ATP/IL-33-Co-Sensing by Mast Cells (MCs) Requires Activated c-Kit to Ensure Effective Cytokine Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Bone Marrow-Derived Mast Cell (BMMC) Generation and Culture
2.3. Culture of p65/RelA-EGFP and NFAT-EGFP Reporter MC/9 Cells
2.4. Stimulation of BMMC
2.5. BMMC Staining and Flow Cytometry
2.6. Detection of EGFP in p65/RelA-EGFP- or NFAT-EGFP-MC/9 Mast Cells
2.7. Statistical Analysis
3. Results
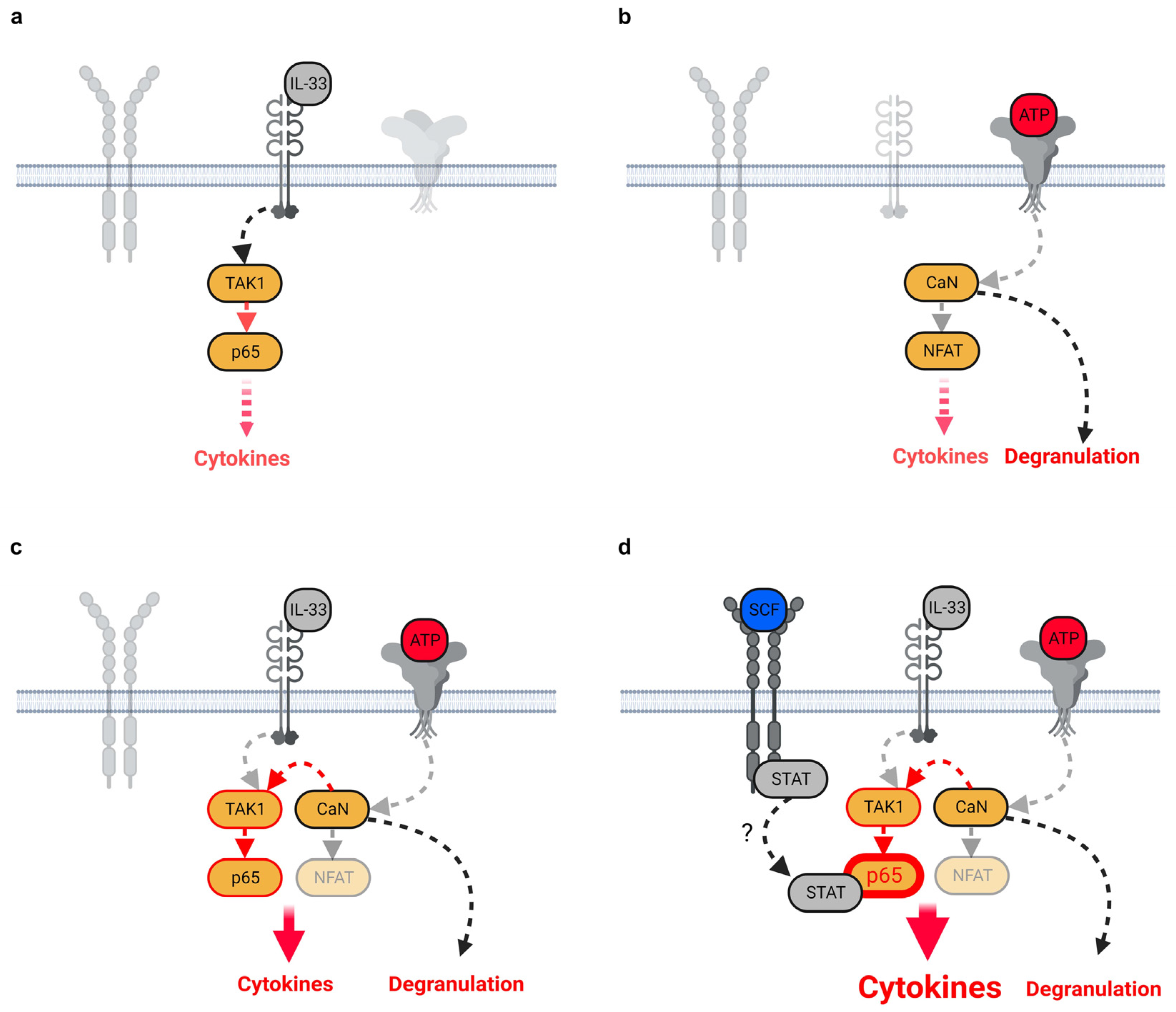
3.1. ATP/IL-33-Co-Sensing by MCs
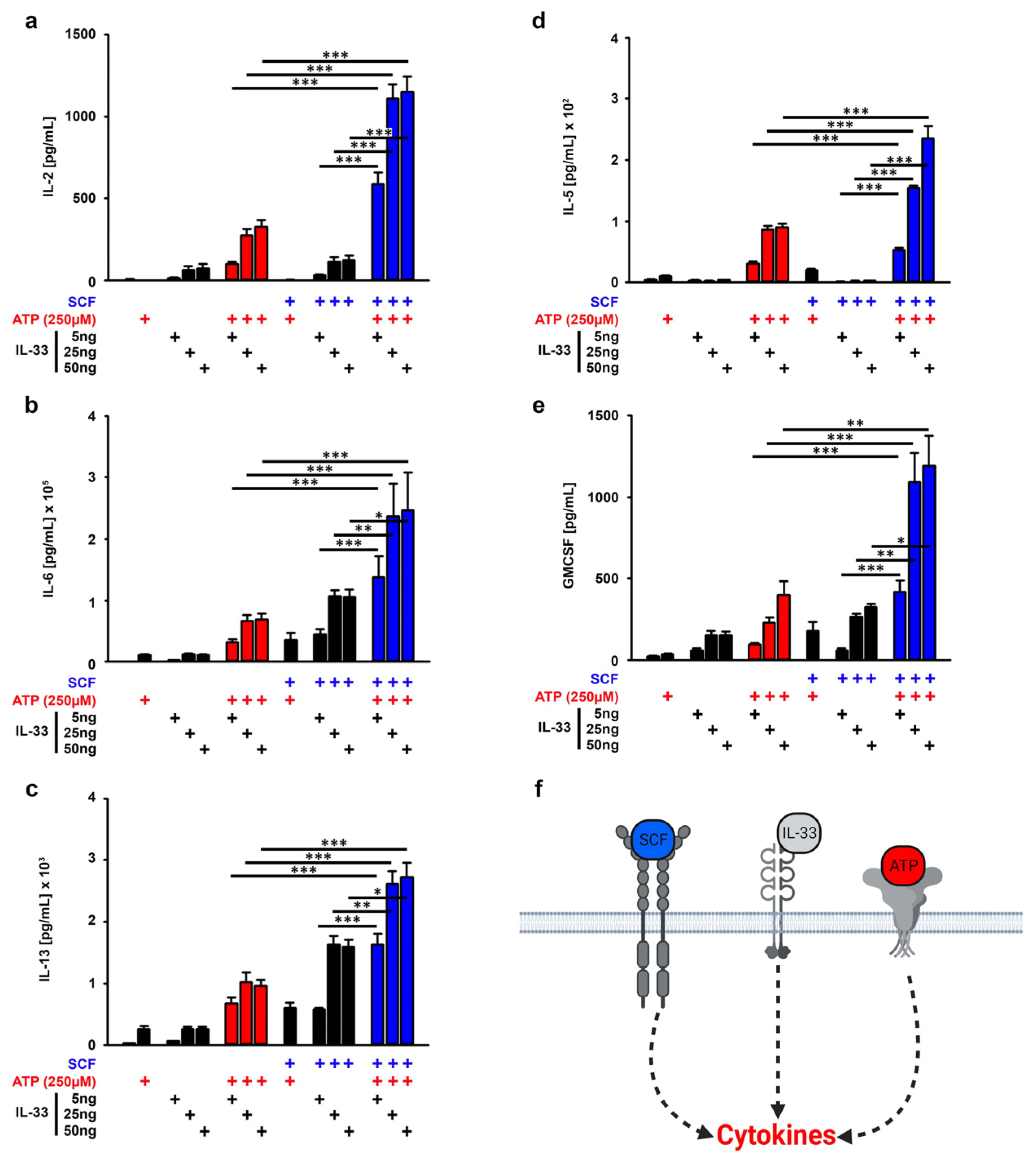
3.2. SCF Supports the Cytokine Production Induced by ATP/IL-33 Co-Sensing
3.3. The SCF and/or IL-33 Did Not Influence the ATP-Induced MC Degranulation
3.4. TAK1 Is Essential for the SCF-Supported ATP/IL-33 Co-Sensing
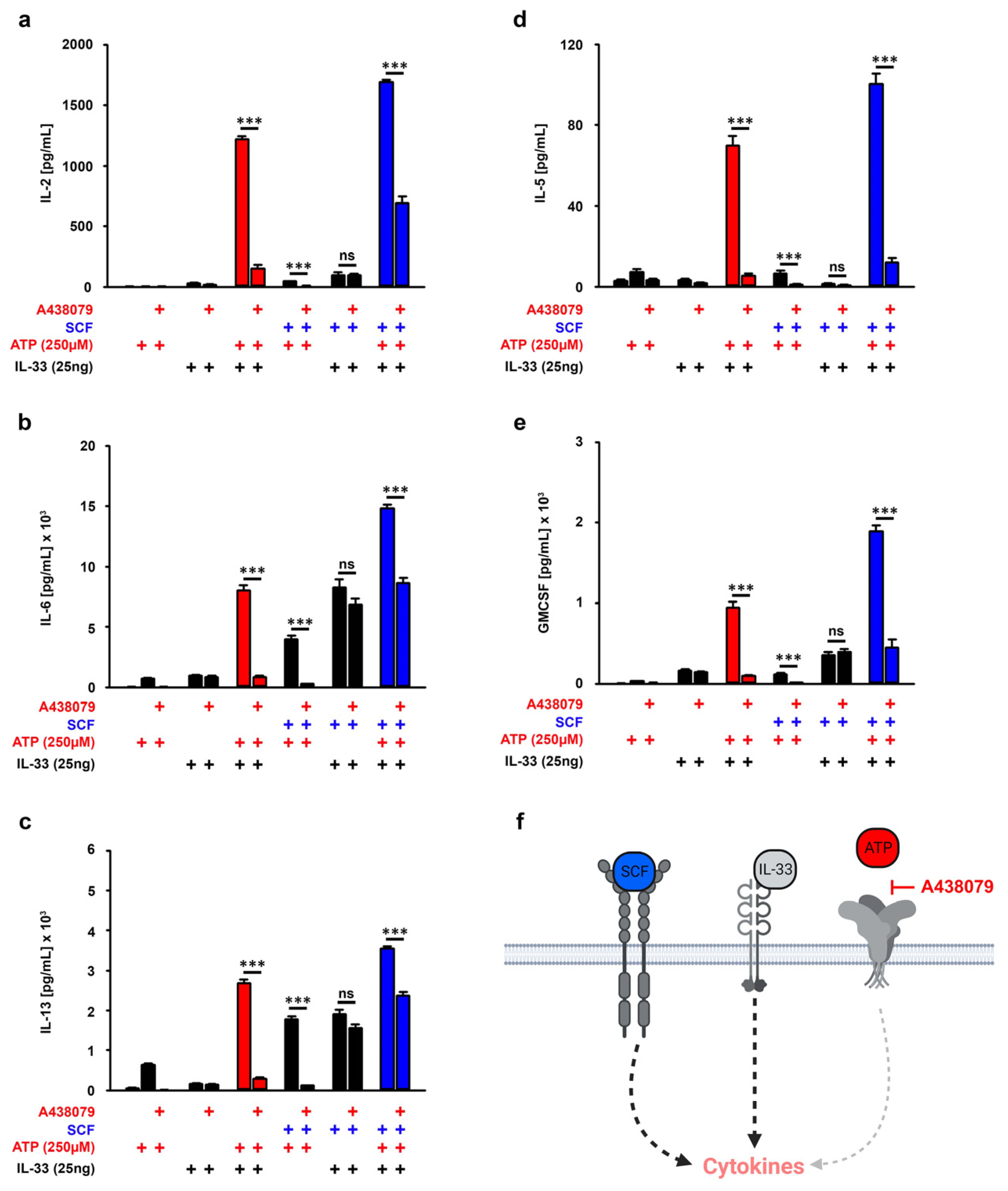
3.5. P2X7 Is Essential for the SCF-Supported ATP/IL-33 Co-Sensing
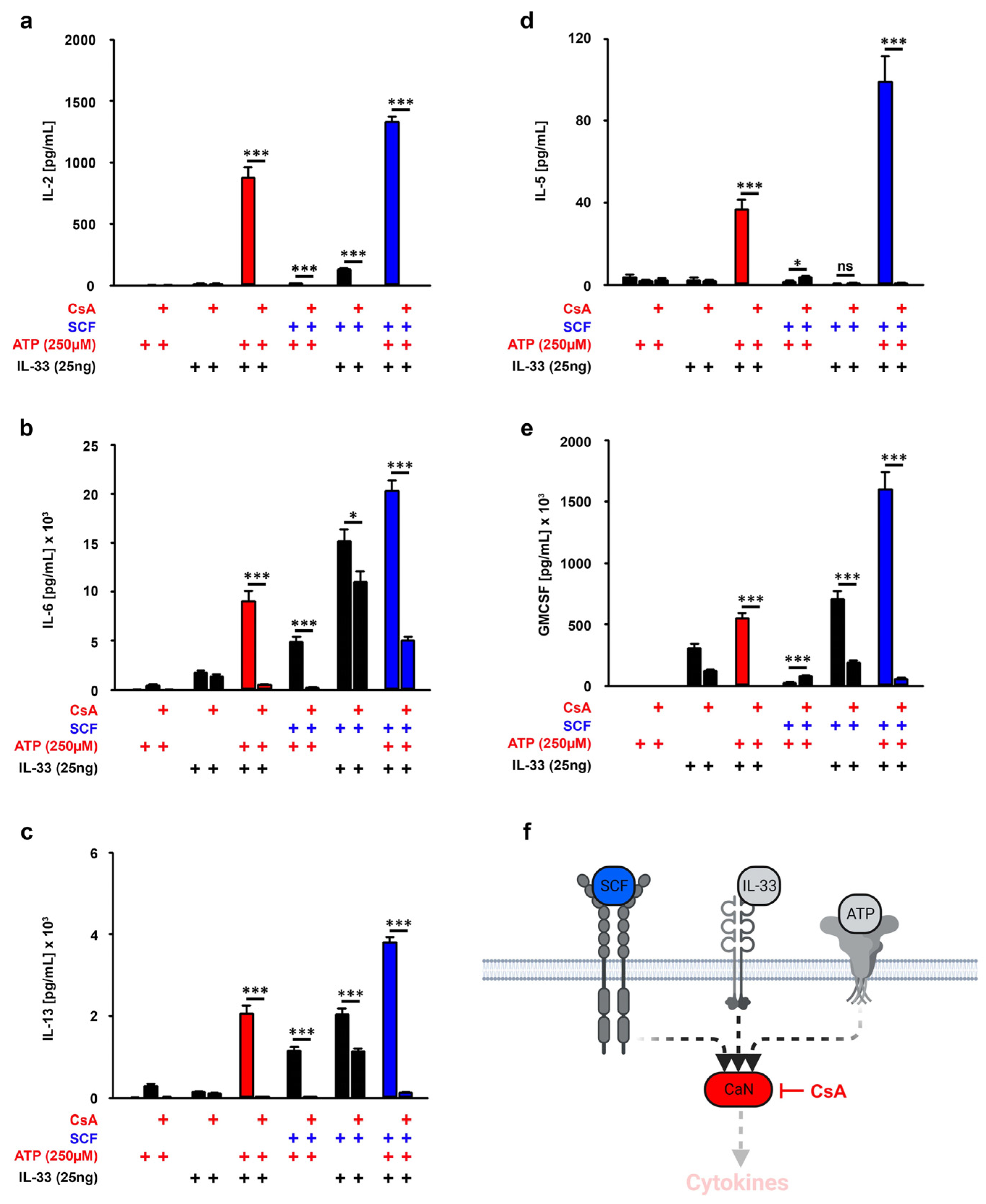
3.6. CaN Downstream of P2X7 Is Essential for the SCF-Supported ATP/IL-33 Co-Sensing
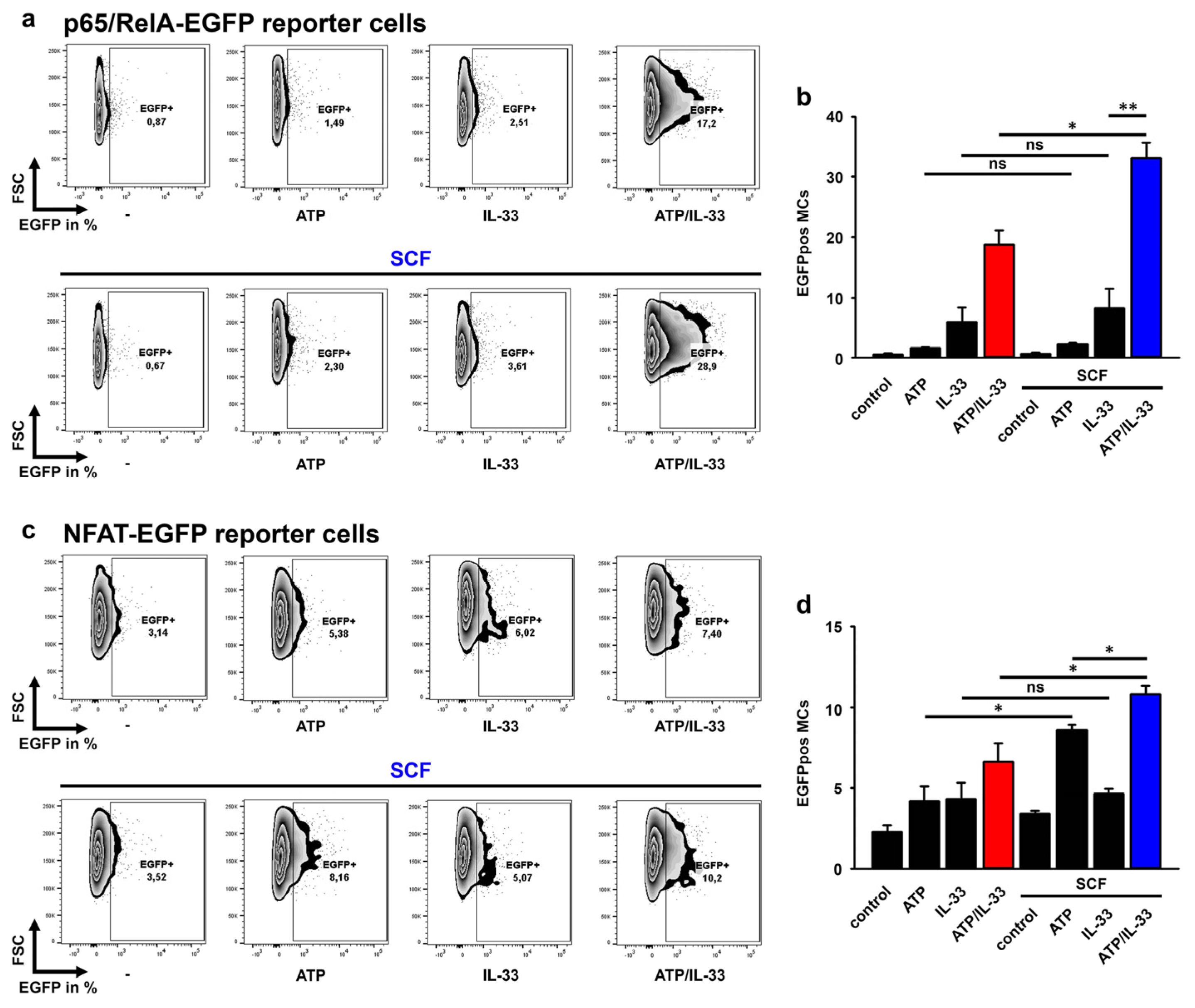
3.7. SCF Predominantly Enhances the p65/RelA Activation Induced by ATP/IL-33 Co-Sensing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef]
- Hoppe, A.; Katsoulis-Dimitriou, K.; Edler, H.J.; Dudeck, J.; Drube, S.; Dudeck, A. Mast cells initiate the vascular response to contact allergens by sensing cell stress. J. Allergy Clin. Immunol. 2020, 145, 1476–1479.e3. