20.500.12348/1540 (accessed on 30 May 2019).
- Izumida, D.; Kobayashi, Y.; Soyano, K. Chapter 1: Reproductive biology of groupers. In Frontiers of Fisheries Science in Groupers; Soyano, K., Teruya, K., Chuda, H., Eds.; Kouseisha: Kouseikaku, Tokyo, 2015; pp. 9–20. (In Japanese) [Google Scholar]
- Chuda, H.; Tsuchihashi, Y.; Tsuji, M. Chapter 6: Technique and issues of seed production and aquaculture in the longtooth grouper and sevenband grouper. In Frontiers of Fisheries Science in Groupers; Soyano, K., Teruya, K., Chuda, H., Eds.; Kouseisha: Kouseikaku, Tokyo, 2015; pp. 81–95. (In Japanese) [Google Scholar]
- Borella, M.I.; Chehade, C.; Costa, F.G.; De Jesus, L.W.O.; Cassel, M.; Batlouni, S.R. Chapter 14: The brain-pituitary-gonad axis and the gametogenesis. In Biology and Physiology of Freshwater Neotropical Fish; Baldisserotto, B., Urbinati, E.C., Cyrino, J.E.P., Eds.; Academic Press: London, UK, 2020; pp. 315–341. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, Y. Sex differentiation, sex change, and sex control in groupers. Part XI: Sex Determination and Sex Control in Other Fish Species. In Sex Control in Aquaculture; Wang, H.P., Piferrer, F., Chen, S.L., Shen, Z.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 735–750. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, Q.; Huang, B.; Jia, Y. Molecular cloning, characterization, and mRNA expression of gonadotropins during larval development in turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2019, 45, 1697–1707. [Google Scholar] [CrossRef]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef]
- Miranda, L.A.; Strüssmann, C.A.; Somoza, G.M. Immunocytochemical identification of GtH1 and GtH2 cells during the temperature-sensitive period for sex differentiation in pejerrey, Odontesthes bonariensis. Gen. Comp. Endocrinol. 2001, 124, 45–52. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, J.H.; Baek, H.J.; Kwon, J.Y. Gene expression of aromatases, steroid receptor, GnRH and GTHs in the brain during the formation of ovarian cavity in red spotted grouper, Epinephelus akaara. Dev. Reprod. 2016, 20, 367–377. [Google Scholar] [CrossRef]
- Wong, T.T.; Zohar, Y. Novel expression of gonadotropin subunit genes in oocytes of the gilthead seabream (Sparus aurata). Endocrinology 2004, 145, 5210–5220. [Google Scholar] [CrossRef]
- Wu, Y.; He, Z.; Zhang, L.; Jiang, H.; Zhang, W. Ontogeny of immunoreactive Lh and Fsh cells in relation to early ovarian differentiation and development in protogynous hermaphroditic ricefield eel Monopterus albus. Biol. Reprod. 2012, 86, 93. [Google Scholar] [CrossRef]
- Prat, F.; Sumpter, J.P.; Tyler, C.R.; Graham, C.H.; Watson, J.D.; Blumenfeld, A.J.; Pang, S.C. Validation of radioimmunoassays for two salmon gonadotropins (GTH I and GTH II) and their plasma concentrations throughout the reproductive cycle in male and female rainbow trout (Oncorhynchus mykiss). Biol. Reprod. 1996, 54, 1375–1382. [Google Scholar] [CrossRef]
- Tyler, C.R.; Pottinger, T.G.; Coward, K.; Prat, F.; Beresford, N.; Maddix, S. Salmonid follicle-stimulating hormone (GtH I) mediates vitellogenic development of oocytes in the rainbow trout, Oncorhynchus mykiss. Biol. Reprod. 1997, 57, 1238–1244. [Google Scholar] [CrossRef]
- Planas, J.V.; Athos, J.; Goetz, F.W.; Swanson, P. Regulation of ovarian steroidogenesis in vitro by follicle-stimulating hormone and luteinizing hormone during sexual maturation in salmonid fish. Biol. Reprod. 2000, 62, 1262–1269. [Google Scholar] [CrossRef]
