Pulmonary Hypertension-Associated Right Ventricular Cardiomyocyte Remodelling Reduces Treprostinil Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animal Ethics
2.3. Generation of the MCT-Induced Rat PH Model
2.4. Ventricular Cardiomyocyte Isolation and Culture
2.5. Assessment of Ventricular Cardiomyocyte Morphological Changes
2.6. Measuring Cell Contraction Using CytoCypher–HTS
2.7. Measuring Nuclear cAMP/PKA Levels Using Multi-Cell FRET Microscopy
2.8. Statistics
3. Results
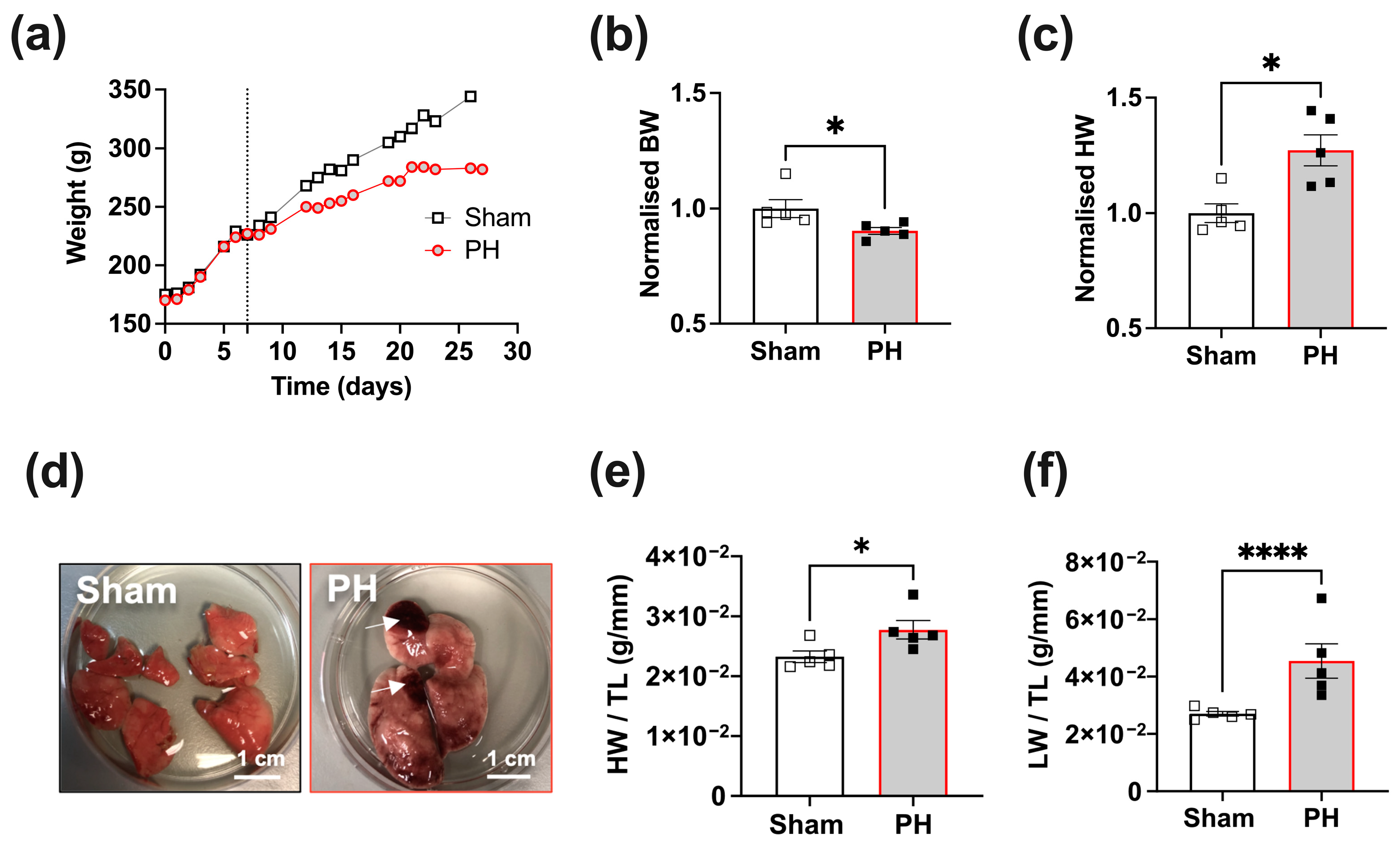
3.1. Rats with Established PH Exhibit Increased Heart and Lung Weights 3 Weeks Post-MCT Treatment
3.2. RV Cardiomyocytes from Rats with Established PH Exhibit Hypertrophy and Reduction in Sarcomere Regularity
3.3. RV Cardiomyocytes from Rats with Established PH Exhibit Impaired Contraction and Ca2+ Transients (CaTs)
3.4. RV Cardiomyocytes from PH Animals Exhibit a Reduced CaT
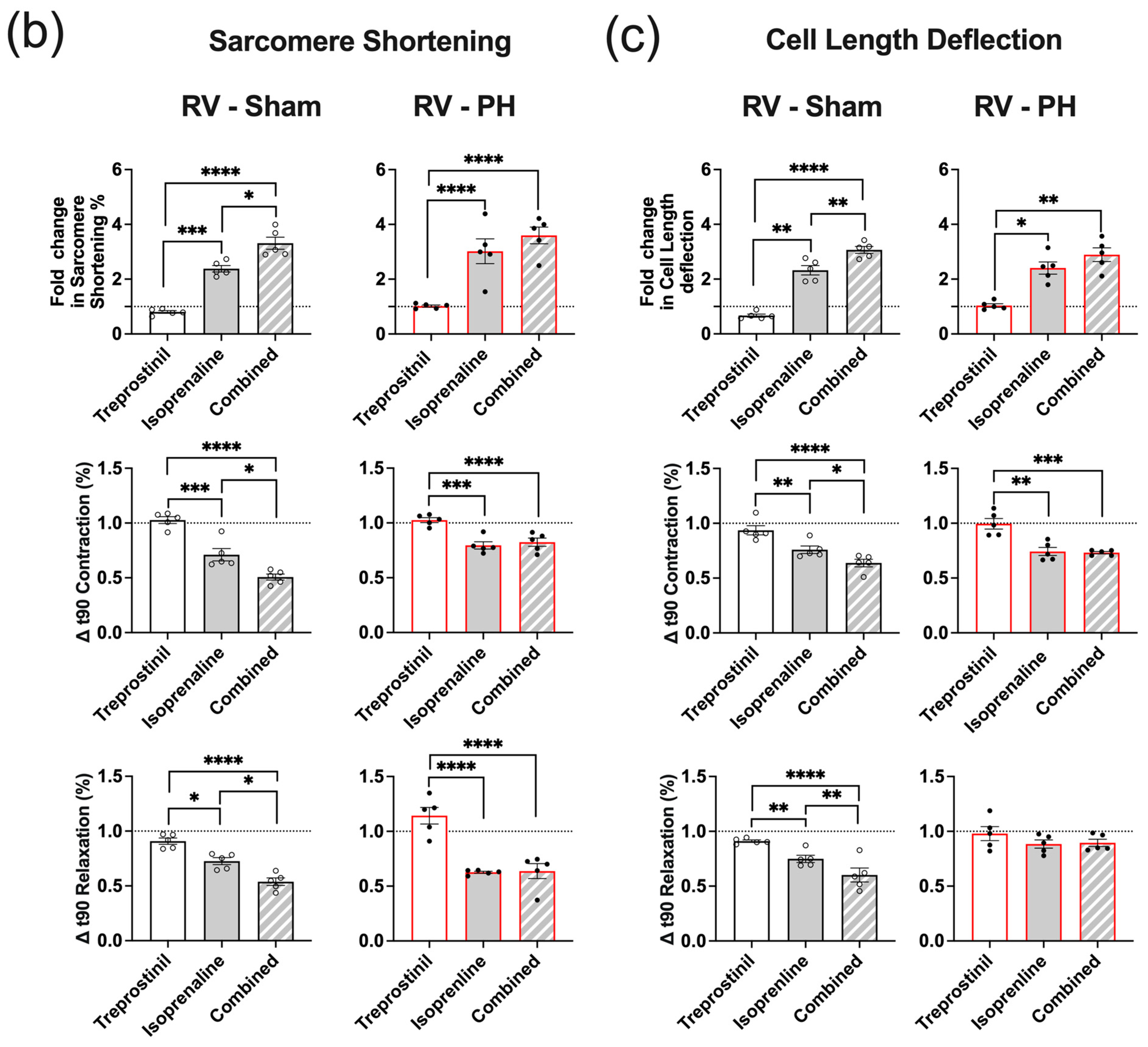
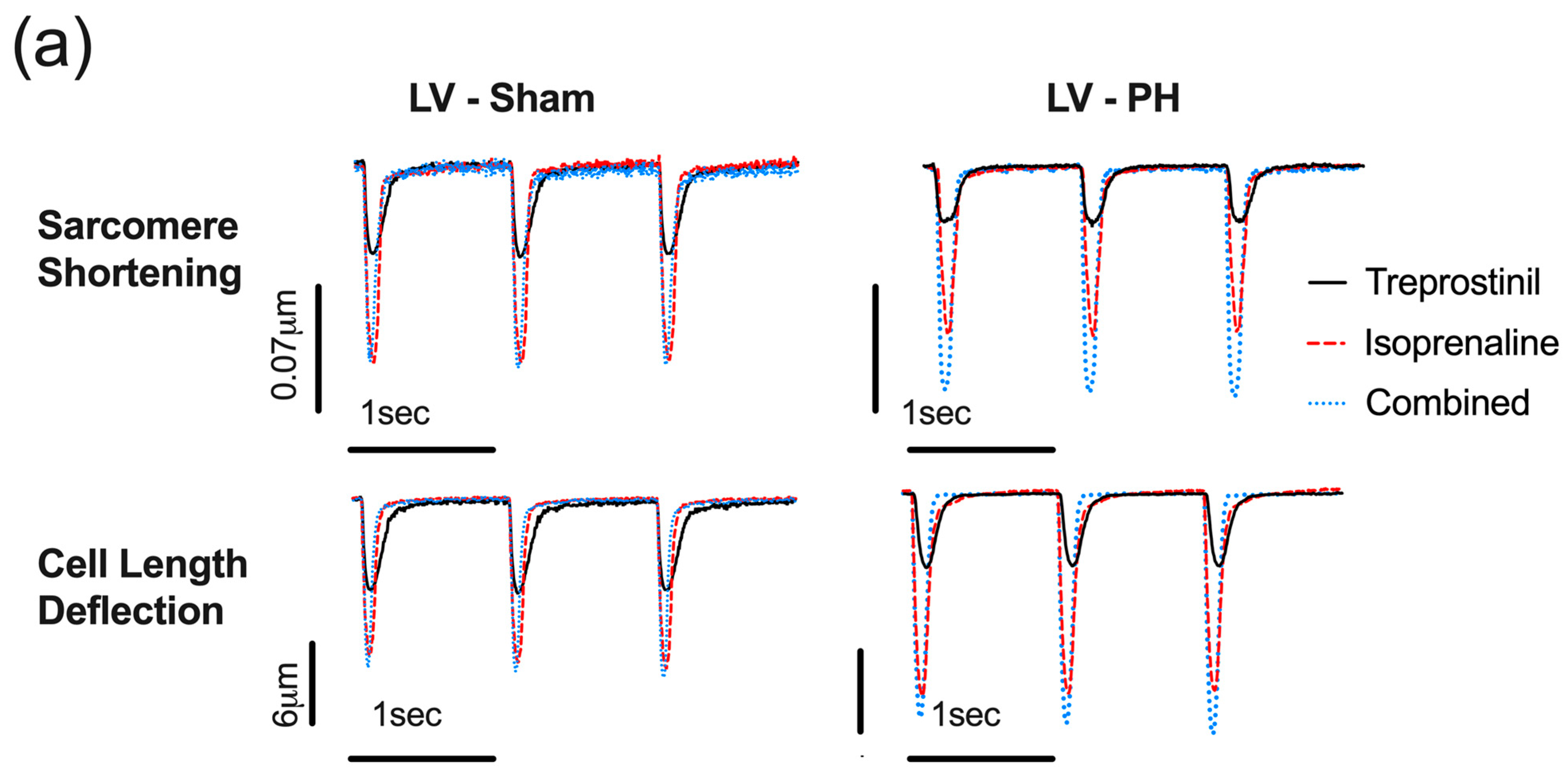
3.5. Potentiation of the Isoprenaline Positive Inotropic Effect by Treprostinil Is Lost in PH RV Cardiomyocytes
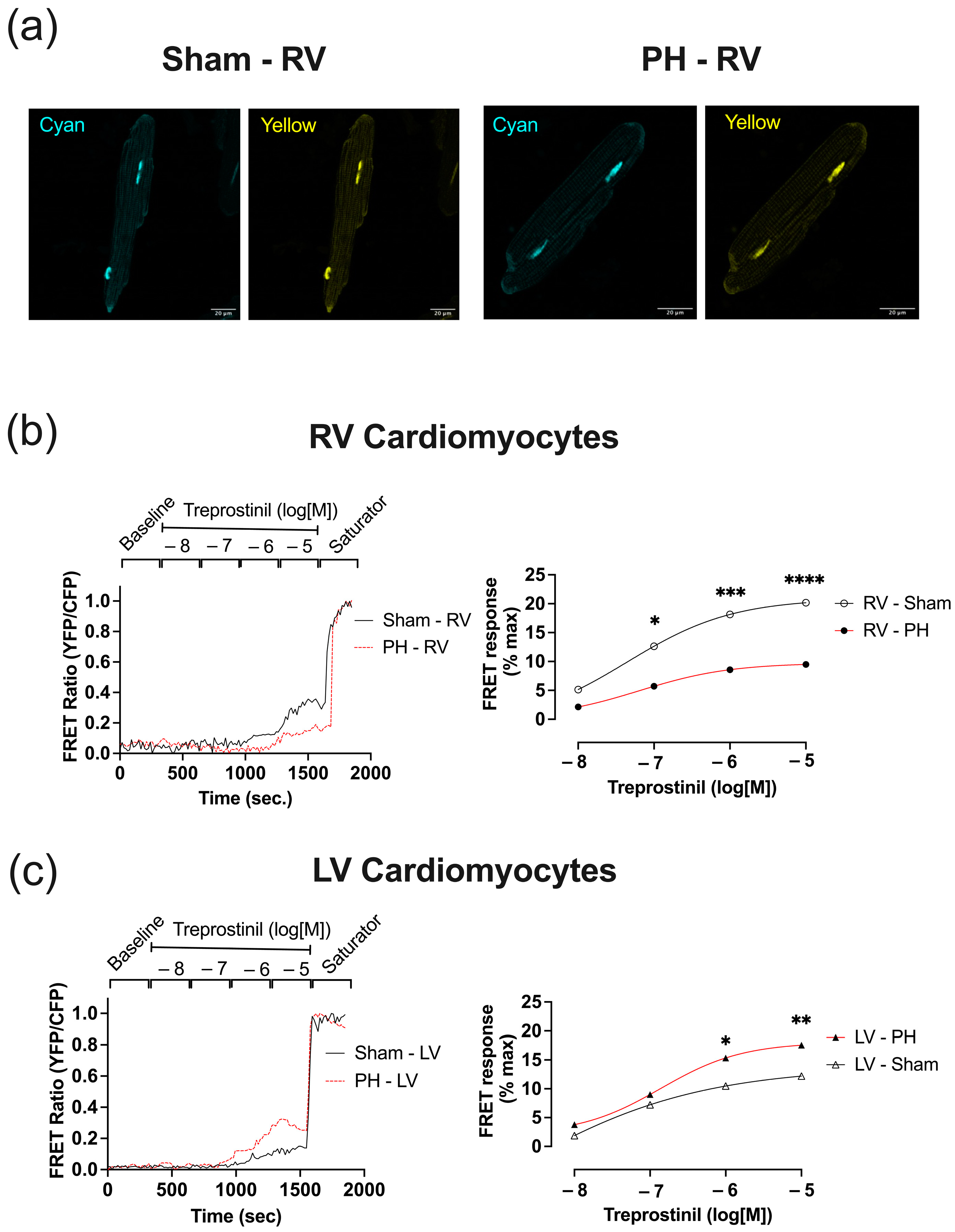
3.6. In Established PH RV Cardiomyocytes, Treprostinil-Induced PKA Activation Is Reduced
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondo, T.; Okumura, N.; Adachi, S.; Murohara, T. Pulmonary Hypertension: Diagnosis, Management, and Treatment. Nagoya J. Med. Sci. 2019, 81, 19–30. [Google Scholar] [CrossRef]
- Geraci, M.W.; Gao, B.; Shepherd, D.C.; Moore, M.D.; Westcott, J.Y.; Fagan, K.A.; Alger, L.A.; Tuder, R.M.; Voelkel, N.F. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J. Clin. Investig. 1999, 103, 1509–1515. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Ahmetaj-Shala, B.; Kirkby, N.S.; Wright, W.R.; Mackenzie, L.S.; Reed, D.M.; Mohamed, N. Role of prostacyclin in pulmonary hypertension. Glob. Cardiol. Sci. Prac. 2014, 2014, 382–393. [Google Scholar] [CrossRef]
- Santos-Ribeiro, D.; Mendes-Ferreira, P.; Maia-Rocha, C.; Adão, R.; Leite-Moreira, A.F.; Brás-Silva, C. Pulmonary arterial hypertension: Basic knowledge for clinicians. Arch. Cardiovasc. Dis. 2016, 109, 550–561. [Google Scholar] [CrossRef]
- Handoko, M.L.; de Man, F.S.; Allaart, C.P.; Paulus, W.J.; Westerhof, N.; Vonk-Noordegraaf, A. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: Lessons from the left heart. Eur. Respir. Rev. 2010, 19, 72–82. [Google Scholar] [CrossRef]