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.C.; Phair, I.R.; Greger, C.; Pardali, K.; McGuire, V.A.; Clark, A.R.; Gaestel, M.; Arthur, J.S.C. IL-33 regulates cytokine production and neutrophil recruitment via the p38 MAPK-activated kinases MK2/3. Immunol. Cell Biol. 2019, 97, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Enoksson, M.; Moller-Westerberg, C.; Wicher, G.; Fallon, P.G.; Forsberg-Nilsson, K.; Lunderius-Andersson, C.; Nilsson, G. Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent. Blood 2013, 121, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A.; Chen, C.C.; Piliponsky, A.M.; Tsai, M.; Tam, S.Y.; Galli, S.J. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005, 167, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Mallen-St Clair, J.; Lewis, C.C.; Villalta, S.A.; Baluk, P.; Erle, D.J.; Caughey, G.H. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin. Exp. Allergy 2005, 35, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, X.; Liu, B.; Huang, H. The STAT5-GATA2 pathway is critical in basophil and mast cell differentiation and maintenance. J. Immunol. 2015, 194, 4328–4338. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.P.; McCoy, M.E.; Piekorz, R.; Sexl, V.; Roh, K.H.; Jacobs-Helber, S.M.; Gillespie, S.R.; Bailey, D.P.; Mirmonsef, P.; Mann, M.N.; et al. Stat5 expression is critical for mast cell development and survival. Blood 2003, 102, 1290–1297. [Google Scholar] [CrossRef]
