Animal Models for the Study of Keratoconus
Abstract
:1. Introduction
2. Mouse Models
2.1. Treatment-Induced Mouse Models
2.2. Spontaneous Mouse Models
2.3. Genetic Mouse Models
3. Rat Models
Treatment-Induced Rat Models
4. Rabbit Models
Treatment-Induced Rabbit Models
5. Proposed Novel Animals
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian. J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Scroggs, M.W.; Proia, A.D. Histopathological variation in keratoconus. Cornea 1992, 11, 553–559. [Google Scholar] [CrossRef]
- Naderan, M.; Jahanrad, A.; Balali, S. Histopathologic findings of keratoconus corneas underwent penetrating keratoplasty according to topographic measurements and keratoconus severity. Int. J. Ophthalmol. 2017, 10, 1640–1646. [Google Scholar] [CrossRef]
- Lozano, V.; Martín, C.; Blanco, N.; Alcalde, I.; Fernández-Vega Cueto, L.; Merayo-Lloves, J.; Quirós, L.M. Exosomes Released by Corneal Stromal Cells Show Molecular Alterations in Keratoconus Patients and Induce Different Cellular Behavior. Biomedicines 2022, 10, 2348. [Google Scholar] [CrossRef]
- Sykakis, E.; Carley, F.; Irion, L.; Denton, J.; Hillarby, M.C. An in depth analysis of histopathological characteristics found in keratoconus. Pathology 2012, 44, 234–239. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Cont. Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef]
- Gordon-Shaag, A.; Millodot, M.; Shneor, E.; Liu, Y. The genetic and environmental factors for keratoconus. Biomed. Res. Int. 2015, 2015, 795738. [Google Scholar] [CrossRef]
- Hashemi, H.; Heydarian, S.; Hooshmand, E.; Saatchi, M.; Yekta, A.; Aghamirsalim, M.; Valadkhan, M.; Mortazavi, M.; Hashemi, A.; Khabazkhoob, M. The Prevalence and Risk Factors for Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2020, 39, 263–270. [Google Scholar] [CrossRef]
- Wang, Y.; Rabinowitz, Y.S.; Rotter, J.I.; Yang, H. Genetic epidemiological study of keratoconus: Evidence for major gene determination. Am. J. Med. Genet. 2000, 93, 403–409. [Google Scholar] [CrossRef]
- Kriszt, Á.; Losonczy, G.; Berta, A.; Vereb, G.; Takács, L. Segregation analysis suggests that keratoconus is a complex non-mendelian disease. Acta Ophthalmol. 2014, 92, e562–e568. [Google Scholar] [CrossRef]
- Bykhovskaya, Y.; Rabinowitz, Y.S. Update on the genetics of keratoconus. Exp. Eye Res. 2021, 202, 108398. [Google Scholar] [CrossRef]
- Li, X.; Bykhovskaya, Y.; Canedo, A.L.C.; Haritunians, T.; Siscovick, D.; Aldave, A.J.; Szczotka-Flynn, L.; Iyengar, S.K.; Rotter, J.I.; Taylor, K.D.; et al. Genetic Association of COL5A1 Variants in Keratoconus Patients Suggests a Complex Connection between Corneal Thinning and Keratoconus. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2696–2704. [Google Scholar] [CrossRef]
- Dudakova, L.; Palos, M.; Jirsova, K.; Stranecky, V.; Krepelova, A.; Hysi, P.G.; Liskova, P. Validation of rs2956540:G>C and rs3735520:G>A association with keratoconus in a population of European descent. Eur. J. Human Genet. 2015, 23, 1581–1583. [Google Scholar] [CrossRef]
- Burdon, K.P.; Macgregor, S.; Bykhovskaya, Y.; Javadiyan, S.; Li, X.; Laurie, K.J.; Muszynska, D.; Lindsay, R.; Lechner, J.; Haritunians, T.; et al. Association of Polymorphisms in the Hepatocyte Growth Factor Gene Promoter with Keratoconus. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8514–8519. [Google Scholar] [CrossRef]