- Chang, K.Y.; Duval, S.; Badesch, D.B.; Bull, T.M.; Chakinala, M.M.; De Marco, T.; Frantz, R.P.; Hemnes, A.; Mathai, S.C.; Rosenzweig, E.B.; et al. Mortality in Pulmonary Arterial Hypertension in the Modern Era: Early Insights from the Pulmonary Hypertension Association Registry. J. Am. Heart Assoc. 2022, 11, e024969. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Wiesen, J.; Dweik, R.; Chaisson, N.F. Evaluating suspected pulmonary hypertension: A structured approach. Clevel. Clin. J. Med. 2018, 85, 468–480. [Google Scholar] [CrossRef]
- Gayat, E.; Mebazaa, A. Pulmonary hypertension in critical care. Curr. Opin. Crit. Care 2011, 17, 439–448. [Google Scholar] [CrossRef]
- Poor, H.D.; Ventetuolo, C.E. Pulmonary Hypertension in the Intensive Care Unit. Prog. Cardiovasc. Dis. 2012, 55, 187–198. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Surdo, N.C.; Pantano, S.; Zaccolo, M. Imaging cAMP nanodomains in the heart. Biochem. Soc. Trans. 2019, 47, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.J.; Krause-Steinrauf, H.; Massie, B.M.; Deedwania, P.; Follmann, D.; Kovar, D.; Murray, D.; Oren, R.; Rosenberg, Y.; Young, J.; et al. A comparative analysis of the results from 4 trials of β-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J. Card. Fail. 2003, 9, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Gabriel-Costa, D. The pathophysiology of myocardial infarction-induced heart failure. Pathophysiology 2018, 25, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Schobesberger, S.; Wright, P.; Tokar, S.; Bhargava, A.; Mansfield, C.; Glukhov, A.V.; Poulet, C.; Buzuk, A.; Monszpart, A.; Sikkel, M.; et al. T-tubule remodelling disturbs localized β2-adrenergic signalling in rat ventricular myocytes during the progression of heart failure. Cardiovasc. Res. 2017, 113, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, C.C.T.; Tian, L.; Xiong, P.Y.; Potus, F.; Bentley, R.E.T.; Al-Qazazi, R.; Prins, K.W.; Archer, S.L. An integrated proteomic and transcriptomic signature of the failing right ventricle in monocrotaline induced pulmonary arterial hypertension in male rats. Front. Physiol. 2022, 13, 966454. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V.; Pellegrino, T.; Bosso, G.; Campi, G.; Parrella, P.; Piscopo, V.; Tocchetti, C.G.; Hassoun, P.M.; Petretta, M.; Cuocolo, A.; et al. Cardiac sympathetic dysfunction in pulmonary arterial hypertension: Lesson from left-sided heart failure. Pulm. Circ. 2019, 9, 2045894019868620. [Google Scholar] [CrossRef] [PubMed]