- Erlich, T.H.; Yagil, Z.; Kay, G.; Peretz, A.; Migalovich-Sheikhet, H.; Tshori, S.; Nechushtan, H.; Levi-Schaffer, F.; Saada, A.; Razin, E. Mitochondrial STAT3 plays a major role in IgE-antigen-mediated mast cell exocytosis. J. Allergy Clin. Immunol. 2014, 134, 460–469. [Google Scholar] [CrossRef]
- Ma, P.; Vemula, S.; Munugalavadla, V.; Chen, J.; Sims, E.; Borneo, J.; Kondo, T.; Ramdas, B.; Mali, R.S.; Li, S.; et al. Balanced interactions between Lyn, the p85alpha regulatory subunit of class I(A) phosphatidylinositol-3-kinase, and SHIP are essential for mast cell growth and maturation. Mol. Cell. Biol. 2011, 31, 4052–4062. [Google Scholar] [CrossRef]
- Ma, P.; Mali, R.S.; Munugalavadla, V.; Krishnan, S.; Ramdas, B.; Sims, E.; Martin, H.; Ghosh, J.; Li, S.; Chan, R.J.; et al. The PI3K pathway drives the maturation of mast cells via microphthalmia transcription factor. Blood 2011, 118, 3459–3469. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Carlesso, N.; Xu, M.; Liu, Y.; Nebreda, A.R.; Takemoto, C.; Kapur, R. Genetic evidence for critical roles of P38alpha protein in regulating mast cell differentiation and chemotaxis through distinct mechanisms. J. Biol. Chem. 2012, 287, 20258–20269. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, D.W. IgE-dependent signaling as a therapeutic target for allergies. Trends Pharmacol. Sci. 2012, 33, 502–509. [Google Scholar] [CrossRef]