- Sahebjada, S.; Schache, M.; Richardson, A.J.; Snibson, G.; MacGregor, S.; Daniell, M.; Baird, P.N. Evaluating the Association Between Keratoconus and the Corneal Thickness Genes in an Independent Australian Population. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8224–8228. [Google Scholar] [CrossRef]
- Simcoe, M.J.; Khawaja, A.P.; Hysi, P.G.; Hammond, C.J.; Eye, U.B.; Consortium, V. Genome-wide association study of corneal biomechanical properties identifies over 200 loci providing insight into the genetic etiology of ocular diseases. Human. Mol. Genet. 2020, 29, 3154–3164. [Google Scholar] [CrossRef]
- Lu, Y.; Vitart, V.; Burdon, K.P.; Khor, C.C.; Bykhovskaya, Y.; Mirshahi, A.; Hewitt, A.W.; Koehn, D.; Hysi, P.G.; Ramdas, W.D.; et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat. Genet. 2013, 45, 155–163. [Google Scholar] [CrossRef]
- Jaskiewicz, K.; Maleszka-Kurpiel, M.; Michalski, A.; Ploski, R.; Rydzanicz, M.; Gajecka, M. Non-allergic eye rubbing is a major behavioral risk factor for keratoconus. PLoS ONE 2023, 18, e0284454. [Google Scholar] [CrossRef]
- Delic, N.C.; Lyons, J.G.; Di Girolamo, N.; Halliday, G.M. Damaging Effects of Ultraviolet Radiation on the Cornea. Photochem. Photobiol. 2017, 93, 920–929. [Google Scholar] [CrossRef]
- Hughes, A.E.; Dash, D.P.; Jackson, A.J.; Frazer, D.G.; Silvestri, G. Familial Keratoconus with Cataract: Linkage to the Long Arm of Chromosome 15 and Exclusion of Candidate Genes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5063–5066. [Google Scholar] [CrossRef]
- Mathan, J.J.; Gokul, A.; Simkin, S.K.; Meyer, J.J.; Patel, D.V.; McGhee, C.N.J. Topographic screening reveals keratoconus to be extremely common in Down syndrome. Clin. Exp. Ophthalmol. 2020, 48, 1160–1167. [Google Scholar] [CrossRef]
- Robertson, I. Keratoconus and the Ehlers-Danlos syndrome: A new aspect of keratoconus. Med. J. Aust. 1975, 1, 571–573. [Google Scholar] [CrossRef]
- Newkirk, K.M.; Chandler, H.L.; Parent, A.E.; Young, D.C.; Colitz, C.M.; Wilkie, D.A.; Kusewitt, D.F. Ultraviolet radiation-induced corneal degeneration in 129 mice. Toxicol. Pathol. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Ebihara, N.; Funaki, T.; Matsuda, H.; Okumura, K.; Murakami, A.; Ra, C. Corneal abnormalities in the NC/Nga mouse: An atopic dermatitis model. Cornea 2008, 27, 923–929. [Google Scholar] [CrossRef]
- Moghadam, F.A.; Hadipour Jahromy, M.; Fazelipour, S.; Khakpour, S.; Younesian, M. Induction of experimental keratoconus in mice using collagenase. Physiol. Pharmacol. 2009, 13, 209–215. [Google Scholar]
- Bech, F.; Alcalde, I.; Íñigo-Portugués, A.; Braga, P.; Artime, E.; Garcia, B.; Alfonso, J.; Merayo-Lloves, J. Induction of experimental keratoconic ectasias by Endo-β-Galactosidase extracellular matrix digestion. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2998. [Google Scholar]
- Tachibana, M.; Okamoto, M.; Sakamoto, M.; Matsushima, Y. Hereditary keratoconus-like keratopathy in Japanese wild mice mapped to mouse Chromosome 13. Mamm. Genome 2002, 13, 692–695. [Google Scholar] [CrossRef]