- Sadushi-Kolici, R.; Jansa, P.; Kopec, G.; Torbicki, A.; Skoro-Sajer, N.; Campean, I.-A.; Halank, M.; Simkova, I.; Karlocai, K.; Steringer-Mascherbauer, R.; et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): A double-blind, phase 3, randomised controlled trial. Lancet Respir. Med. 2019, 7, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H.; et al. A Comparison of Continuous Intravenous Epoprostenol (Prostacyclin) with Conventional Therapy for Primary Pulmonary Hypertension. N. Engl. J. Med. 1996, 334, 296–301. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Shillington, A.; Rich, S. Survival in Primary Pulmonary Hypertension. Circulation 2002, 106, 1477–1482. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Akhmedov, D.; Shala, F.; Berdeaux, R.; Mitchell, J.A. Abstract 16362: The Right Heart is Specifically Targeted by Intravenous Administration of Treprostinil: Implications for Our Understanding of How Prostacyclin Drugs Work to Treat Pulmonary Arterial Hypertension. Circulation 2018, 138, A16362. [Google Scholar]
- Zhao, L.; Oliver, E.; Maratou, K.; Atanur, S.S.; Dubois, O.D.; Cotroneo, E.; Chen, C.-N.; Wang, L.; Arce, C.; Chabosseau, P.L.; et al. The zinc transporter ZIP12 regulates the pulmonary vascular response to chronic hypoxia. Nature 2015, 524, 356–360. [Google Scholar] [CrossRef]
- Ashek, A.; Spruijt, O.A.; Harms, H.J.; Lammertsma, A.A.; Cupitt, J.; Dubois, O.; Wharton, J.; Dabral, S.; Pullamsetti, S.S.; Huisman, M.C.; et al. 3′-Deoxy-3′-[18F]Fluorothymidine Positron Emission Tomography Depicts Heterogeneous Proliferation Pathology in Idiopathic Pulmonary Arterial Hypertension Patient Lung. Circ. Cardiovasc. Imaging 2018, 11, e007402. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, R.; Sanchez-Alonso, J.L.; Alvarez-Laviada, A.; Rossi, S.; Dries, E.; Schorn, T.; Abdul-Salam, V.B.; Trayanova, N.; Wojciak-Stothard, B.; Miragoli, M.; et al. Nanoscale Study of Calcium Handling Remodeling in Right Ventricular Cardiomyocytes Following Pulmonary Hypertension. Hypertension 2021, 77, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Kreisselmeier, K.P.; Ghofrani, H.A.; Samidurai, A.; Pullamsetti, S.; Weissmann, N.; Schudt, C.; Ermert, L.; Seeger, W.; Grimminger, F. Antiremodeling