Galectins in Protozoan Parasitic Diseases: Potential Applications in Diagnostics and Therapeutics
Abstract
:1. Introduction
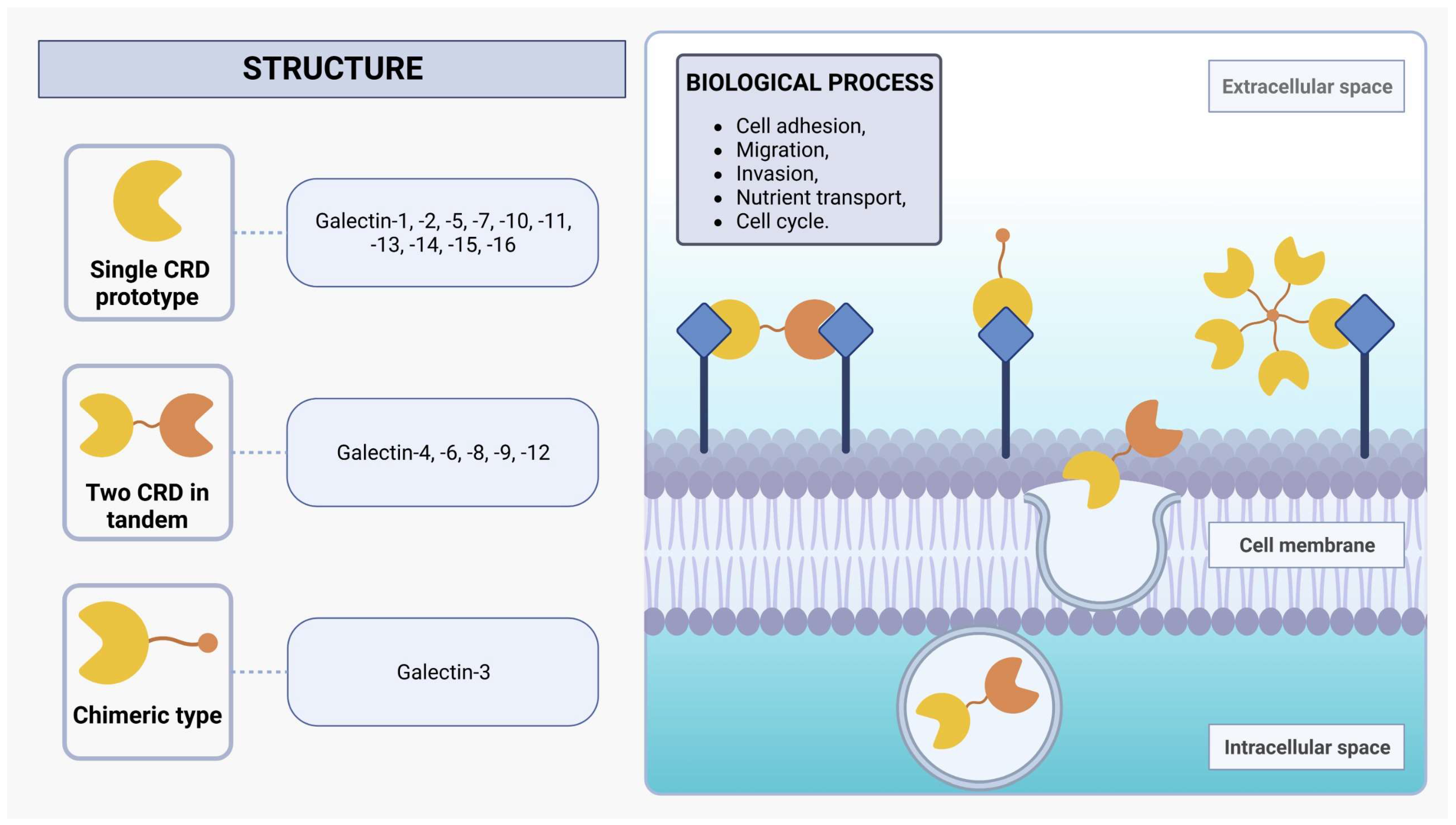
2. Galectins
3. The Role of Galectins in Plasmodium Infection
| Reference | Experimental Model | Species | Main Results |
|---|---|---|---|
| [61] | Mice | P. berghei ANKA strain | Treatment with alpha (α)-lactose reduced host survival rates and increased peripheral blood parasitemia. However, an unexpected outcome emerged as the pro-inflammatory cytokine levels in the lungs and liver were more pronounced in the alpha (α)-lactose-treated group compared to control-infected mice. This suggests that the blockade of galectin-receptor interactions by α-lactose exacerbates the inflammatory responses in the liver and lungs during P. berghei infection. |
| [62] | Human | P. falciparum and P. vivax | Gal-2 has been associated with an increased susceptibility to severe malaria in age-related populations. |
| [63] | Mice | P. falciparum and P. vivax | Gal-3 has the potential to alter the pathogenic course of experimental cerebral malaria (CM) through its binding to the endogenous oligosaccharides on matrix proteins and its release after the lysis of brain-infiltrating macrophages. |
| [56] | Mice | P. yoelii, P. berghei and P. chabaudi | Upon P. yoelii infection, the Gal-3 knockout mice exhibited a significant reduction in parasitemia compared to wild-type (WT) mice; however, in the cases of P. berghei and P. chabaudi infections, the parasitemia levels observed in the knockout mice were similar to those in WT mice. This finding suggests that Gal-3 stimulates specifically P. yoelii replication or infectivity. Also, additional experiments with P. yoelii revealed that a more robust immune reaction against the parasite occurs in the absence of Gal-3. |
| [66] | Human | P. falciparum | Gal-9 levels in the blood plasma of malaria patients were markedly higher in cases of severe malaria compared to uncomplicated cases, potentially serving as a biomarker for disease severity. Additionally, in both severe and uncomplicated malaria, Gal-9 levels were associated with various pro- and anti-inflammatory cytokines and chemokines, including TNF, IL-6, IFN-α2, IFN-γ, IL-1Ra, and IL-10. |
| [67] | Mice | P. berghei ANKA strain | The Tim-3/Gal-9 pathway plays a crucial role as a key regulator in the inflammatory pathways within the liver, leading to liver injury as the malaria infection progresses. |
| [70] | Mice | P. berghei | The upregulation of Tim-3 and Gal-9 during malaria infection can lead to their overexpression, which is associated with the severity of malaria and tissue damage, particularly in the liver and lungs. |