- Tachibana, M.; Adachi, W.; Kinoshita, S.; Kobayashi, Y.; Honma, Y.; Hiai, H.; Matsushima, Y. Androgen-Dependent Hereditary Mouse Keratoconus: Linkage to an MHC Region. Investig. Ophthalmol. Vis. Sci. 2002, 43, 51–57. [Google Scholar]
- Quantock, A.J.; Young, R.D.; Akama, T.O. Structural and biochemical aspects of keratan sulphate in the cornea. Cell. Mol. Life Sci. 2010, 67, 891–906. [Google Scholar] [CrossRef]
- Tost, F.; Wolfinger, J.; Giebel, J.; Buselmaier, W. Corneal anomalies in murine trisomy 16. Ophthalmologe 2005, 102, 64–69. [Google Scholar] [CrossRef]
- Parapuram, S.K.; Huh, K.; Liu, S.; Leask, A. Integrin β1 is necessary for the maintenance of corneal structural integrity. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7799–7806. [Google Scholar] [CrossRef]
- Stanton, C.M.; Findlay, A.S.; Drake, C.; Mustafa, M.Z.; Gautier, P.; McKie, L.; Jackson, I.J.; Vitart, V. A mouse model of brittle cornea syndrome caused by mutation in Zfp469. Dis. Model. Mech. 2021, 14, dmm049175. [Google Scholar] [CrossRef]
- Khaled, M.L.; Bykhovskaya, Y.; Gu, C.; Liu, A.; Drewry, M.D.; Chen, Z.; Mysona, B.A.; Parker, E.; McNabb, R.P.; Yu, H.; et al. PPIP5K2 and PCSK1 are Candidate Genetic Contributors to Familial Keratoconus. Sci. Rep. 2019, 9, 19406. [Google Scholar] [CrossRef]
- Terceiro, L.E.L.; Blanchard, A.A.A.; Edechi, C.A.; Freznosa, A.; Triggs-Raine, B.; Leygue, E.; Myal, Y. Generation of prolactin-inducible protein (Pip) knockout mice by CRISPR/Cas9-mediated gene engineering. Can. J. Physiol. Pharmacol. 2022, 100, 86–91. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Zolnik, O.B.; Yasoda, S.; Yeh, L.-K.; Yuan, Y.; Kao, W.; Saika, S.; Liu, C.-Y. Transforming growth factor beta receptor 2 (Tgfbr2) deficiency in keratocytes results in corneal ectasia. Ocul. Surf. 2023, 29, 557–565. [Google Scholar] [CrossRef]
- Joseph, R.; Boateng, A.; Srivastava, O.P.; Pfister, R.R. Role of Fibroblast Growth Factor Receptor 2 (FGFR2) in Corneal Stromal Thinning. Investig. Ophthalmol. Vis. Sci. 2023, 64, 40. [Google Scholar] [CrossRef]
- Kronschläger, M.; Talebizadeh, N.; Yu, Z.; Meyer, L.M.; Löfgren, S. Apoptosis in Rat Cornea After In Vivo Exposure to Ultraviolet Radiation at 300 nm. Cornea 2015, 34, 945–949. [Google Scholar] [CrossRef]
- Peterson, C.; Kim, Y.C.; Ensign, L.M.; Jun, A.S.; Foster, J. Induction of the integrated stress response in the rat cornea. Exp. Eye Res. 2021, 210, 108722. [Google Scholar] [CrossRef]
- Mutch, J.R.; Richards, M.B. Keratoconus Experimentally Produced in the Rat by Vitamin A Deficiency. Br. J. Ophthalmol. 1939, 23, 381–387. [Google Scholar] [CrossRef]
- Qiao, J.; Li, H.; Tang, Y.; Song, W.; Rong, B.; Yang, S.; Wu, Y.; Yan, X. A rabbit model of corneal Ectasia generated by treatment with collagenase type II. BMC Ophthalmol. 2018, 18, 94. [Google Scholar] [CrossRef]
- Liu, R.; Yan, X. Sulforaphane protects rabbit corneas against oxidative stress injury in keratoconus through activation of the Nrf-2/HO-1 antioxidant pathway. Int. J. Mol. Med. 2018, 42, 2315–2328. [Google Scholar] [CrossRef]
- Kobashi, H.; Yano, T.; Tsubota, K. Combination of violet light irradiation and collagenase treatments in a rabbit model of keratoconus. Front. Med. 2023, 10, 1109689. [Google Scholar] [CrossRef]