- Jordan, P.M.; Andreas, N.; Groth, M.; Wegner, P.; Weber, F.; Jager, U.; Kuchler, C.; Werz, O.; Serfling, E.; Kamradt, T.; et al. ATP/IL-33-triggered hyperactivation of mast cells results in an amplified production of pro-inflammatory cytokines and eicosanoids. Immunology 2021, 164, 541–554. [Google Scholar] [CrossRef]
- Kuhny, M.; Hochdorfer, T.; Ayata, C.K.; Idzko, M.; Huber, M. CD39 is a negative regulator of P2X7-mediated inflammatory cell death in mast cells. Cell Commun. Signal. 2014, 12, 40. [Google Scholar] [CrossRef]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef]
- Li, H.N.; Yang, Q.Q.; Wang, W.T.; Tian, X.; Feng, F.; Zhang, S.T.; Xia, Y.T.; Wang, J.X.; Zou, Y.W.; Wang, J.Y.; et al. Red nucleus IL-33 facilitates the early development of mononeuropathic pain in male rats by inducing TNF-alpha through activating ERK, p38 MAPK, and JAK2/STAT3. J. Neuroinflamm. 2021, 18, 150. [Google Scholar] [CrossRef]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Anderson, D.E.; Rathore, A.P.; O’Neill, A.; Mantri, C.K.; Saron, W.A.; Lee, C.Q.; Cui, C.W.; Kang, A.E.; Foo, R.; et al. Mast cell activation in lungs during SARS-CoV-2 infection associated with lung pathology and severe COVID-19. J. Clin. Investig. 2023, 133, 19. [Google Scholar] [CrossRef]
- Krysko, O.; Bourne, J.H.; Kondakova, E.; Galova, E.A.; Whitworth, K.; Newby, M.L.; Bachert, C.; Hill, H.; Crispin, M.; Stamataki, Z.; et al. Severity of SARS-CoV-2 infection is associated with high numbers of alveolar mast cells and their degranulation. Front. Immunol. 2022, 13, 968981. [Google Scholar] [CrossRef]