| [72] | Mice | P. berghei ANKA strain | Gal-9 is also involved in the aggregation of P. berghei-infected red blood cells, which is crucial for blood–brain barrier injuries and, hence, in the aggravation of CM. |
4. The Role of Galectins in Leishmaniasis
| Reference | Experimental Model | Main Results |
|---|---|---|
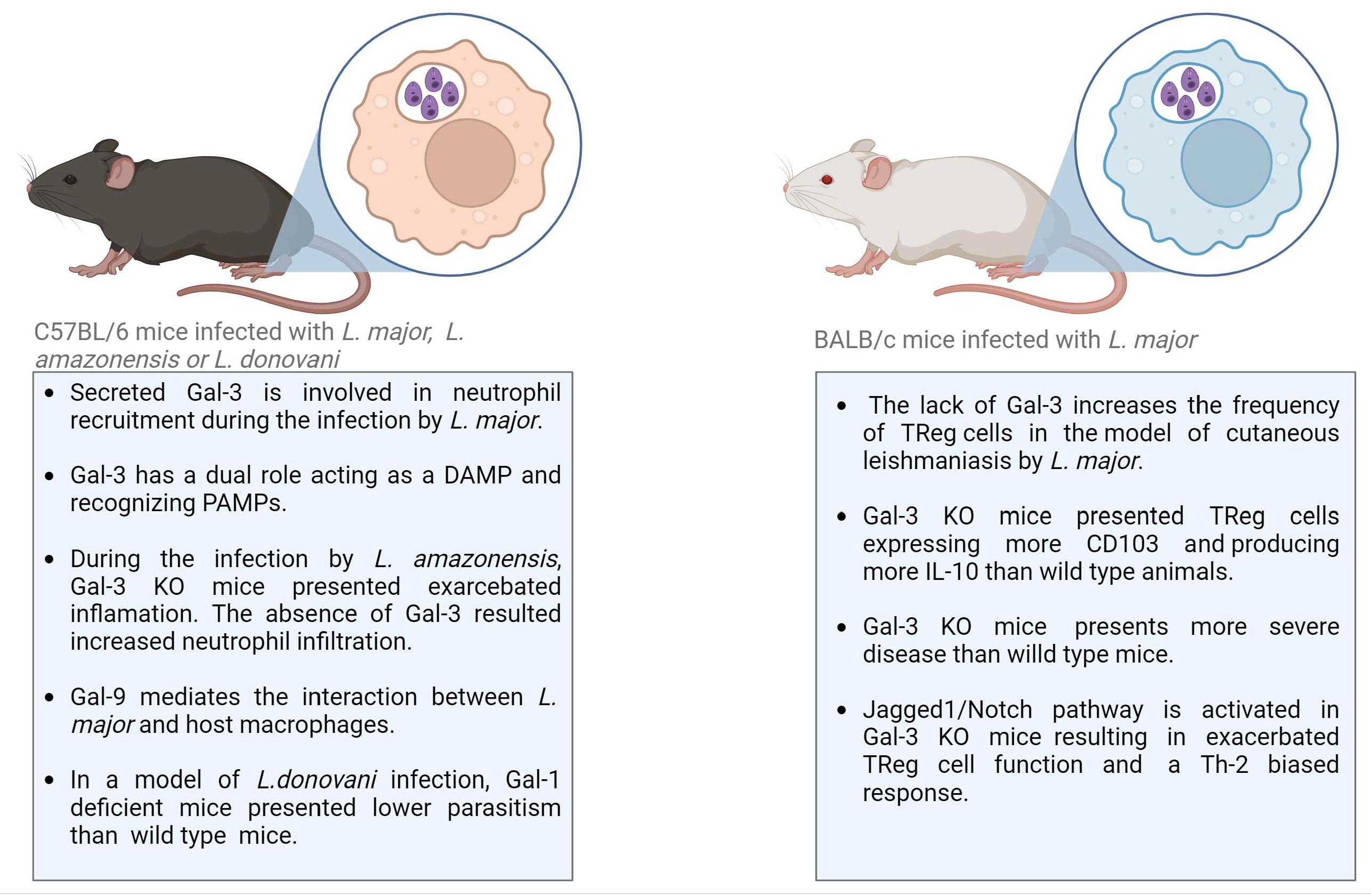
| [76] | Mice | Gal-1 knockout mice presented lower parasitism compared to wild-type mice, indicating infection by L. donovani. |
| [78] | Mice | Gal-3 deficiency resulted in reduced neutrophil infiltration in a model of cutaneous Leishmaniasis by infection with L. major |
| [79] | Mice | It was demonstrated that Gal-3 acted as a chemoattractant for neutrophils in vitro. |
| [80] | Mice | Gal-3 recognizes and binds to lipophosphoglycan from L. major but not from L. donovani. The binding of Gal-3 to L. major leads to truncated Gal-3 |
| [81] | Human | Significant increase in circulating Gal-3 in samples from patients with PKDL as compared to health control |
| [83] | Mice | The lack of Gal-3 increases the frequency of TReg cells in the site of infection, as well as in the draining lymph nodes, in a mouse model of cutaneous Leishmaniasis by L. major infection |
| [86] | Mice | Gal-3 modulates T helper responses during L. major infection |
| [57] | Mice | Recongnition of L. major by Gal-9 through binding to the L. major-specific polygalactosyl epitope |
| [87] | Mice | During the experimental infection of mice with L. amazonensis, Gal-3 was involved in the control of parasite invasion, replication, recruitment of leukocytes, and the biogenesis of endocytic vesicles |
| [37] | Cell culture | Galectin isolated from the marine sponge Chondrilla caribensis presented anti-Leishmania activity against L. infantum promastigotes in vitro |
5. The Role of Galectins in Chagas Disease
| Reference | Experimental Model | Main Results |
|---|---|---|
| [90] | Human | Observed the occurrence of anti-Gal-1 autoAb in sera from patients in the acute and chronic stages of Chagas’ disease. |
| [92] | Mice | Gal-1 was expressed on activated B cells from T. cruzi-infected (Tulahuen strain) mice, and it induced apoptosis (programmed cell death) specifically in T cells. |
| [94] | Mice | Lack of Galectin-3 Prevents Cardiac Fibrosis and effective Immune Responses in a Murine model of Trypanosoma cruzi infection |
| [93] | Human and mice | Gal-1 (Lgals1−/−) exhibited higher parasitemia in the acute phase, diminished signs of inflammation in heart and skeletal muscle tissues, and lower survival rates compared to wild-type (WT) mice when intraperitoneally infected with the T. cruzi Tulahuen strain. |