Effects of Iloprost and the Dual-Selective Phosphodiesterase 3/4 Inhibitor Tolafentrine in Chronic Experimental Pulmonary Hypertension. Circ. Res. 2004, 94, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; O’Gara, P.; Harding, S.E.; Fuller, S.J. Enhancement of adenoviral gene transfer to adult rat cardiomyocytes in vivo by immobilization and ultrasound treatment of the heart. Gene Ther. 2005, 12, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.T.; Tsui, S.F.; Francis, A.J.; MacLeod, K.T.; Marston, S.B. Approaches to High-Throughput Analysis of Cardiomyocyte Contractility. Front. Physiol. 2020, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.T.; Bhogal, N.K.; Diakonov, I.; Pannell, L.M.K.; Perera, R.K.; Bork, N.I.; Schobesberger, S.; Lucarelli, C.; Faggian, G.; Alvarez-Laviada, A.; et al. Cardiomyocyte Membrane Structure and cAMP Compartmentation Produce Anatomical Variation in β2AR-cAMP Responsiveness in Murine Hearts. Cell Rep. 2018, 23, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kartasalo, K.; Pölönen, R.-P.; Ojala, M.; Rasku, J.; Lekkala, J.; Aalto-Setälä, K.; Kallio, P. CytoSpectre: A tool for spectral analysis of oriented structures on cellular and subcellular levels. BMC Bioinform. 2015, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- MFontana, M.; Olschewski, H.; Olschewski, A.; Schlüter, K. Treprostinil potentiates the positive inotropic effect of catecholamines in adult rat ventricular cardiomyocytes. Br. J. Pharmacol. 2007, 151, 779–786. [Google Scholar] [CrossRef]
- Allen, M.D.; Zhang, J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun. 2006, 348, 716–721. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Lu, T.-W.; Stolerman, L.M.; Tenner, B.; Yang, J.R.; Zhang, J.-F.; Falcke, M.; Rangamani, P.; Taylor, S.S.; Mehta, S.; et al. Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling. Cell 2020, 182, 1531–1544.e15. [Google Scholar] [CrossRef]
- Wright, P.T.; Nikolaev, V.O.; O’Hara, T.; Diakonov, I.; Bhargava, A.; Tokar, S.; Schobesberger, S.; Shevchuk, A.I.; Sikkel, M.B.; Wilkinson, R.; et al. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J. Mol. Cell. Cardiol. 2014, 67, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ramuz, M.; Hasan, A.; Gruscheski, L.; Diakonov, I.; Pavlaki, N.; Nikolaev, V.O.; Harding, S.; Dunsby, C.; Gorelik, J. A Software Tool for High-Throughput Real-Time Measurement of Intensity-Based Ratio-Metric FRET. Cells 2019, 8, 1541. [Google Scholar] [CrossRef] [PubMed]