- Cano-Gómez, L.E.; Casillas-Casillas, E.; Andrade-Lozano, P.; Ventura-Juárez, J.; Barba-Gallardo, L.F. Animal model of corneal ectasia in rabbits by intrastromal injection of type II collagenase. Arch. Soc. Esp. Oftalmol. Engl. Ed. 2023, 98, 206–212. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Chen, Y.; Ye, C.; Wei, W.; Feng, Y.; Mi, S. Study on patterned photodynamic cross-linking for keratoconus. Exp. Eye Res. 2021, 204, 108450. [Google Scholar] [CrossRef]
- Wei, J.; He, R.; Wang, X.; Song, Y.; Yao, J.; Liu, X.; Yang, X.; Chen, W.; Li, X. The Corneal Ectasia Model of Rabbit: A Validity and Stability Study. Bioengineering 2023, 10, 479. [Google Scholar] [CrossRef]
- Yu, J.-G.; Bao, F.-J.; Joda, A.; Fu, X.-A.; Zhou, S.; Wang, J.; Hu, X.-L.; Wang, Q.-M.; Elsheikh, A. Influence of glucocorticosteroids on the biomechanical properties of in-vivo rabbit cornea. J. Mech. Behav. Biomed. Mater. 2014, 29, 350–359. [Google Scholar] [CrossRef]
- Bitgood, J.J.; Whitley, R.D. Pop-eye: An inherited Z-linked keratoglobus in the chicken. J. Hered. 1986, 77, 123–125. [Google Scholar] [CrossRef]
- Henriksson, J.T.; McDermott, A.M.; Bergmanson, J.P.G. Dimensions and Morphology of the Cornea in Three Strains of Mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3648–3654. [Google Scholar] [CrossRef]
- Rüfer, F.; Schröder, A.; Erb, C. White-to-white corneal diameter: Normal values in healthy humans obtained with the Orbscan II topography system. Cornea 2005, 24, 259–261. [Google Scholar] [CrossRef]
- Smith, R.S.; John, S.W.; Nishina, P.M.; Sundberg, J.P. Systematic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wilson, S.E. Bowman’s layer in the cornea—Structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef]
- Henriksson, J.T.; Bron, A.J.; Bergmanson, J.P. An explanation for the central to peripheral thickness variation in the mouse cornea. Clin. Exp. Ophthalmol. 2012, 40, 174–181. [Google Scholar] [CrossRef]
- Schulz, D.; Iliev, M.E.; Frueh, B.E.; Goldblum, D. In vivo pachymetry in normal eyes of rats, mice and rabbits with the optical low coherence reflectometer. Vision Res. 2003, 43, 723–728. [Google Scholar] [CrossRef]
- Doughty, M.J.; Zaman, M.L. Human Corneal Thickness and Its Impact on Intraocular Pressure Measures: A Review and Meta-analysis Approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Hanlon, S.D.; Patel, N.B.; Burns, A.R. Assessment of postnatal corneal development in the C57BL/6 mouse using spectral domain optical coherence tomography and microwave-assisted histology. Exp. Eye Res. 2011, 93, 363–370. [Google Scholar] [CrossRef]
- He, J.; Pham, T.L.; Bazan, H.E.P. Neuroanatomy and neurochemistry of rat cornea: Changes with age. Ocul. Surf. 2021, 20, 86–94. [Google Scholar] [CrossRef]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef]
- Peiffer, R.L.; Pohm-Thorsen, L.; Corcoran, K. Chapter 19—Models in Ophthalmology and Vision Research*. In The Biology of the Laboratory Rabbit, 2nd ed.; Manning, P.J., Ringler, D.H., Newcomer, C.E., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 409–433. [Google Scholar] [CrossRef]