| [91] | Mice | Alongside the heightened expression of immune inhibitory mediators and programmed death ligand 2, the infection of the T. cruzi RA strain triggered an early elevation of Gal-1 expression within living organisms. When compared to the wild-type (WT) mice, Gal-1-deficient (Lgals1−/−) mice demonstrated decreased mortality rates and lower parasite levels in their muscle tissue. |
| [95] | Cell culture | The passage states that galectins have a preference for binding to forms of a parasite that are present in the host (trypanosomatid trypomastigotes and amastigotes) compared to the non-infective epimastigote present in the intestinal tract of the vector. This is due to changes in glycosylation that occur during the metacyclogenesis and amastigogenesis processes. |
| [96] | Mice | Association of cardiac galectin-3 expression, myocarditis, and fibrosis in CCC |
| [97] | Mice and cell culture | Gal-3 is important to survival, migration, and immunomodulatory action, and Gal-3 knockdown MSC treatment does not reduce cardiac inflammation and fibrosis. |
| [98] | Human | There is no correlation between the degree of myocardial fibrosis and the concentration of Gal-3 in plasma samples from subjects with Chagas disease. |
| [99] | Human | Compared to non-chagasic patients, chagasic patients exhibited elevated expression of Gal-1, Gal-3, and Gal-9 in the myenteric plexus ganglia. The heightened presence of Gal-1 in the myenteric plexus ganglia of chagasic patients might play a role in the regeneration of ganglion cells, as Gal-1 is recognized for its ability to enhance axon plasticity and suppress macrophages. |
| [100] | Mice | DMS treatment reduces Gal-3 expression in the heart and serum of mice with chronic Chagas cardiomyopathy |
| [101] | Human | Higher levels of Gal-3 were significantly associated with severe forms of disease and a higher long-term mortality rate. |
| [102] | Mice | During the chronic phase, Gal-8-deficient mice exhibited widespread inflammation in the heart, skeletal muscle, and liver, leading to extensive fibrosis, independent of tissue parasite loads. Remarkably, there was a notable increase in the occurrence of neutrophils and macrophages as well. |
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Akinokun, R.T.; Ilesanmi, E.B.; Adebisi, Y.A.; Akingbade, O. The status of neglected tropical diseases amidst COVID-19 in Africa: Current evidence and recommendations. Health Promot. Perspect. 2021, 11, 430–433. [Google Scholar] [CrossRef]
- Doenças Tropicais Negligenciadas: OPAS Pede Fim dos Atrasos no Tratamento Nas Américas—OPAS/OMS | Organização Pan-Americana da Saúde. Available online: www.paho.org (accessed on 11 July 2023).