- Wu, M.L.; Liu, F.L.; Sun, J.; Li, X.; He, X.Y.; Zheng, H.Y.; Zhou, Y.H.; Yan, Q.; Chen, L.; Yu, G.Y.; et al. SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal Transduct. Target. Ther. 2021, 6, 428. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Sanin, D.E.; Apostolova, P.; Nerz, G.; Lampaki, D.; Hofmann, M.; Steinmann, D.; Krohn-Grimberghe, M.; Thimme, R.; Mittler, G.; et al. IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals. Nat. Commun. 2021, 12, 2133. [Google Scholar] [CrossRef]
- Markovic, S.S.; Jovanovic, M.; Gajovic, N.; Jurisevic, M.; Arsenijevic, N.; Jovanovic, M.; Jovanovic, M.; Mijailovic, Z.; Lukic, S.; Zornic, N.; et al. IL 33 Correlates With COVID-19 Severity, Radiographic and Clinical Finding. Front. Med. 2021, 8, 749569. [Google Scholar] [CrossRef]
- Vicentino, A.R.R.; Fraga-Junior, V.D.S.; Palazzo, M.; Tasmo, N.R.A.; Rodrigues, D.A.S.; Barroso, S.P.C.; Ferreira, S.N.; Neves-Borges, A.C.; Allonso, D.; Fantappie, M.R.; et al. High mobility group box 1, ATP, lipid mediators, and tissue factor are elevated in COVID-19 patients: HMGB1 as a biomarker of worst prognosis. Clin. Transl. Sci. 2023, 16, 631–646. [Google Scholar] [CrossRef]
- Wilkinson, T.; De Soyza, A.; Carroll, M.; Chalmers, J.D.; Crooks, M.G.; Griffiths, G.; Shankar-Hari, M.; Ho, L.P.; Horsley, A.; Kell, C.; et al. A randomised phase 2a study to investigate the effects of blocking interleukin-33 with tozorakimab in patients hospitalised with COVID-19: ACCORD-2. ERJ Open Res. 2023, 9, 00249–02023. [Google Scholar] [CrossRef]