- Wilson, S.E. The Cornea: No Difference in the Wound Healing Response to Injury Related to Whether, or Not, There’s a Bowman’s Layer. Biomolecules 2023, 13, 771. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, X.; Cao, X.; Zhang, D.; Li, L. Age-Related Variations of Rabbit Corneal Geometrical and Clinical Biomechanical Parameters. Biomed. Res. Int. 2017, 2017, 3684971. [Google Scholar] [CrossRef]
- Ritchey, E.R.; Code, K.; Zelinka, C.P.; Scott, M.A.; Fischer, A.J. The chicken cornea as a model of wound healing and neuronal re-innervation. Mol. Vis. 2011, 17, 2440–2454. [Google Scholar]
- Fowler, W.C.; Chang, D.H.; Roberts, B.C.; Zarovnaya, E.L.; Proia, A.D. A new paradigm for corneal wound healing research: The white leghorn chicken (Gallus gallus domesticus). Curr. Eye Res. 2004, 28, 241–250. [Google Scholar] [CrossRef]
- Wisely, C.E.; Sayed, J.A.; Tamez, H.; Zelinka, C.; Abdel-Rahman, M.H.; Fischer, A.J.; Cebulla, C.M. The chick eye in vision research: An excellent model for the study of ocular disease. Prog. Retin. Eye Res. 2017, 61, 72–97. [Google Scholar] [CrossRef]
- Bitgood, J.J.; Rozum, J.J.; Rozum, J.J. Close Linkage Relationship of the Z-Linked Pop-Eye and Silver Plumage Color Loci in the Chicken 1. Poult. Sci. 1996, 75, 1067–1068. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, R.; Chen, C.S. Tree shrew (Tupaia belangeri) as a novel laboratory disease animal model. Zool. Res. 2017, 38, 127–137. [Google Scholar] [CrossRef]
- Jasien, J.V.; Read, A.T.; van Batenburg-Sherwood, J.; Perkumas, K.M.; Ethier, C.R.; Stamer, W.D.; Samuels, B.C. Anterior Segment Anatomy and Conventional Outflow Physiology of the Tree Shrew (Tupaia belangeri). Investig. Ophthalmol. Vis. Sci. 2022, 63, 21. [Google Scholar] [CrossRef]
- Knox Cartwright, N.E.; Tyrer, J.R.; Marshall, J. Age-related differences in the elasticity of the human cornea. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4324–4329. [Google Scholar] [CrossRef]
- Koehn, D.R.; Meyer, K.J.; Anderson, M.G. Genetic Evidence for Differential Regulation of Corneal Epithelial and Stromal Thickness. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5599–5607. [Google Scholar] [CrossRef]
- Mackiewicz, Z.; Määttä, M.; Stenman, M.; Konttinen, L.; Tervo, T.; Konttinen, Y.T. Collagenolytic proteinases in keratoconus. Cornea 2006, 25, 603–610. [Google Scholar] [CrossRef]
- Ashwin, P.T.; McDonnell, P.J. Collagen cross-linkage: A comprehensive review and directions for future research. Br. J. Ophthalmol. 2010, 94, 965–970. [Google Scholar] [CrossRef]
- Fukuda, M.N.; Matsumura, G. Endo-beta-galactosidase of Escherichia freundii. Purification and endoglycosidic action on keratan sulfates, oligosaccharides, and blood group active glycoprotein. J. Biol. Chem. 1976, 251, 6218–6225. [Google Scholar] [CrossRef]
- Liu, C.Y.; Birk, D.E.; Hassell, J.R.; Kane, B.; Kao, W.W. Keratocan-deficient mice display alterations in corneal structure. J. Biol. Chem. 2003, 278, 21672–21677. [Google Scholar] [CrossRef]
- Whitelock, R.B.; Li, Y.; Zhou, L.L.; Sugar, J.; Yue, B.Y. Expression of transcription factors in keratoconus, a cornea-thinning disease. Biochem. Biophys. Res. Commun. 1997, 235, 253–258. [Google Scholar] [CrossRef]