- Sikkel, M.B.; Francis, D.P.; Howard, J.; Gordon, F.; Rowlands, C.; Peters, N.S.; Lyon, A.R.; Harding, S.E.; MacLeod, K.T. Hierarchical statistical techniques are necessary to draw reliable conclusions from analysis of isolated cardiomyocyte studies. Cardiovasc. Res. 2017, 113, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Koschinski, A.; Zaccolo, M. Activation of PKA in cell requires higher concentration of cAMP than in vitro: Implications for compartmentalization of cAMP signalling. Sci. Rep. 2017, 7, 14090. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.O.; Moshkov, A.; Lyon, A.R.; Miragoli, M.; Novak, P.; Paur, H.; Lohse, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. Beta2-Adrenergic Receptor Redistribution in Heart Failure Changes cAMP Compartmentation. Science 2010, 327, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Polanowska-Grabowska, R.K.; Smith, J.S.; Shields, C.W.; Saucerman, J.J. PKA catalytic subunit compartmentation regulates contractile and hypertrophic responses to β-adrenergic signaling. J. Mol. Cell. Cardiol. 2014, 66, 83–93. [Google Scholar] [CrossRef]
- Pieske, B.; Houser, S.R. [Na+]i handling in the failing human heart. Cardiovasc. Res. 2003, 57, 874–886. [Google Scholar] [CrossRef]
- Sabourin, J.; Beauvais, A.; Luo, R.; Montani, D.; Benitah, J.-P.; Masson, B.; Antigny, F. The SOCE Machinery: An Unbalanced Knowledge between Left and Right Ventricular Pathophysiology. Cells 2022, 11, 3282. [Google Scholar] [CrossRef]
- Lin, C.-I.; Loh, S.-H.; Luk, H.-N.; Wei, J. Depressant effects of prostacyclin in human atrial fibers and cardiomyocytes. J. Biomed. Sci. 1994, 1, 139–146. [Google Scholar] [CrossRef]
- Holmboe, S.; Andersen, A.; Jensen, R.V.; Kimose, H.H.; Ilkjær, L.B.; Shen, L.; Clapp, L.H.; Nielsen-Kudsk, J.E. Prostacyclins have no direct inotropic effect on isolated atrial strips from the normal and pressure-overloaded human right heart. Pulm. Circ. 2017, 7, 339–347. [Google Scholar] [CrossRef]