- Solana, J.C.; Moreno, J.; Iborra, S.; Soto, M.; Requena, J.M. Live attenuated vaccines, a favorable strategy to provide long-term immunity against protozoan diseases. Trends Parasitol. 2022, 38, 316–334. [Google Scholar] [CrossRef]
- Junior, M.C.d.S.; Araújo, J.S.C.; Oliveira, L.d.M.; de Andrade, K.V.F.; Benevides, R.G.; Leite, F.H.A. Superoxide Dismutase Inhibitors against Malaria, Leishmaniasis, and Chagas Disease: Systematic Review. Curr. Drug Targets 2023, 24, 201–210. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 723–735. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Anaemia and malaria. Malar. J. 2018, 17, 371. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sahi, P.K. Malaria: An Update. Indian J. Pediatr. 2017, 84, 521–528. [Google Scholar] [CrossRef]
- Moxon, C.A.; Gibbins, M.P.; McGuinness, D.; Milner, D.A.; Marti, M. New Insights into Malaria Pathogenesis. Annu. Rev. Pathol. 2020, 15, 315–343. [Google Scholar] [CrossRef]
- Rehman, A.; Abbas, N.; Saba, T.; Mehmood, Z.; Mahmood, T.; Ahmed, K.T. Microscopic malaria parasitemia diagnosis and grading on benchmark datasets. Microsc. Res. Tech. 2018, 81, 1042–1058. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.S. Malaria. Clin. Lab. Med. 2010, 30, 93–129. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L. Council on Chagas Disease of the Interamerican Society of Cardiology. Chagas disease: An overview of clinical and epidemiological aspects. J. Am. Col. Cardio. 2013, 62, 767–776. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chagas Disease (American Trypanosomiasis). World Health Organization. Available online: https://www.who.int/health-topics/chagas-disease (accessed on 5 April 2023).
- Chao, C.; Leone, J.L.; Vigliano, C.A. Chagas disease: Historic perspective. Biochim. Biophy. Acta Mol. Basis Dis. 2020, 1866, 165689. [Google Scholar] [CrossRef] [PubMed]
- Balouz, V.; Agüero, F.; Buscaglia, C.A. Chagas Disease Diagnostic Applications: Present Knowledge and Future Steps. Adv. Parasitol. 2017, 97, 1–45. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leishmaniasis. World Health Organization. Available online: https://www.who.int/health-topics/Leishmaniasis (accessed on 5 April 2023).
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in humans: Drug or vaccine therapy? Drug Des. Devel. Ther. 2017, 12, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Harris, D.R.; Alshammari, S.O.; Gugssa, A.; Young, T.; Lee, C.M. Leishmaniasis: Recent epidemiological studies in the Middle East. Front Microbiol. 2023, 13, 1052478. [Google Scholar] [CrossRef]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Aspects Med. 2017, 57, 1–29. [Google Scholar] [CrossRef]
- Weng, H.-B.; Chen, H.-X.; Wang, M.-W. Innovation in neglected tropical disease drug discovery and development. Infect. Dis. Poverty 2018, 7, 67. [Google Scholar] [CrossRef]
- Van Vugt, M.; Van Beest, A.; Sicuri, E.; Van Tulder, M.; Grobusch, M.P. Malaria treatment and prophylaxis in endemic and nonendemic countries: Evidence on strategies and their cost–effectiveness. Future Microbiol. 2011, 6, 1485–1500. [Google Scholar] [CrossRef]
- Nordmann, T.; Borrmann, S.; Ramharter, M. Drug-induced hypersensitivity to artemisinin-based therapies for malaria. Trends Parasitol. 2021, 38, 136–146. [Google Scholar] [CrossRef]
- WHO. Malaria. World Health Organization, 2022. Available online: https://www.who.int/news-room/questions-and-answers/item/malaria (accessed on 5 April 2023).
- Guarner, J. Chagas disease as an example of a reemerging parasite. Semin. Diagn. Pathol. 2019, 36, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lascano, F.; García Bournissen, F.; Altcheh, J. Review of pharmacological options for the treatment of Chagas disease. Br. J. Clin. Pharmacol. 2021, 88, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, Y.; Zahedifard, F.; Rafati, S. Leishmaniasis and various immunotherapeutic approaches. Parasitology 2016, 145, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Moar, P.; Tandon, R. Galectin-9 as a biomarker of disease severity. Cell Immunol. 2021, 361, 104287. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Shi, W.; Xue, C.; Su, X.Z.; Lu, F. The roles of galectins in parasitic infections. Acta Trop. 2018, 177, 97–104. [Google Scholar] [CrossRef]
- Preston, S.; Dunphy, J.; Beddoe, T.; Meeusen, E.; Young, A. Evaluation of the Role of Galectins in Parasite Immunity. Methods Mol. Biol. 2015, 1207, 371–395. [Google Scholar] [CrossRef]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef]