- Quantock, A.J.; Dennis, S.; Adachi, W.; Kinoshita, S.; Boote, C.; Meek, K.M.; Matsushima, Y.; Tachibana, M. Annulus of collagen fibrils in mouse cornea and structural matrix alterations in a murine-specific keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1906–1911. [Google Scholar] [CrossRef]
- Stepp, M.A. Corneal integrins and their functions. Exp. Eye Res. 2006, 83, 3–15. [Google Scholar] [CrossRef]
- Bao, J.; Yu, X.; Ping, X.; Shentu, X.; Zou, J. Znf469 Plays a Critical Role in Regulating Synthesis of ECM: A Zebrafish Model of Brittle Cornea Syndrome. Investig. Ophthalmol. Vis. Sci. 2023, 64, 29. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Hjortdal, J.; Sarker-Nag, A.; Sejersen, H.; Asara, J.M.; Karamichos, D. Gross cystic disease fluid protein-15/prolactin-inducible protein as a biomarker for keratoconus disease. PLoS ONE 2014, 9, e113310. [Google Scholar] [CrossRef]
- Guan, T.; Liu, C.; Ma, Z.; Ding, S. The point mutation and polymorphism in keratoconus candidate gene TGFBI in Chinese population. Gene 2012, 503, 137–139. [Google Scholar] [CrossRef]
- Wilson, S.E.; Walker, J.W.; Chwang, E.L.; He, Y.G. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2544–2561. [Google Scholar]
- Joseph, R.; Srivastava, O.P.; Pfister, R.R. Modeling Keratoconus Using Induced Pluripotent Stem Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3685–3697. [Google Scholar] [CrossRef]
- Ortega, S.; Ittmann, M.; Tsang, S.H.; Ehrlich, M.; Basilico, C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA 1998, 95, 5672–5677. [Google Scholar] [CrossRef]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Chapter 7—Commonly Used Animal Models. In Principles of Animal Research for Graduate and Undergraduate Students; Academic Press: Cambridge, MA, USA, 2017; pp. 117–175. [Google Scholar] [CrossRef]
- Foster, J.W.; Shinde, V.; Soiberman, U.S.; Sathe, G.; Liu, S.; Wan, J.; Qian, J.; Dauoud, Y.; Pandey, A.; Jun, A.S.; et al. Integrated Stress Response and Decreased ECM in Cultured Stromal Cells From Keratoconus Corneas. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2977–2986. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Gudjónsson, S.V. Experiments on Vitamin A Deficiency in Rats and the Quantitative Determination of Vitamin A. Acta Pathol. Microbiol. Scand. 1930, Supplementum IV. [Google Scholar]
- Marcovich, A.L.; Brandis, A.; Daphna, O.; Feine, I.; Pinkas, I.; Goldschmidt, R.; Kalchenko, V.; Berkutzki, T.; Wagner, H.D.; Salomon, Y.; et al. Stiffening of rabbit corneas by the bacteriochlorophyll derivative WST11 using near infrared light. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6378–6388. [Google Scholar] [CrossRef]
- Liu, R.; Yan, X. Oxidative stress in corneal stromal cells contributes to the development of keratoconus in a rabbit model. Eur. J. Ophthalmol. 2021, 31, 3518–3524. [Google Scholar] [CrossRef]
- Spoerl, E.; Zubaty, V.; Terai, N.; Pillunat, L.E.; Raiskup, F. Influence of High-Dose Cortisol on the Biomechanics of Incubated Porcine Corneal Strips. J. Refract. Surg. 2009, 25, S794–S798. [Google Scholar] [CrossRef]
- Hocking, P.M.; Guggenheim, J.A. The chick as an animal model of eye disease. Drug Discov. Today Dis. Models 2013, 10, e225–e230. [Google Scholar] [CrossRef]