- Barbu, E.A.; Zhang, J.; Siraganian, R.P. The limited contribution of Fyn and Gab2 to the high affinity IgE receptor signaling in mast cells. J. Biol. Chem. 2010, 285, 15761–15768. [Google Scholar] [CrossRef]
- Totzke, J.; Gurbani, D.; Raphemot, R.; Hughes, P.F.; Bodoor, K.; Carlson, D.A.; Loiselle, D.R.; Bera, A.K.; Eibschutz, L.S.; Perkins, M.M.; et al. Takinib, a Selective TAK1 Inhibitor, Broadens the Therapeutic Efficacy of TNF-alpha Inhibition for Cancer and Autoimmune Disease. Cell Chem. Biol. 2017, 24, 1029–1039.e7. [Google Scholar] [CrossRef]
- Drube, S.; Weber, F.; Gopfert, C.; Loschinski, R.; Rothe, M.; Boelke, F.; Diamanti, M.A.; Lohn, T.; Ruth, J.; Schutz, D.; et al. TAK1 and IKK2, novel mediators of SCF-induced signaling and potential targets for c-Kit-driven diseases. Oncotarget 2015, 6, 28833–28850. [Google Scholar] [CrossRef] [PubMed]
- Drube, S.; Heink, S.; Walter, S.; Lohn, T.; Grusser, M.; Gerbaulet, A.; Berod, L.; Schons, J.; Dudeck, A.; Freitag, J.; et al. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood 2010, 115, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Grutzkau, A.; Smorodchenko, A.; Lippert, U.; Kirchhof, L.; Artuc, M.; Henz, B.M. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytom. A 2004, 61, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ito, M.; Matsuoka, I. Divergent regulatory roles of extracellular ATP in the degranulation response of mouse bone marrow-derived mast cells. Int. Immunopharmacol. 2017, 43, 99–107. [Google Scholar] [CrossRef]