- Auclair, M.C.; Vernimmen, C.; Lechat, P. Influence of Prostacyclin and two metabolites on the contractility of cultured rat heart cells. Prostaglandins Leukot. Essent. Fat. Acids 1988, 32, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Fassina, G.; Tessari, F.; Dorigo, P. Positive inotropic effect of a stable analogue of PGI2 and of PGI2 on isolated guinea pig atria. Mechanism of action. Pharmacol. Res. Commun. 1983, 15, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Türker, R. Evidence for a prostacyclin-mediated chronotropic effect of Angiotensin II in the isolated cat right atria. Eur. J. Pharmacol. 1982, 83, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Riise, J.; Nguyen, C.H.; Hussain, R.I.; Dahl, C.P.; Ege, M.S.; Osnes, J.-B.; Skomedal, T.; Sandnes, D.L.; Levy, F.O.; Krobert, K.A. Prostanoid-mediated inotropic responses are attenuated in failing human and rat ventricular myocardium. Eur. J. Pharmacol. 2012, 686, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Leineweber, K.; Seyfarth, T.; Abraham, G.; Gerbershagen, H.-P.; Heinroth-Hoffmann, I.; Pönicke, K.; Brodde, O.-E. Cardiac β-Adrenoceptor Changes in Monocrotaline-Treated Rats: Differences Between Membrane Preparations from Whole Ventricles and Isolated Ventricular Cardiomyocytes. J. Cardiovasc. Pharmacol. 2003, 41, 333–342. [Google Scholar] [CrossRef]
- Irannejad, R.; Pessino, V.; Mika, D.; Huang, B.; Wedegaertner, P.B.; Conti, M.; von Zastrow, M. Functional selectivity of GPCR-directed drug action through location bias. Nat. Chem. Biol. 2017, 13, 799–806. [Google Scholar] [CrossRef]
- Irannejad, R.; Tomshine, J.C.; Tomshine, J.R.; Chevalier, M.; Mahoney, J.P.; Steyaert, J.; Rasmussen, S.G.F.; Sunahara, R.K.; El-Samad, H.; Huang, B.; et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature 2013, 495, 534–538. [Google Scholar] [CrossRef]
- Yoo, B.; Lemaire, A.; Mangmool, S.; Wolf, M.J.; Curcio, A.; Mao, L.; Rockman, H.A.; Woulfe, K.C.; Wilson, C.E.; Nau, S.; et al. β1-Adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am. J. Physiol. Circ. Physiol. 2009, 297, H1377–H1386. [Google Scholar] [CrossRef]
- Couttenye, M.M.; De Clerck, N.M.; Herman, A.G.; Brutsaert, D.L. Effects of Prostacyclin on Contractile Properties of Isolated Mammalian Cardiac Muscle. J. Cardiovasc. Pharmacol. 1985, 7, 971–976. [Google Scholar] [CrossRef]
- Pavlovic, M.; Petkovic, D.; Cvetkovic, M.; Zdjelar, K.; Starcevic, V.; Bosnic, O. Study of the mechanism of prostacyclin (PgI2) action on myocardial contractility. Agents Actions Suppl. 1992, 37, 171–175. [Google Scholar] [CrossRef]