- John, S.; Mishra, R. Galectin-9: From cell biology to complex disease dynamics. J. Biosci. 2016, 41, 507–534. [Google Scholar] [CrossRef]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef]
- Kaminker, J.D.; Timoshenko, A.V. Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues. Biomolecules 2021, 11, 1909. [Google Scholar] [CrossRef]
- Nonaka, Y.; Ogawa, T.; Yoshida, H.; Shoji, H.; Nishi, N.; Kamitori, S.; Nakamura, T. Crystal structure of a Xenopus laevis skin proto-type galectin, close to but distinct from galectin-1. Glycobiology 2015, 25, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.R.d.O.; Andrade, F.R.N.; Chaves, R.P.; de Sousa, B.L.; de Lima, D.B.; Souza, R.O.d.S.; da Silva, C.G.L.; Teixeira, C.S.; Sampaio, A.H.; Nagano, C.S.; et al. Structural characterization of a galectin isolated from the marine sponge Chondrilla caribensis with leishmanicidal potential. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129992. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Chen, Y.; Li, M.; Wu, J.; Fang, Z.; Wang, J.; Liu, J. Coprinopsis cinerea Galectin CGL1 induces apoptosis and inhibits tumor growth in colorectal cancer cells. Int. J. Mol. Sci. 2022, 24, 235. [Google Scholar] [CrossRef] [PubMed]
- Caballero, G.G.; Beckwith, D.; Shilova, N.V.; Gabba, A.; Kutzner, T.J.; Ludwig, A.-K.; Manning, J.C.; Kaltner, H.; Sinowatz, F.; Cudic, M.; et al. Influence of protein (human galectin-3) design on aspects of lectin activity. Histochem. Cell Biol. 2020, 154, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef]
- Nielsen, M.I.; Stegmayr, J.; Grant, O.C.; Yang, Z.; Nilsson, U.J.; Boos, I.; Carlsson, M.C.; Woods, R.J.; Unverzagt, C.; Leffler, H.; et al. Galectin binding to cells and glycoproteins with genetically modified glycosylation reveals galectin-glycan specificities in a natural context. J. Biol. Chem. 2018, 293, 20249–20262. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a next-generation biomarker for detecting the early stage of various diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- de Souza, M.L.; Lapierre, T.J.W.J.D.; Marques, G.V.L.; Ferraz, W.R.; Penteado, A.B.; Trossini, G.H.G.; Murta, S.M.F.; de Oliveira, R.B.; Rezende, C.O.; Ferreira, R.S. Molecular targets for Chagas disease: Validation, challenges and lead compounds for widely exploited targets. Expert Opin. Ther. Targets 2023, 27, 911–925. [Google Scholar] [CrossRef]
- Ramu, D.; Singh, S. Potential molecular targets of Leishmania pathways in developing novel antileishmanials. Future Microbiol. 2022, 17, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.C.; de Sousa, L.R.F.; Garcia, C.R.S.; Oliva, G.; Guido, R.V.C. New Molecular Targets and Strategies for Antimalarial Discovery. Curr. Med. Chem. 2019, 26, 4380–4402. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, Z.; Li, L.; Yan, J.; Shao, C.; Bao, Z.; Jing, L.; Pang, Q.; Geng, Y.; Zhang, L. RAGE/galectin-3 yields intraplaque calcification transformation via sortilin. Acta Diabetol. 2019, 56, 457–472. [Google Scholar] [CrossRef]
- Liang, T.; Ma, C.; Wang, T.; Deng, R.; Ding, J.; Wang, W.; Xu, Z.; Li, X.; Li, H.; Sun, Q.; et al. Galectin-9 promotes neuronal restoration via binding TLR-4 in a rat intracerebral hemorrhage model. Neuromolecular Med. 2021, 23, 267–284. [Google Scholar] [CrossRef]
- Lee, M.; Hamilton, J.A.G.; Talekar, G.R.; Ross, A.J.; Michael, L.; Rupji, M.; Dwivedi, B.; Raikar, S.S.; Boss, J.; Scharer, C.D.; et al. Obesity-induced galectin-9 is a therapeutic target in B-cell acute lymphoblastic leukemia. Nat. Commun. 2022, 13, 1157. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yu, D.; Hu, Y.; Jin, W.; Qin, Y.; Kong, D.; Wang, H.; Li, G.; Alessandrini, A.; et al. Galectin-9 is required for endometrial regenerative cells to induce long-term cardiac allograft survival in mice. Stem Cell Res. Ther. 2020, 11. [Google Scholar] [CrossRef]
- Ikeda, M.; Katoh, S.; Shimizu, H.; Hasegawa, A.; Ohashi-Doi, K.; Oka, M. Beneficial effects of Galectin-9 on allergen-specific sublingual immunotherapy in a Dermatophagoides farinae-induced mouse model of chronic asthma. Allergol. Int. 2017, 66, 432–439. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef]
- Si, Y.; Yao, Y.; Ayala, G.J.; Li, X.; Han, Q.; Zhang, W.; Xu, X.; Tai, G.; Mayo, K.H.; Zhou, Y.; et al. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129755. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins in host–pathogen interactions: Structural, functional and evolutionary aspects. Adv. Exp. Med. Biol. 2020, 1204, 169–196. [Google Scholar] [CrossRef]