- Kajimura, S.; Yoshiura, Y.; Suzuki, M.; Utoh, T.; Horie, N.; Oka, H.; Aida, K. Changes in the levels of mRNA coding for gonadotropin Iβ and IIβ subunits during vitellogenesis in the common Japanese conger Conger myriaster. Fish. Sci. 2001, 67, 1053–1062. [Google Scholar] [CrossRef]
- Suetake, H.; Okubo, K.; Sato, N.; Yoshiura, Y.; Suzuki, Y.; Aida, K. Differential expression of two gonadotropin (GTH) β subunit genes during ovarian maturation induced by repeated injection of salmon GTH in the Japanese eel Anguilla japonica. Fish. Sci. 2002, 68, 290–298. [Google Scholar] [CrossRef]
- Molés, G.; Gómez, A.; Carrillo, M.; Rocha, A.; Mylonas, C.C.; Zanuy, S. Determination of Fsh quantity and bioactivity during sex differentiation and oogenesis in European sea bass. Biol. Reprod. 2011, 85, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.A.; Peleteiro, J.B.; García Martín, L.O.; Aldegunde, M. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858). Aquaculture 2009, 288, 147–150. [Google Scholar] [CrossRef]
- Xu, W.G.; Manabe, S.; Mushirobira, Y.; Nagae, M.; Soyano, K. Changes in expression of reproduction-related hormones in the brain and pituitary during early ovarian differentiation and development in the red spotted grouper Epinephelus akaara, with emphasis on FSHβ and LHβ. Aquaculture 2020, 514, 734497. [Google Scholar] [CrossRef]
- Xu, W.G.; Soyano, K.; Zou, H.F.; Nagae, M. Kisspeptins, gonadotropins, and their receptors in the brain-pituitary-gonadal axis of Epinephelus akaara: Their relationship with oogonial proliferation and primary oocyte development. Aquacult. Rep. 2022, 27, 101360. [Google Scholar] [CrossRef]
- Sao, P.N.; Hur, S.W.; Lee, C.H.; Lee, Y.D. Gonadal sex differentiation of hatchery reared longtooth grouper (Epinephelus bruneus). Dev. Reprod. 2012, 16, 185–193. Available online: https://koreascience.kr/article/JAKO201232736361764.pdf (accessed on 21 June 2020).
- Shimizu, A.; Yamashita, M. Purification of mummichog (Fundulus heteroclitus) gonadotropins and their subunits, using an immunochemical assay with antisera raised against synthetic peptides. Gen. Comp. Endocrinol. 2002, 125, 79–91. [Google Scholar] [CrossRef]
- Ryu, Y.W.; Hur, S.W.; Hur, S.P.; Lee, C.H.; Lim, B.S.; Lee, Y.D. Characterization of pubertal development phases in female Longtooth grouper, Epinephelus bruneus via classification of bodyweight. Dev. Reprod. 2013, 17, 55–62. [Google Scholar] [CrossRef]
- Karnatak, G.; Sarkar, U.K.; Naskar, M.; Roy, K.; Gupta, S.; Nandy, S.K.; Srivastava, P.K.; Das Sarkar, S.; Sudheesan, D.; Bose, A.K.; et al. Understanding the role of climatic and environmental variables in gonadal maturation and spawning periodicity of spotted snakehead, Channa punctata (Bloch, 1793) in a tropical floodplain wetland, India. Environ. Biol. Fish. 2018, 101, 595–607. [Google Scholar] [CrossRef]
- Akhoundian, M.; Salamat, N.; Savari, A.; Movahedinia, A.; Salari, M.A. Influence of photoperiod and temperature manipulation on gonadal development and spawning in Caspian roach (Rutilus rutilus caspicus): Implications for artificial propagation. Aquac. Res. 2020, 51, 1623–1642. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Murata, R.; Karimata, H.; Alam, M.A.; Nakamura, M. Gonadal sex differentiation in the Malabar grouper, Epinephelus malabaricus. Aquaculture 2009, 293, 286–289. [Google Scholar] [CrossRef]