- Borda, L.S.; Cangas, L.; Gimeno, M.F.; Gimeno, A.L. An adrenergic participation subserving a positive inotropism and chronotropism of prostacyclin on isolated rat atria. Cell. Mol. Life Sci. 1979, 35, 529–530. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Canga, L.; Borda, E.S.; Gimeno, M.F.; Gimeno, A.L. Inotropic effect of prostacyclin (PGI2) on isolated rat atria at different contraction frequencies. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1980, 313, 95–100. [Google Scholar] [CrossRef]
- Mentz, P.; Mest, H.J.; Anger, H. Interaction of Iloprost and Indomethacin with the Cardiac Effects of Isoprenaline, Ouabain and Trapidil. Biomed. Biochim. Acta 1988, 47, S109–S112. [Google Scholar]
- Medvedev, R.Y.; Sanchez-Alonso, J.L.; Mansfield, C.A.; Judina, A.; Francis, A.J.; Pagiatakis, C.; Trayanova, N.; Glukhov, A.V.; Miragoli, M.; Faggian, G.; et al. Local hyperactivation of L-type Ca2+ channels increases spontaneous Ca2+ release activity and cellular hypertrophy in right ventricular myocytes from heart failure rats. Sci. Rep. 2021, 11, 4840. [Google Scholar] [CrossRef]
- Dries, E.; Santiago, D.J.; Gilbert, G.; Lenaerts, I.; Vandenberk, B.; Nagaraju, C.K.; Johnson, D.M.; Holemans, P.; Roderick, H.L.; Macquaide, N.; et al. Hyperactive ryanodine receptors in human heart failure and ischaemic cardiomyopathy reside outside of couplons. Cardiovasc. Res. 2018, 114, 1512–1524. [Google Scholar] [CrossRef]
- Bedioune, I.; Lefebvre, F.; Lechêne, P.; Varin, A.; Domergue, V.; Kapiloff, M.S.; Fischmeister, R.; Vandecasteele, G. PDE4 and mAKAPβ are nodal organizers of β2-ARs nuclear PKA signalling in cardiac myocytes. Cardiovasc. Res. 2018, 114, 1499–1511. [Google Scholar] [CrossRef]
- Mika, D.; Bobin, P.; Pomérance, M.; Lechêne, P.; Westenbroek, R.E.; Catterall, W.A.; Vandecasteele, G.; Leroy, J.; Fischmeister, R. Differential regulation of cardiac excitation–contraction coupling by cAMP phosphodiesterase subtypes. Cardiovasc. Res. 2013, 100, 336–346. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M.; Carlsen, J.; Coats, A.J.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2022, 61, 2200879. [Google Scholar] [CrossRef]





| Chamber | Sham (Mean ± SEM) | PH (Mean ± SEM) | p-Value Hierarchical |
|---|---|---|---|
| Calcium Transients Amplitude (Fura-2AM ratio) | |||
| RV | 0.031 ± 0.005 | 0.020 ± 0.003 | 0.0108 (*) |
| LV | 0.037 ± 0.003 | 0.030 ± 0.003 | 0.2046 (n.s.) |
| Calcium TTP90 (sec.) | |||