- Almubrad, T.; Akhtar, S. Structure of corneal layers, collagen fibrils, and proteoglycans of tree shrew cornea. Mol. Vis. 2011, 17, 2283–2291. [Google Scholar]
- Wu, M.; Kuang, D.X.; Huang, Y.Q.; Miao, Y.R.; Liu, X.C.; Dai, J.J. Age-related changes of corneal endothelial cell in healthy Chinese tree shrew measured by non-contact specular microscope. Int. J. Ophthalmol. 2017, 10, 1798–1804. [Google Scholar] [CrossRef]


| Author (Year) | Species | Strain | Genetic Modification | Treatment | KC Features |
|---|---|---|---|---|---|
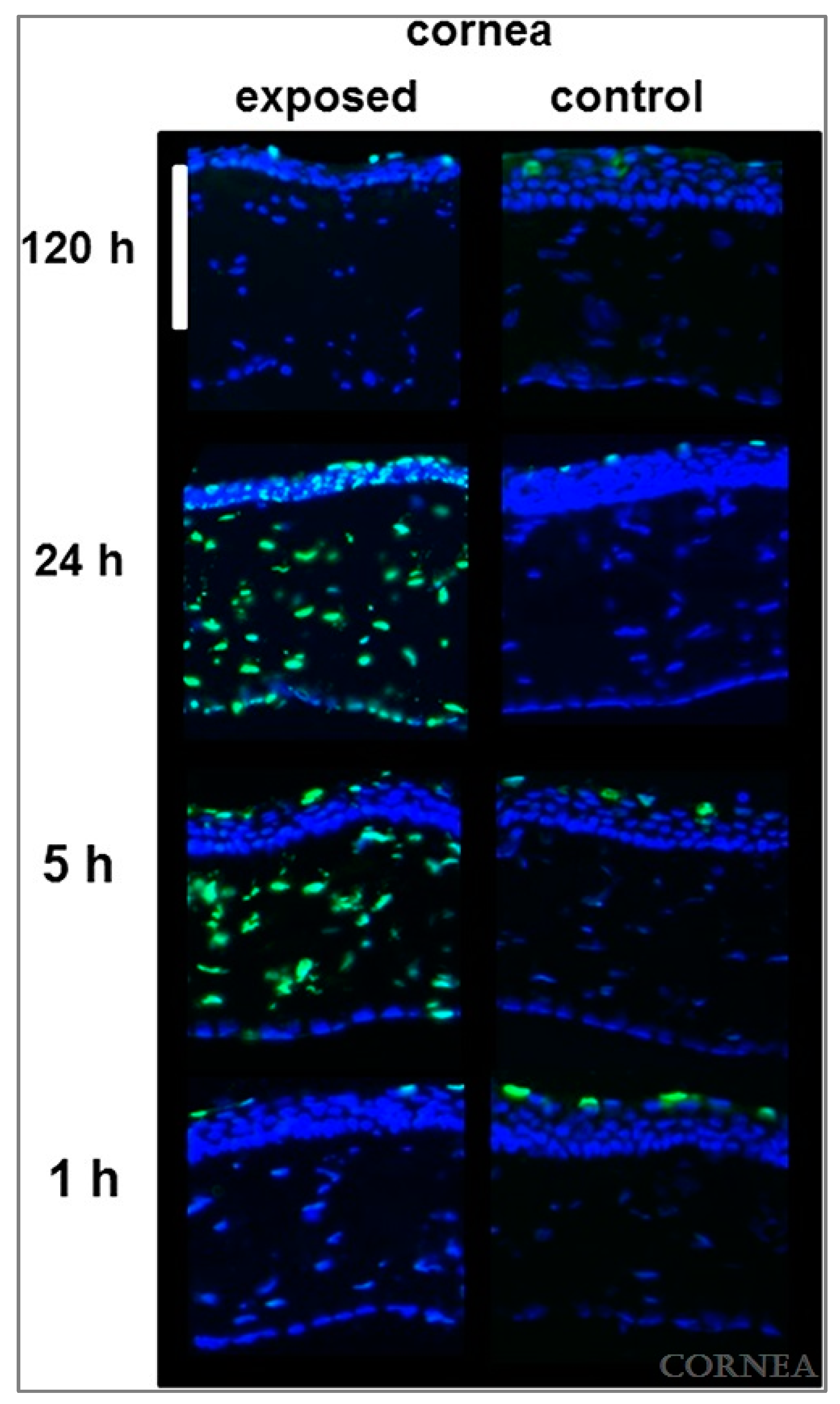
| Newkirk et al. (2007) [26] | Mouse | 129S1/SvImJ | None | 60% UVA 40% UVB exposure | Cone-like protrusion, loss of keratocytes, stromal thinning, corneal vascularization, corneal fibrosis, keratitis |
| Ebihara et al. (2008) [27] | Mouse | NC/Nga | None | Eye rubbing/scratching | Cone-like protrusion, epithelial thinning; irregular interface between the epithelium and stroma; epithelial fibrosis; hemidesmosome accumulation in basal cells; deposition of material under epithelial cells; keratocyte deformity; disorganization of stromal collagen fibers; stromal neovascularization |
| Moghadam et al. (2009) [28] | Mouse | BALB/c | None | Collagenase | Damaged collagen fibrils, epithelial thinning, corneal rupture |
| Bech et al. (2005) [29] | Mouse | Not specified | Not specified | Photorefractive keratectomy endo-β-galactosidase | Cone-like protrusion, epithelial and stromal thinning, keratocan and β-catenin expression |
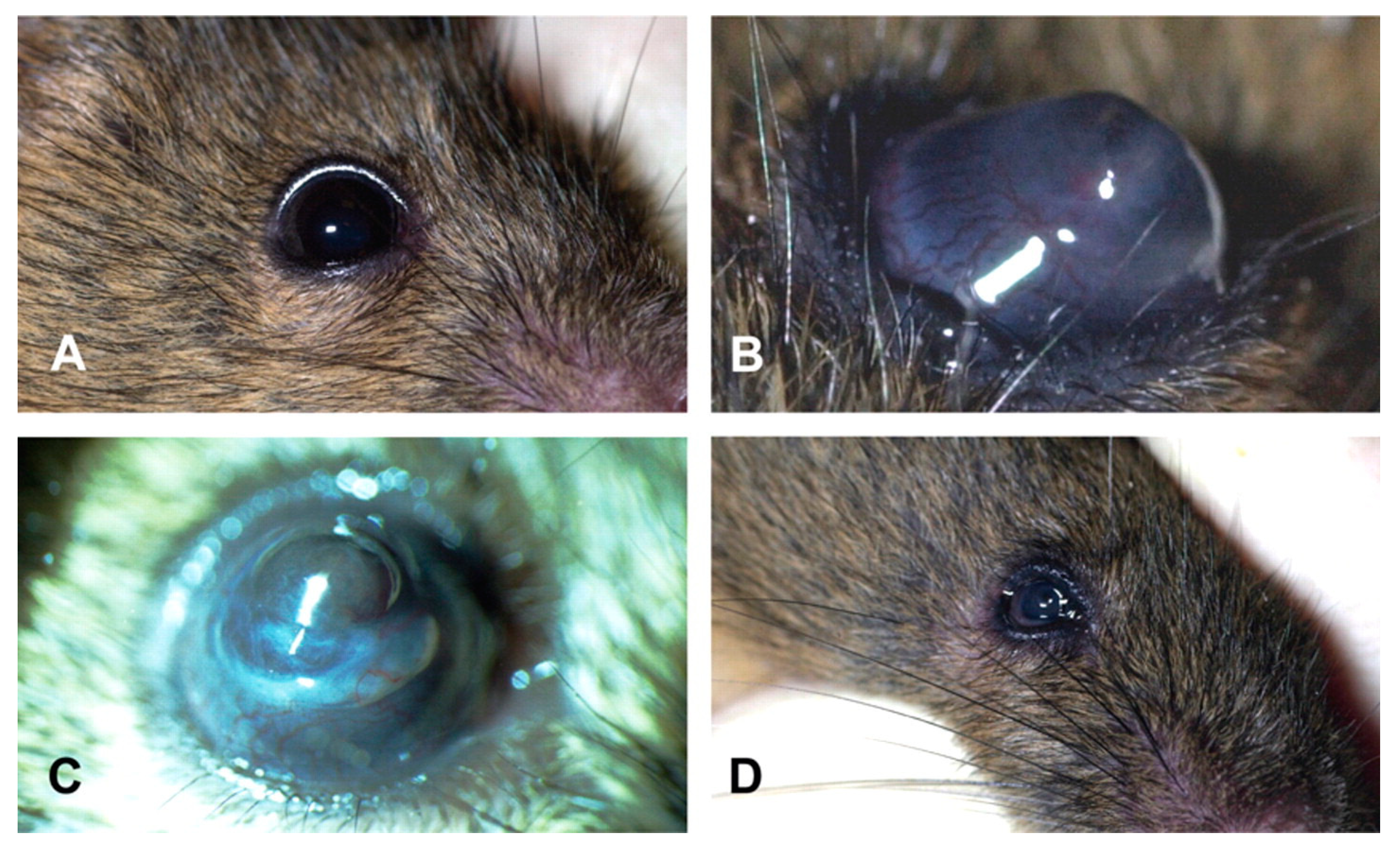
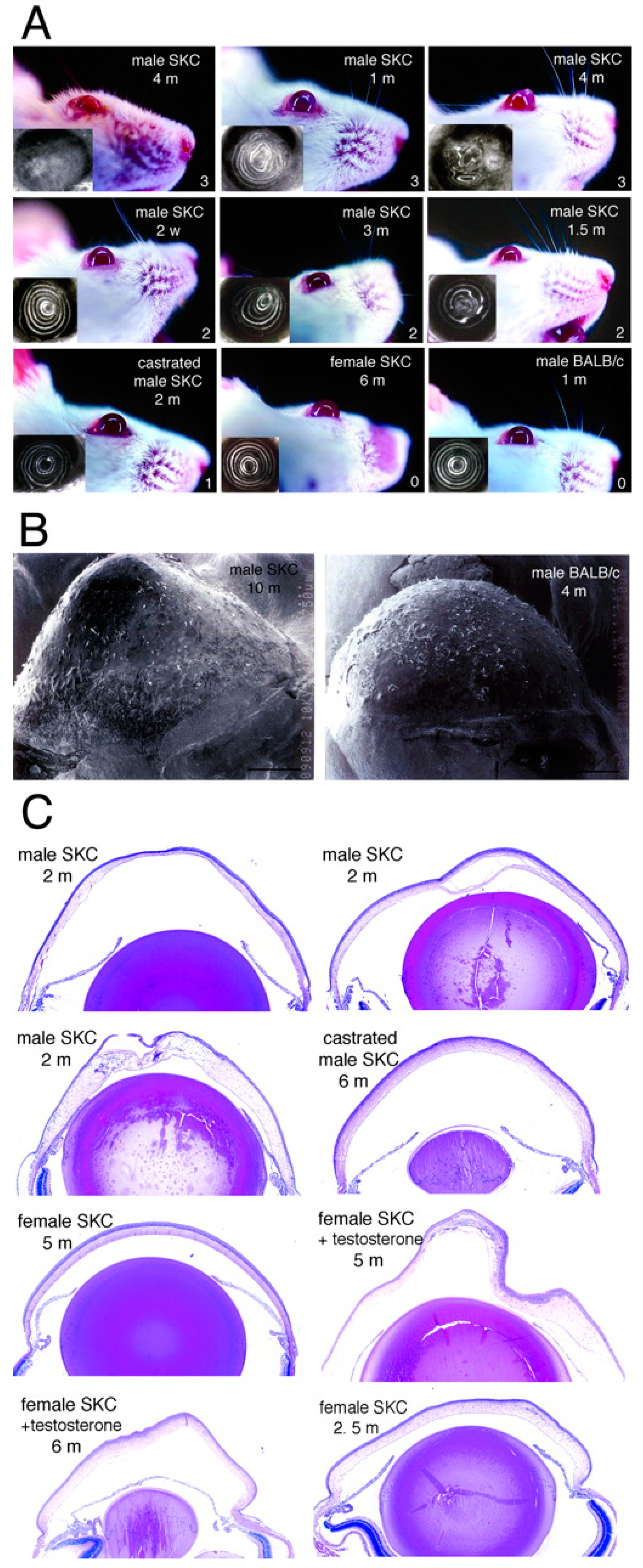
| Tachibana et al. (2002) [30] | Mouse | SKC | None | Castration of males, androgen for females | Cone-like protrusion; large, widely spaced collagen fibrils |
| Tachibana et al. (2002) [31] | Mouse | Japanese keratoconus (JKC) | None | None | |
| Quantock et al. (2003) [32] | Mouse | SKC | None | None | |
| Tost et al. (2005) [33] | Mouse | Not specified | Transgenic murine trisomy 16 | None | Corneal hypoplasia, stromal fibrosis, degradation of lens fibers, and loss of compaction in stromal lamellae |
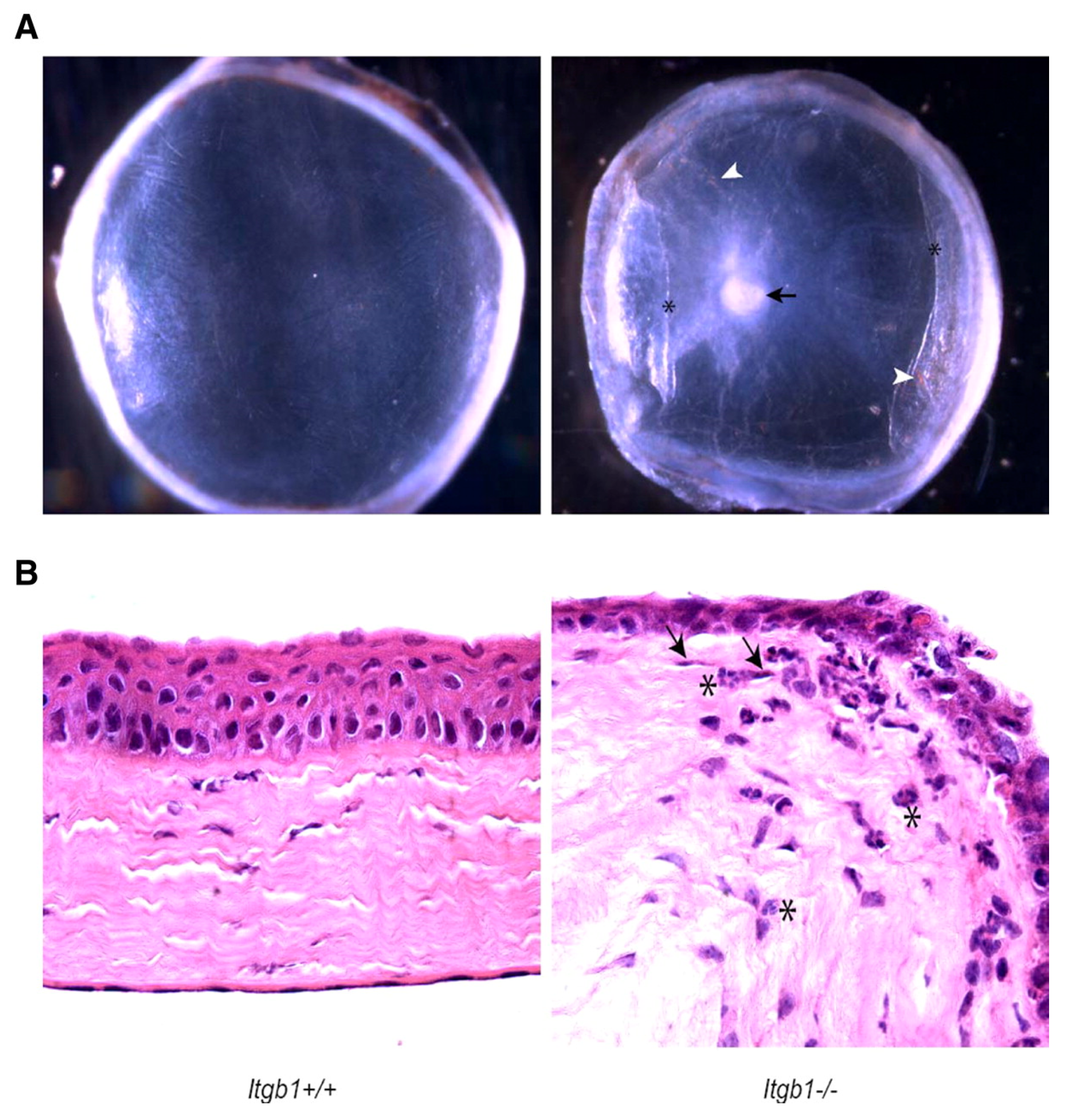
| Parapuram et al. (2011) [34] | Mouse | Itgb1 f/f | Tamoxifen-induced Cre recombinase | None | Stromal thinning, loss of epithelial cell layers, edema, scarring, stromal haze |
| Stanton et al. (2021) [35] | Mouse | Zfp469 BCS/BCS | CRISPR-Cas9-mediated genome editing | None | Stromal thinning |
| Khaled et al. (2019) [36] | Mouse | B6N (Cg)-Ppip5k2tm1b (EUCOMM)Wtsi/J | Gene trap | None | Abnormal corneal surfaces, changes in anterior chamber depth, abnormal corneal curvature, thinning of CCT |
| Terceiro et al. (2022) [37] | Mouse | C57Bl/6 | CRISPR-Cas9-mediated knockout | None | N/A |
| Wang et al. (2023) [38] | Mouse | Tgfbr2kera-cko | Conditional knockout | Eye rubbing | Stromal-specific thinning, reduced COL1a1 expression, diminished stromal collagen fibril density |
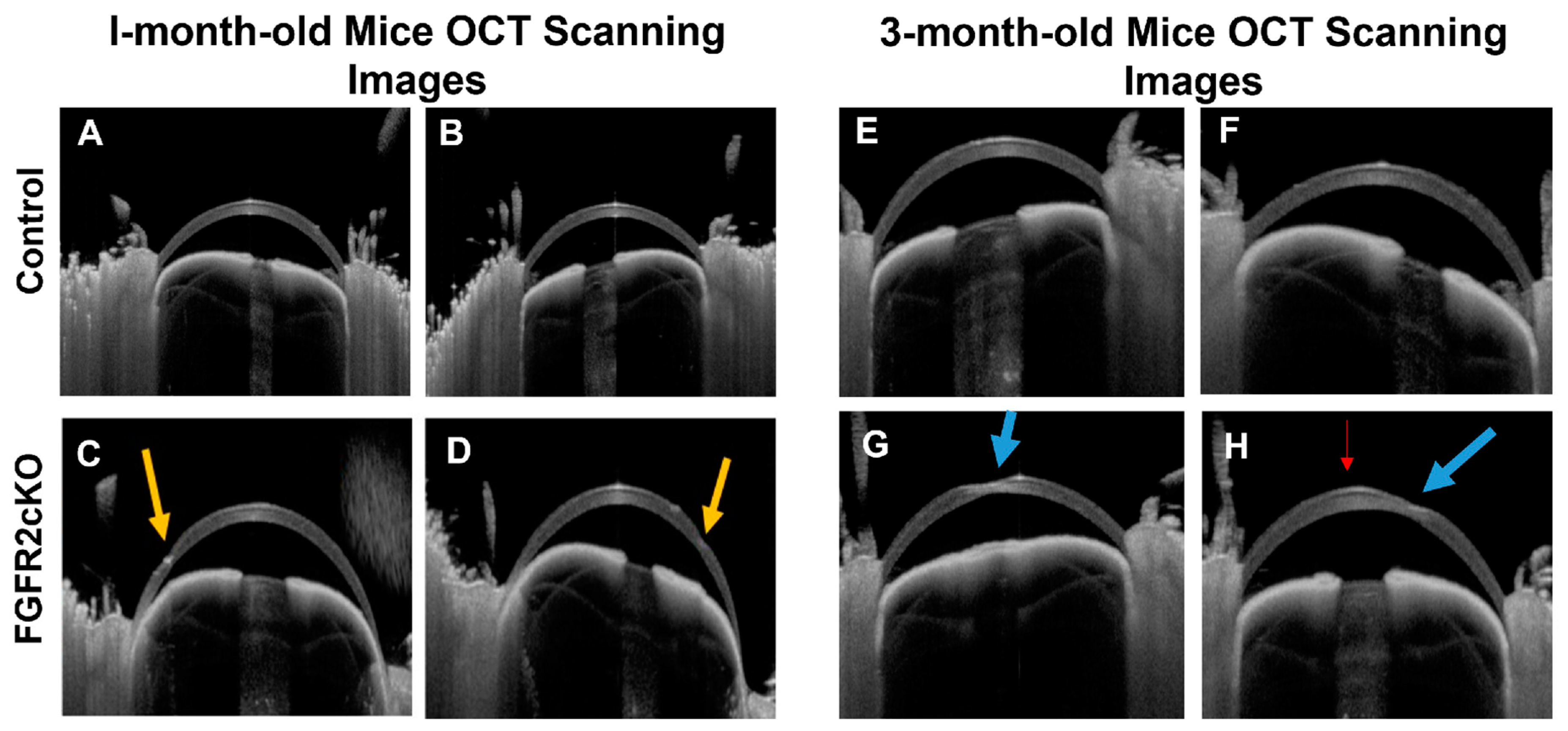
| Joseph et al. (2023) [39] | Mouse | FGFR2 KO | Inducible keratocyte-specific Cre mice | None | Localized stromal thinning |
| Kronschläger et al. (2015) [40] | Rat | Sprague-Dawley | None | UVR | Apoptosis in all corneal layers and neutrophil infiltration in the stroma |