- Murata, R.; Karimata, H.; Kobayashi, Y.; Horiguchi, R.; Kishimoto, K.; Kimura, M.; Kobayashi, T.; Soyano, K.; Nakamura, M. Differentiation of steroid-producing cells during ovarian differentiation in the protogynous Malabar grouper, Epinephelus malabaricus. Int. J. Dev. Biol. 2011, 55, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, T.; Uesaka, M.; Inaoka, Y.; Mizutani, T.; Sekiguchi, T.; Kajitani, T.; Kitano, T.; Umezawa, A.; Miyamoto, K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: Conservation and evolution of the androgen metabolic pathway. Endocrinology 2008, 149, 1786–1792. [Google Scholar] [CrossRef]
- Callard, G.V.; Petro, Z.; Ryan, K.J.; Claiborne, J.B. Estrogen synthesis in vitro and in vivo in the brain of a marine teleost (Myoxocephalus). Gen. Comp. Endocrinol. 1981, 43, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Callard, G.V.; Petro, Z.; Ryan, K.J. Conversion of androgen to estrogen and other steroids in the vertebrate brain. Am. Zool. 1978, 18, 511–523. [Google Scholar] [CrossRef]
- Sundararaj, B.I.; Goswami, S.V.; Lamba, V.J. Role of testosterone, estradiol-17β, and cortisol during vitellogenin synthesis in the catfish, Heteropneustes fossilis (Bloch). Gen. Comp. Endocrinol. 1982, 48, 390–397. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Grimm, A.S. Seasonal changes in plasma cortisol, testosterone and oestradiol-17β in the plaice, Pleuronectes platessa L. Gen. Comp. Endocrinol. 1977, 31, 1–11. [Google Scholar] [CrossRef]
- Yaron, Z.; Levavi-Sivan, B. Endocrine regulation of fish reproduction. In Encyclopedia of Fish Physiology: From Genome to Environment; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; Volume 2, pp. 1500–1508. [Google Scholar] [CrossRef]
- Miura, C.; Higashino, T.; Miura, T. A progestin and an estrogen regulate early stages of oogenesis in fish. Biol. Reprod. 2007, 77, 822–828. [Google Scholar] [CrossRef]
- Frantzen, M.; Jaohnsen, H.K.; Mayer, I. Gonadal development and sex steroids in a female Arctic charr broodstock. J. Fish Biol. 1997, 51, 697–709. [Google Scholar] [CrossRef]
- Barannikova, I.A.; Bayunova, L.V.; Semenkova, T.B. Serum levels of testosterone, 11-ketotestosterone and oestradiol-17β in three species of sturgeon during gonadal development and final maturation induced by hormonal treatment. J. Fish Biol. 2004, 64, 1330–1338. [Google Scholar] [CrossRef]
- Rodrigues-Filho, J.A.; Araújo, B.C.; Mello, P.H.; Garcia, C.E.O.; Silva, V.F.D.; Li, W.; Levavi-Sivan, B.; Moreira, R.G. Hormonal profile during the reproductive cycle and induced breeding of the dusky grouper Epinephelus marginatus (Teleostei: Serranidae) in captivity. Aquaculture 2023, 566, 739150. [Google Scholar] [CrossRef]
- Masuma, S.; Kusunoki, Y.; Aoki, R. Seasonal changes of plasma sex steroids in captive longtooth grouper Epinephelus bruneus (Bloch) in subtropical regions. Aquac. Res. 2022, 53, 1268–1275. [Google Scholar] [CrossRef]
- Baron, D.; Houlgatte, R.; Fostier, A.; Guiguen, Y. Large-scale temporal gene expression profiling during gonadal differentiation and early gametogenesis in rainbow trout. Biol. Reprod. 2005, 73, 959–966. [Google Scholar] [CrossRef]