- Hu, X.M.; Shi, N.R.; Zhang, J.Z.; Zuo, Y.Q.; Wang, X.; Zhao, Y.F.; Wu, J.S. CD73: Friend or Foe in Lung Injury. Int. J. Mol. Sci. 2023, 24, 5545. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Y.; Xu, Y.; Xie, L.; Yu, Z.; Zheng, J. Function of the P2X7 receptor in hematopoiesis and leukemogenesis. Exp. Hematol. 2021, 104, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Molfetta, R.; Lecce, M.; Milito, N.D.; Putro, E.; Pietropaolo, G.; Marangio, C.; Scarno, G.; Moretti, M.; De Smaele, E.; Santini, T.; et al. SCF and IL-33 regulate mouse mast cell phenotypic and functional plasticity supporting a pro-inflammatory microenvironment. Cell Death Dis. 2023, 14, 616. [Google Scholar] [CrossRef] [PubMed]
- Barnstein, B.O.; Li, G.; Wang, Z.; Kennedy, S.; Chalfant, C.; Nakajima, H.; Bunting, K.D.; Ryan, J.J. Stat5 expression is required for IgE-mediated mast cell function. J. Immunol. 2006, 177, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Pullen, N.A.; Falanga, Y.T.; Morales, J.K.; Ryan, J.J. The Fyn-STAT5 Pathway: A New Frontier in IgE- and IgG-Mediated Mast Cell Signaling. Front. Immunol. 2012, 3, 117. [Google Scholar] [CrossRef]
- Andrade, M.V.; Iwaki, S.; Ropert, C.; Gazzinelli, R.T.; Cunha-Melo, J.R.; Beaven, M.A. Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur. J. Immunol. 