- Toscano, M.A.; Tongren, J.E.; De Souza, J.B.; Liu, F.-T.; Riley, E.M.; Rabinovich, G.A. Endogenous galectin-3 controls experimental malaria in a species-specific manner. Parasite Immunol. 2012, 34, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, I.; Hashidate, T.; Urashima, T.; Nishi, N.; Nakamura, T.; Futai, M.; Arata, Y.; Kasai, K.-I.; Hirashima, M.; Hirabayashi, J.; et al. Specific recognition of Leishmania major poly-β-galactosyl epitopes by galectin-9: Possible implication of galectin-9 in the interaction between L. major and host cells. J. Biol. Chem. 2003, 278, 22223–22230. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.M.; Silva, D.A.; Azzolini, A.E.C.; Marzocchi-Machado, C.M.; Carvalho, J.V.; Pajuaba, A.C.A.; Lucisano-Valim, Y.M.; Chammas, R.; Liu, F.-T.; Roque-Barreira, M.C.; et al. Galectin-3 plays a modulatory role in the lifespan and activation of murine neutrophils during early Toxoplasma gondii infection. Immunobiology 2010, 215, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, T.K.; Honing, H.; Franke, N.; Van Remoortere, A.; Schiphorst, W.E.C.M.; Liu, F.T.; Deelder, A.M.; Cummings, R.D.; Hokke, C.H.; Van Die, I. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 2004, 173, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Kumar, V.; Bhave, A.; Singh, V.; Gogtay, N.J.; Thatte, U.M.; Talukdar, A.; Kochar, S.K.; Patankar, S.; Srivastava, S. Proteomic analysis of Plasmodium falciparum-induced alterations in humans from different endemic regions of India to decipher malaria pathogenesis and identify surrogate markers of severity. J. Proteom. 2015, 127 Pt A, 103–113. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Su, X.Z.; Song, J.; Lu, F. Blockage of Galectin-receptor Interactions by α-lactose Exacerbates Plasmodium berghei-induced Pulmonary Immunopathology. Sci. Rep. 2016, 6, 32024. [Google Scholar] [CrossRef]
- Randall, L.M.; Kenangalem, E.; Lampah, D.A.; Tjitra, E.; Mwaikambo, E.D.; Handojo, T.; Piera, K.A.; Zhao, Z.Z.; de Labastida Rivera, F.; Zhou, Y.; et al. Age-related susceptibility to severe malaria associated with galectin-2 in highland Papuans. J. Infect. Dis. 2010, 202, 117–124. [Google Scholar] [CrossRef]
- Oakley, M.S.; Majam, V.; Mahajan, B.; Gerald, N.; Anantharaman, V.; Ward, J.M.; Faucette, L.J.; McCutchan, T.F.; Zheng, H.; Terabe, M.; et al. Pathogenic roles of CD14, galectin-3, and OX40 during experimental cerebral malaria in mice. PLoS ONE 2009, 4, e6793. [Google Scholar] [CrossRef]
- Alaro, J.R.; Angov, E.; Lopez, A.M.; Zhou, H.; Long, C.A.; Burns, J.M., Jr. Evaluation of the immunogenicity and vaccine potential of recombinant Plasmodium falciparum merozoite surface protein 8. Infect. Immun. 2012, 80, 2473–2484. [Google Scholar] [CrossRef]
- Seki, M.; Oomizu, S.; Sakata, K.M.; Sakata, A.; Arikawa, T.; Watanabe, K.; Ito, K.; Takeshita, K.; Niki, T.; Saita, N.; et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 2008, 127, 78–88. [Google Scholar] [CrossRef]
- Dembele, B.P.; Chagan-Yasutan, H.; Niki, T.; Ashino, Y.; Tangpukdee, N.; Shinichi, E.; Krudsood, S.; Kano, S.; Hattori, T. Plasma levels of Galectin-9 reflect disease severity in malaria infection. Malar. J. 2016, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Liu, J.; Huang, S.; Lu, F. Increased Gal-9 and Tim-3 expressions during liver damage in a murine malarial model. Parasitol. Res. 2016, 115, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, N.; Zhang, T.; Chen, R.; Feng, Y.; Sang, X.; Yang, N.; Chen, Q. Tim-3 signaling blockade with α-lactose induces compensatory TIGIT expression in Plasmodium berghei ANKA-infected mice. Parasit. Vectors 2019, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, S.; Xiao, S.; He, J.; Lu, F. Impact of Galectin-Receptor Interactions on Liver Pathology During the Erythrocytic Stage of Plasmodium berghei Malaria. Front. Immunol. 2021, 12, 758052. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Contribution of the TIM-3/Gal-9 immune checkpoint to tropical parasitic diseases. Acta Trop. 2023, 238, 106792. [Google Scholar] [CrossRef]
- Iwasaki-Hozumi, H.; Chagan-Yasutan, H.; Ashino, Y.; Hattori, T. Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious Diseases. Biomolecules 2021, 11, 430. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.; Huang, S.; Pei, F.; Lu, F. Upregulated Tim-3/galectin-9 expressions in acute lung injury in a murine malarial model. Parasitol. Res. 2016, 115, 587–595. [Google Scholar] [CrossRef]
- Duan, H.; Zhao, S.; Xiang, J.; Ju, C.; Chen, X.; Gramaglia, I.; Yan, X. Targeting the CD146/Galectin-9 axis protects the integrity of the blood-brain barrier in experimental cerebral malaria. Cell Mol. Immunol. 2021, 18, 2443–2454. [Google Scholar] [CrossRef]