- Shimizu, A.; Hamaguchi, M.; Ito, H.; Ohkubo, M.; Udagawa, M.; Fujii, K.; Kobayashi, T.; Nakamura, M. Appearances and chronological changes of mummichog Fundulus heteroclitus FSH cells and LH cells during ontogeny, sexual differentiation, and gonadal development. Gen. Comp. Endocrinol. 2008, 156, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Kobayashi, T. Relationships between gonadal and gonadotrophic differentiation during early gonadal sex differentiation in several strains from the southern population of japanese medaka (Oryzias latipes). J. Exp. Zool. 2014, 321, 472–477. [Google Scholar] [CrossRef]
- Murata, R.; Kobayashi, Y.; Karimata, H.; Kishimoto, K.; Kimura, M.; Shimizu, A.; Nakamura, M. The role of pituitary gonadotropins in gonadal sex differentiation in the protogynous Malabar grouper, Epinephelus malabaricus. Gen. Comp. Endocrinol. 2012, 178, 587–592. [Google Scholar] [CrossRef]
- Nyuji, M.; Shiraishi, T.; Selvaraj, S.; Van In, V.; Kitano, H.; Yamaguchi, A.; Okamoto, K.; Onoue, S.; Shimizu, A.; Matsuyama, M. Immunoreactive changes in pituitary FSH and LH cells during seasonal reproductive and spawning cycles of female chub mackerel Scomber japonicus. Fish. Sci. 2011, 77, 731–739. [Google Scholar] [CrossRef]



| Sampling Date | dph | n | TL (cm) | BW (g) | GW (g) | GSI (%) |
|---|---|---|---|---|---|---|
| 7 August 2015 | 53 | 20 | 3.69 ± 0.14 | 0.53 ± 0.04 | UD | UD |
| 7 December 2015 | 174 | 15 | 15.74 ± 1.21 | 58.73 ± 3.40 | UD | UD |
| 13 May 2016 | 333 | 15 | 18.11 ± 1.25 | 92.59 ± 11.54 | UD | UD |
| 2 February 2017 | 590 | 15 | 25.29 ± 2.57 | 219.20 ± 20.65 | 0.06 ± 0.02 | 0.03 ± 0.01 |
| 9 May 2017 | 687 | 15 | 26.96 ± 1.31 | 257.50 ± 12.56 | 1.12 ± 0.02 | 0.05 ± 0.02 |
| 12 May 2018 | 1050 | 15 | 31.87 ± 2.63 | 448.44 ± 30.61 | 0.74 ± 0.18 | 0.16 ± 0.04 |
| 30 May 2019 | 1435 | 15 | 39.98 ± 2.76 | 899.90 ± 56.64 | 1.59 ± 061 | 0.18 ± 0.04 |
| Gene | Accession No. | Sequence (5′ → 3′) | Amplicon (bp) | Efficiency | |
|---|---|---|---|---|---|
| gnrh1 | FJ380047 | Forward | CCACTGTCAGCTCTGGTCAT | 60 | 1.998 |
| Reverse | AGGCTGTCCAGATCCCTCTT | ||||
| fshβ | EF583919 | Forward | CTGCCACTCCGACTGTCATC | 101 | 2.044 |
| Probe | ACCAGCATCAGCATCCCTGTGGAGA | ||||
| Reverse | GGTAACACTGTCCTTCACATATGG | ||||
| lhβ | EF583920 | Forward | TTTGAGCTTCCTGACTGTCCTC | 115 | 2.015 |
| Probe | ACCCGACTGTCACCTACCCTGTGGC | ||||
| Reverse | GGCTCTCGAAGGTGCAGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Chuda, H.; Soyano, K.; Zeng, J.; Mei, W.; Zou, H. Chronological Changes in Gonadotropin-Releasing Hormone 1, Gonadotropins, and Sex Steroid Hormones along the Brain–Pituitary–Gonadal Axis during Gonadal Sex Differentiation and Development in the Longtooth Grouper, Epinephelus bruneus. Cells 2023, 12, 2634. https://doi.org/10.3390/cells12222634
Xu W, Chuda H, Soyano K, Zeng J, Mei W, Zou H. Chronological Changes in Gonadotropin-Releasing Hormone 1, Gonadotropins, and Sex Steroid Hormones along the Brain–Pituitary–Gonadal Axis during Gonadal Sex Differentiation and Development in the Longtooth Grouper, Epinephelus bruneus. Cells. 2023; 12(22):2634. https://doi.org/10.3390/cells12222634
Chicago/Turabian StyleXu, Wengang, Hisashi Chuda, Kiyoshi Soyano, Jun Zeng, Weiping Mei, and Huafeng Zou. 2023. "Chronological Changes in Gonadotropin-Releasing Hormone 1, Gonadotropins, and Sex Steroid Hormones along the Brain–Pituitary–Gonadal Axis during Gonadal Sex Differentiation and Development in the Longtooth Grouper, Epinephelus bruneus" Cells 12, no. 22: 2634. https://doi.org/10.3390/cells12222634