2011, 41, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Kiwanuka, K.N.; Motunrayo Kolawole, E.; McLeod, J.J.A.; Baker, B.; Paez, P.A.; Zellner, M.P.; Haque, T.T.; Paranjape, A.; Jackson, K.; Kee, S.A.; et al. Stat5B is required for IgE-Mediated mast cell function in vitro and in vivo. Cell Immunol. 2021, 364, 104344. [Google Scholar] [CrossRef]
- Kaltenbach, L.; Martzloff, P.; Bambach, S.K.; Aizarani, N.; Mihlan, M.; Gavrilov, A.; Glaser, K.M.; Stecher, M.; Thunauer, R.; Thiriot, A.; et al. Slow integrin-dependent migration organizes networks of tissue-resident mast cells. Nat. Immunol. 2023, 24, 915–924. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifert, J.; Küchler, C.; Drube, S. ATP/IL-33-Co-Sensing by Mast Cells (MCs) Requires Activated c-Kit to Ensure Effective Cytokine Responses. Cells 2023, 12, 2696. https://doi.org/10.3390/cells12232696
Seifert J, Küchler C, Drube S. ATP/IL-33-Co-Sensing by Mast Cells (MCs) Requires Activated c-Kit to Ensure Effective Cytokine Responses. Cells. 2023; 12(23):2696. https://doi.org/10.3390/cells12232696
Chicago/Turabian StyleSeifert, Johanna, Claudia Küchler, and Sebastian Drube. 2023. "ATP/IL-33-Co-Sensing by Mast Cells (MCs) Requires Activated c-Kit to Ensure Effective Cytokine Responses" Cells 12, no. 23: 2696. https://doi.org/10.3390/cells12232696
APA StyleSeifert, J., Küchler, C., & Drube, S. (2023). ATP/IL-33-Co-Sensing by Mast Cells (MCs) Requires Activated c-Kit to Ensure Effective Cytokine Responses. Cells, 12(23), 2696. https://doi.org/10.3390/cells12232696