- Goerdeler, F.; Seeberger, P.H.; Moscovitz, O. Unveiling the Sugary Secrets of Plasmodium Parasites. Front. Microbiol. 2021, 12, 712538. [Google Scholar] [CrossRef]
- Larregui, J.M.; Croci, D.O.; Bianco, G.A.; Toscano, M.A.; Salatino, M.; Vermeulen, M.E.; Geffner, J.R.; Rabinovich, G.A. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 2009, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- de Oca, M.M.; Kumar, R.; de Labastida Rivera, F.; Amante, F.H.; Sheel, M.; Faleiro, R.J.; Bunn, P.T.; Best, S.E.; Beattie, L.; Ng, S.S.; et al. Blimp-1-Dependent IL-10 Production by Tr1 Cells Regulates TNF-Mediated Tissue Pathology. PLoS Pathog. 2016, 12, e1005398. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, P.; St-Pierre, G.; Milot, V.; St-Pierre, C.; Sato, S. Galectin-3 facilitates neutrophil recruitment as an innate immune response to a parasitic protozoa cutaneous infection. J. Immunol. 2013, 190, 630–640. [Google Scholar] [CrossRef]
- Baseras, B.; Gaida, M.M.; Kahle, N.; Schuppel, A.K.; Kathrey, D.; Prior, B.; Wente, M.; Hänsch, G.M. Galectin-3 inhibits the chemotaxis of human polymorphonuclear neutrophils in vitro. Immunobiology 2012, 217, 83–90. [Google Scholar] [CrossRef]
- Pelletier, I.; Sato, S. Specific Recognition and Cleavage of Galectin-3 by Leishmania major through Species-Specific Polygalactose Epitope. J. Biol. Chem. 2002, 277, 17663–17670. [Google Scholar] [CrossRef]
- Datta, S.; Ghosh, M.; Dewan, K.; Banerjee, N.; Saha, B.; Mukhopadhyay, S. Dermatological Implications of Galectin-3 in Circulation: An Evaluation From the Perspective of Patients With Differential Manifestations of Post-Kala-Azar Dermal Leishmaniasis. Am. J. Dermatopathol. 2019, 41, 897–907. [Google Scholar] [CrossRef]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in Innate Immunity: Dual Functions of Host Soluble β-Galactoside-Binding Lectins as Damage-Associated Molecular Patterns (DAMPs) and as Receptors for Pathogen-Associated Molecular Patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef]
- Fermino, M.L.; Dias, F.C.; Lopes, C.D.; Souza, M.A.; Cruz, K.; Liu, F.-T.; Chammas, R.; Roque-Barreira, M.C.; Rabinovich, G.A.; Bernardes, E.S. Galectin-3 Negatively Regulates the Frequency and Function of CD4(+) CD25(+) Foxp3(+) Regulatory T Cells and Influences the Course of Leishmania major Infection. Eur. J. Immunol. 2013, 43, 1806–1817. [Google Scholar] [CrossRef]
- Radtke, F.; MacDonald, H.R.; Tacchini-Cottier, F. Regulation of Innate and Adaptive Immunity by Notch. Nat. Rev. Immunol. 2013, 13, 427–437. [Google Scholar] [CrossRef]
- Shang, Y.; Smith, S.; Hu, X. Role of Notch Signaling in Regulating Innate Immunity and Inflammation in Health and Disease. Protein Cell. 2016, 7, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Fermino, M.L.; Dylon, L.S.D.; Cecílio, N.T.; Santos, S.N.; Toscano, M.A.; Dias-Baruffi, M.; Roque-Barreira, M.C.; Rabinovich, G.A.; Bernardes, E.S. Lack of Galectin-3 Increases Jagged1/Notch Activation in Bone Marrow-Derived Dendritic Cells and Promotes Dysregulation of T Helper Cell Polarization. Mol. Immunol. 2016, 76, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.M.; Teixeira, T.L.; Rodrigues, C.C.; da Silva, A.; Borges, B.C.; Brígido, R.T.S.; Teixeira, S.C.; A Dos Santos, M.; Servato, J.P.S.; de Santos, D.O.; et al. Galectin-3 Plays a Protective Role in Leishmania (Leishmania) amazonensis Infection. Glycobiology 2021, 31, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Marin-neto, J.A.; Simões, M.V.; Sarabanda, A.V. The Chagas’ Heart Disease. Arq. Bras. Cardiol. 1999, 72, 247–280. [Google Scholar] [CrossRef] [PubMed]
- Seropian, I.M.; González, G.E.; Maller, S.M.; Berrocal, D.H.; Abbate, A.; Rabinovich, G.A. Galectin-1 as an Emerging Mediator of cardiovascular Inflammation: Mechanisms and Therapeutic Opportunities. Mediat. Inflamm. 2018, 20, 8696543. [Google Scholar] [CrossRef]
- Giordanengo, L.; Gea, S.; Barbieri, G.; Rabinovich, G.A. Anti-galectin-1 autoantibodies in human infection: Differential expression of this β-galactoside-binding protein in cardiac Chagas’ disease. Clin. Exp. Immunol. 2001, 124, 266–273. [Google Scholar] [CrossRef]
- Poncini, C.V.; Ilarregui, J.M.; Batalla, E.I.; Engels, S.; Cerliani, J.P.; Cucher, M.A.; van Kooyk, Y.; González-Cappa, S.M.; Rabinovich, G.A. Trypanosoma cruzi infection imparts a regulatory program in dendritic cells and T cells via galectin-1-dependent mechanisms. J. Immunol. 2015, 195, 3311–3324. [Google Scholar] [CrossRef]
- Zuñiga, E.; Rabinovich, G.A.; Iglesias, M.M.; Gruppi, A. Regulated expression of galectin-1 during B-cell activation and implications for T-cell apoptosis. J. Leukoc. Biol. 2001, 70, 73–79. [Google Scholar] [CrossRef]