| Peterson et al. (2021) [41] | Rat | Sprague-Dawley | None | Topical SAL003 nanosuspension | Decreased keratocyte density and reduced Col1A1 transcripts |
| Mutch et al. (1939) [42] | Rat | Not specified | None | Low vitamin A diet | Cone-like protrusion |
| Qiao et al. (2018) [43] | Rabbit | New Zealand White | None | Collagenase type II, epithelial debridement | Steepened ocular surface, central thinning, loss of corneal stiffness, loose association of stromal collagen fibrils |
| Liu and Yan (2018) [44] | Rabbit | New Zealand White | None | Collagenase type II, zinc (II) protoporphryin IX, sulforaphane | Increased corneal steepness, central thinning, loose stromal fiber association |
| Kobashi et al. (2023) [45] | Rabbit | Japanese White | None | Violet light, collagenase type II | Cone-like protrusion, increased corneal steepness, central thinning |
| Cano-Gomez et al. (2023) [46] | Rabbit | New Zealand White | None | Collagenase type II intrastromal injection | Increased corneal steepness, abnormal epithelial arrangement, loss of collagen fibril arrangement, inflamed stroma |
| Hu et al. (2021) [47] | Rabbit | New Zealand White | None | Intrastromal collagenase type I injection | Central thinning and degraded collagen fiber structure |
| Wei et al. (2023) [48] | Rabbit | Japanese White | None | Intrastromal collagenase type I injection | Progressive central thinning and compromised biomechanical integrity |
| Yu et al. (2014) [49] | Rabbit | Japanese White | None | Topical fluorometholone | Decreased biomechanical stiffness |
| Bitgood and Whitley (1986) [50] | Avian chick | Pop-eye (pop) | None | None | Keratoglobus and increased anterior chamber depth |
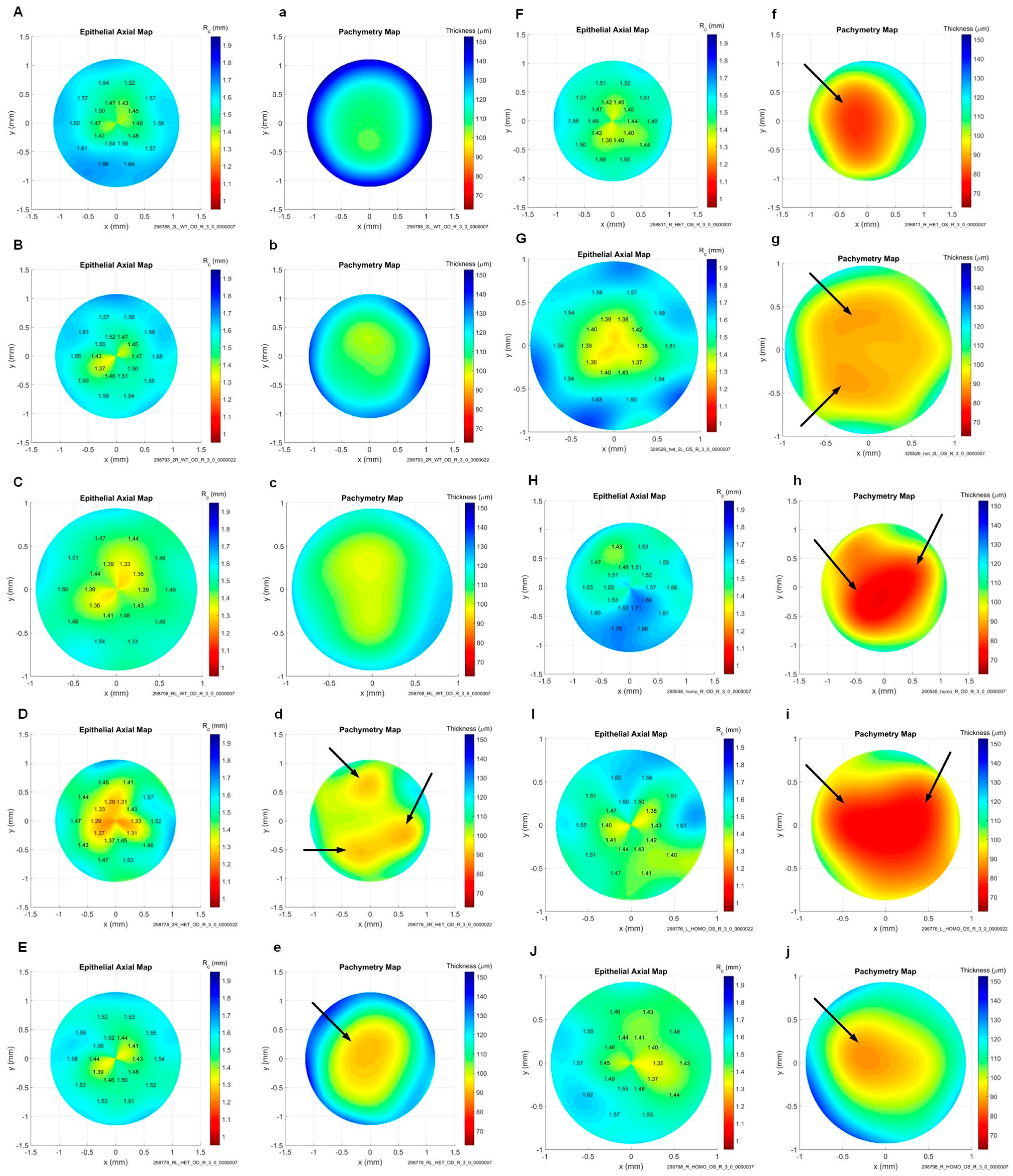
| Species | Cornea Diameter (mm) | Average CCT (μm) | Corneal Layers | Age at Corneal Maturation | References |
|---|---|---|---|---|---|
| Mouse | 2.3–2.6 | 106.0 | 4–5 layers (strain-dependent, some lack Bowman’s layer) | 8 weeks | Henricksson et al. [51], Schulz et al. [56], Hanlon et al. [58], Smith et al. [53], Wilson [54] |
| Rat | 5 | 159.08 | 5 layers | 8–12 weeks | Schulz et al. [56], He et al. [59], Hayashi et al. [60] |
| Rabbit | 15 | 356.11 | 4 layers, lacking Bowman’s layer | 18 months | Schulz et al. [56], Peiffer at al. [61], Wilson et al. [62], Zhang et al. [63] |
| Avian Chick | 9.1 | 405 (overall corneal thickness) | 5 layers | n/a | Ritchey et al. [64], Fowler et al. [65], Wisely et al. [66] |
| Tree Shrew | 8.5 | 202–301 | 5 layers | n/a | Jasien et al. [67], Almubrad et al. [68], Wu et al. [69] |
| Human | 11.7 | 565.0 | 5 layers | ~20 years | Rüfer et al. [52], Doughty et al. [57], Knox Cartwright et al. [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadvina, R.; Estes, A.; Liu, Y. Animal Models for the Study of Keratoconus. Cells 2023, 12, 2681. https://doi.org/10.3390/cells12232681
Hadvina R, Estes A, Liu Y. Animal Models for the Study of Keratoconus. Cells. 2023; 12(23):2681. https://doi.org/10.3390/cells12232681
Chicago/Turabian StyleHadvina, Rachel, Amy Estes, and Yutao Liu. 2023. "Animal Models for the Study of Keratoconus" Cells 12, no. 23: 2681. https://doi.org/10.3390/cells12232681