| RV | 0.028 ± 0.002 | 0.034 ± 0.003 | 0.0231 (*) |
| LV | 0.027 ± 0.002 | 0.032 ± 0.003 | 0.1853 (n.s.) |
| Calcium TTB90 (sec.) | |||
| RV | 0.300 ± 0.010 | 0.409 ± 0.040 | 0.0063 (*) |
| LV | 0.301 ± 0.025 | 0.359 ± 0.014 | 0.1416 (n.s.) |
| Sarcomere Shortening (%) | |||
| RV | 3.899 ± 0.285 | 2.82 ± 0.2048 | 0.0279 (*) |
| LV | 5.259 ± 0.562 | 4.057 ± 0.5356 | 0.1311 (n.s.) |
| Sarcomere Shortening TTP90 (sec.) | |||
| RV | 0.049 ± 0.003 | 0.060 ± 0.006 | 0.0145 (*) |
| LV | 0.050 ± 0.004 | 0.057 ± 0.003 | 0.0238 (*) |
| Sarcomere Shortening TTB90 (sec.) | |||
| RV | 0.193 ± 0.024 | 0.272 ± 0.025 | 0.0096 (**) |
| LV | 0.222 ± 0.019 | 0.266 ± 0.018 | 0.0348 (*) |
| Cell Length Deflection (%) | |||
| RV | 3.702 ± 0.250 | 2.238 ± 0.387 | 0.0003 (***) |
| LV | 5.565 ± 0.782 | 4.541 ± 0.461 | 0.065 (n.s.) |
| Cell Length Deflection TTP90 (sec.) | |||
| RV | 0.050 ± 0.002 | 0.063 ± 0.004 | 0.0009 (***) |
| LV | 0.047 ± 0.003 | 0.055 ± 0.005 | 0.0084 (**) |
| Cell Length Deflection TTB90 (sec.) | |||
| RV | 0.224 ± 0.017 | 0.328 ± 0.045 | 0.0026 (**) |
| LV | 0.250 ± 0.031 | 0.258 ± 0.012 | 0.6909 (n.s.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judina, A.; Niglas, M.; Leonov, V.; Kirkby, N.S.; Diakonov, I.; Wright, P.T.; Zhao, L.; Mitchell, J.A.; Gorelik, J. Pulmonary Hypertension-Associated Right Ventricular Cardiomyocyte Remodelling Reduces Treprostinil Function. Cells 2023, 12, 2764. https://doi.org/10.3390/cells12232764
Judina A, Niglas M, Leonov V, Kirkby NS, Diakonov I, Wright PT, Zhao L, Mitchell JA, Gorelik J. Pulmonary Hypertension-Associated Right Ventricular Cardiomyocyte Remodelling Reduces Treprostinil Function. Cells. 2023; 12(23):2764. https://doi.org/10.3390/cells12232764
Chicago/Turabian StyleJudina, Aleksandra, Marili Niglas, Vladislav Leonov, Nicholas S. Kirkby, Ivan Diakonov, Peter T. Wright, Lan Zhao, Jane A. Mitchell, and Julia Gorelik. 2023. "Pulmonary Hypertension-Associated Right Ventricular Cardiomyocyte Remodelling Reduces Treprostinil Function" Cells 12, no. 23: 2764. https://doi.org/10.3390/cells12232764
APA StyleJudina, A., Niglas, M., Leonov, V., Kirkby, N. S., Diakonov, I., Wright, P. T., Zhao, L., Mitchell, J. A., & Gorelik, J. (2023). Pulmonary Hypertension-Associated Right Ventricular Cardiomyocyte Remodelling Reduces Treprostinil Function. Cells, 12(23), 2764. https://doi.org/10.3390/cells12232764