- Benatar, A.F.; García, G.A.; Bua, J.; Cerliani, J.P.; Postan, M.; Tasso, L.M.; Scaglione, J.; Stupirski, J.C.; Toscano, M.A.; Rabinovich, G.A.; et al. Galectin-1 Prevents Infection and Damage Induced by Trypanosoma cruzi on Cardiac Cells. PLoS Negl. Trop. Dis. 2015, 9, e0004148. [Google Scholar] [CrossRef]
- Pineda, M.A.; Cuervo, H.; Fresno, M.; Soto, M.; Bonay, P. Lack of galectin-3 prevents cardiac fibrosis and effective immune responses in a murine model of Trypanosoma cruzi infection. J. Infect. Dis. 2015, 212, 1160–1171. [Google Scholar] [CrossRef]
- Pineda, M.A.; Corvo, L.; Soto, M.; Fresno, M.; Bonay, P. Interactions of human galectins with Trypanosoma cruzi: Binding profile correlate with genetic clustering of lineages. Glycobiology 2015, 25, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.S.F.; Silva, D.N.; Carvalho, R.H.; Sampaio, G.L.d.A.; Paredes, B.D.; França, L.A.; Azevedo, C.M.; Vasconcelos, J.F.; Meira, C.S.; Neto, P.C.; et al. Association of Cardiac Galectin-3 Expression, Myocarditis, and Fibrosis in Chronic Chagas Disease Cardiomyopathy. Am. J. Pathol. 2017, 187, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.S.d.F.; da Silva, K.N.; Silva, D.N.; Rocha, V.P.C.; Paredes, B.D.; Azevedo, C.M.; Nonaka, C.K.; Carvalho, G.B.; Vasconcelos, J.F.; dos Santos, R.R.; et al. Galectin-3 Knockdown Impairs Survival, Migration, and Immunomodulatory Actions of Mesenchymal Stromal Cells in a Mouse Model of Chagas Disease Cardiomyopathy. Stem Cells Int. 2017, 2017, 3282656. [Google Scholar] [CrossRef] [PubMed]
- Noya-Rabelo, M.M.; Larocca, T.F.; Macêdo, C.T.; Torreão, J.A.; De Freitas Souza, B.S.; Vasconcelos, J.F.; Souza, L.E.; Silva, A.M.; Dos Santos, R.R.; Correia, L.C.L.; et al. Evaluation of Galectin-3 as a Novel Biomarker for Chagas Cardiomyopathy. Cardiology 2016, 136, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Beghini, M.; de Araújo, M.F.; Severino, V.O.; Etchebehere, R.M.; Rocha Rodrigues, D.B.; de Lima Pereira, S.A. Evaluation of the immunohistochemical expression of Gal-1, Gal-3 and Gal-9 in the colon of chronic chagasic patients. Pathol. Res. Pract. 2017, 213, 1207–1214. [Google Scholar] [CrossRef]
- Vasconcelos, J.F.; Meira, C.S.; Silva, D.N.; Nonaka, C.K.V.; Daltro, P.S.; Macambira, S.G.; Domizi, P.D.; Borges, V.M.; Ribeiro-Dos-Santos, R.; De Freitas Souza, B.S.; et al. Therapeutic effects of sphingosine kinase inhibitor N,N-dimethylsphingosine (DMS) in experimental chronic Chagas disease cardiomyopathy. Sci. Rep. 2017, 7, 6171. [Google Scholar] [CrossRef]
- Fernandes, F.; Moreira, C.H.V.; Oliveira, L.C.; Souza-Basqueira, M.; Ianni, B.M.; di Lorenzo, C.; Ramires, F.J.A.; Nastari, L.; Cunha-Neto, E.; Ribeiro, A.L.; et al. Galectina-3 Associada a Formas Graves e Mortalidade em Longo Prazo em Pacientes com Doença de Chagas. Arq. Bras. Cardiol. 2019, 116, 248–256. [Google Scholar] [CrossRef]
- Bertelli, A.; Sanmarco, L.M.; Pascuale, C.A.; Postan, M.; Aoki, M.P.; Leguizamón, M.S. Anti-inflammatory role of galectin-8 during Trypanosoma cruzi chronic infection. Front. Cell. Infect. Microbiol. 2020, 10, 285. [Google Scholar] [CrossRef]
- Elola, M.T.; Ferragut, F.; Cárdenas Delgado, V.M.; Nugnes, L.G.; Gentilini, L.; Laderach, D.; Troncoso, M.F.; Compagno, D.; Wolfenstein-Todel, C.; Rabinovich, G.A. Expression, localization and function of galectin-8, a tandem-repeat lectin, in human tumors. Histol. Histopathol. 2014, 29, 1093–1105. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meira, C.; Silva, J.; Quadros, H.; Silva, L.; Barreto, B.; Rocha, V.; Bomfim, L.; Santos, E.; Soares, M. Galectins in Protozoan Parasitic Diseases: Potential Applications in Diagnostics and Therapeutics. Cells 2023, 12, 2671. https://doi.org/10.3390/cells12232671
Meira C, Silva J, Quadros H, Silva L, Barreto B, Rocha V, Bomfim L, Santos E, Soares M. Galectins in Protozoan Parasitic Diseases: Potential Applications in Diagnostics and Therapeutics. Cells. 2023; 12(23):2671. https://doi.org/10.3390/cells12232671
Chicago/Turabian StyleMeira, Cássio, Jaqueline Silva, Helenita Quadros, Laís Silva, Breno Barreto, Vinícius Rocha, Larissa Bomfim, Emanuelle Santos, and Milena Soares. 2023. "Galectins in Protozoan Parasitic Diseases: Potential Applications in Diagnostics and Therapeutics" Cells 12, no. 23: 2671. https://doi.org/10.3390/cells12232671
APA StyleMeira, C., Silva, J., Quadros, H., Silva, L., Barreto, B., Rocha, V., Bomfim, L., Santos, E., & Soares, M. (2023). Galectins in Protozoan Parasitic Diseases: Potential Applications in Diagnostics and Therapeutics. Cells, 12(23), 2671. https://doi.org/10.3390/cells12232671








