The Role of Retinal Ganglion Cell Structure and Function in Glaucoma
Abstract
:1. Introduction
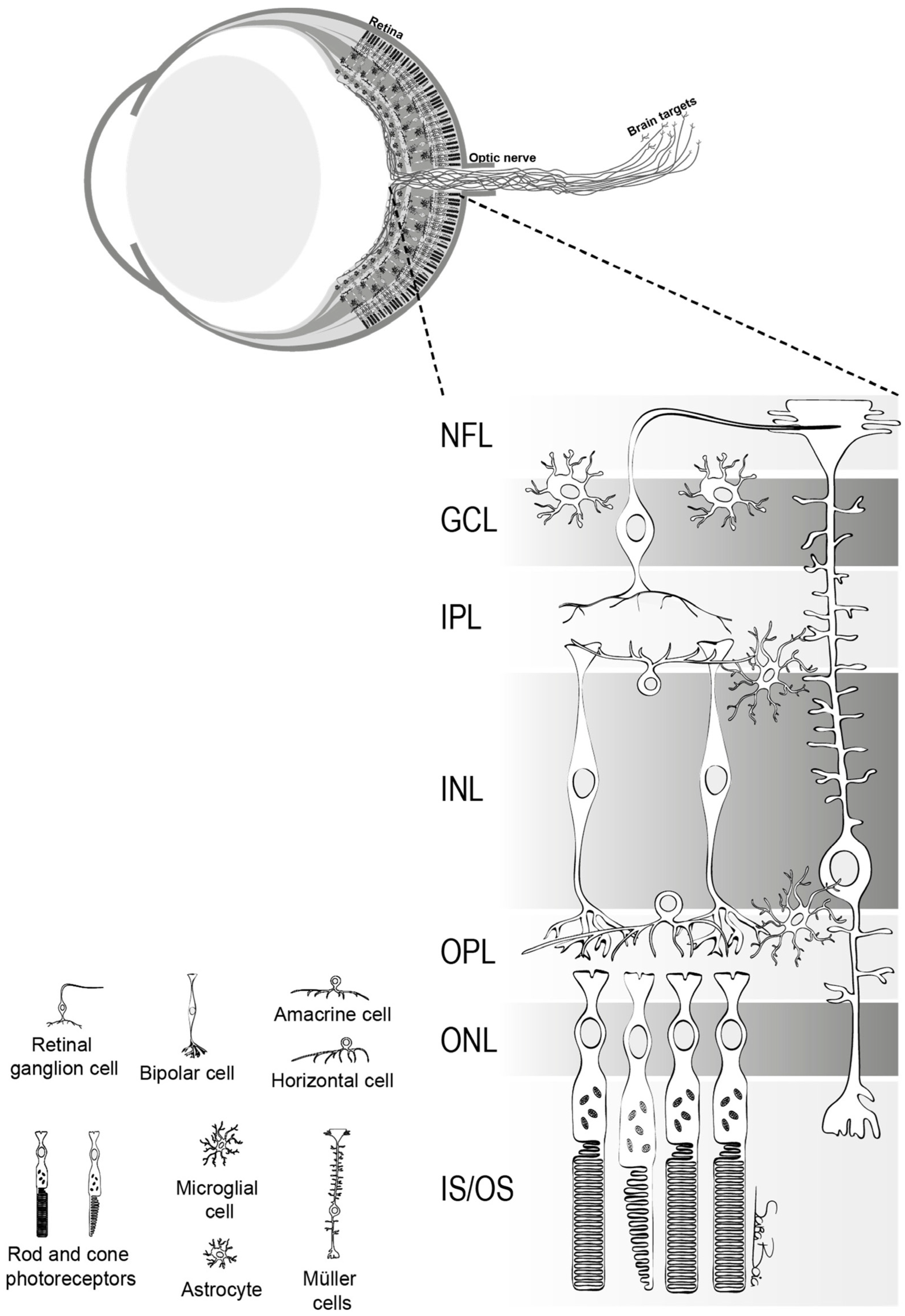
2. Basic Anatomy and Function of Retinal Ganglion Cells
3. Pathophysiology of Glaucoma and Current Theories on Retinal Ganglion Cell Vulnerability and Functionality in Glaucoma
3.1. Mechanical Theories
3.2. Vascular Theories
3.3. Excitotoxicity Theory
3.4. Neurotrophic Factor Deprivation Theory
3.5. Oxidative Stress and Inflammation
4. Imaging Technique for Assessing Retinal Ganglion Cells
4.1. Structural Imaging Modalities
4.2. Functional Imaging Modalities
5. Therapeutic Approaches Targeting Retinal Ganglion Cells
5.1. Neuroprotective Agents
5.2. Calcium Channel Blockers
5.3. Antioxidants
5.4. Neurotrophic Factors
5.5. Gene Therapy
5.6. Stem Cell Therapy
5.7. Intraocular Pressure Management
5.8. Blood Flow Enhancement
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Balendra, S.I.; Normando, E.M.; Bloom, P.A.; Cordeiro, M.F. Advances in retinal ganglion cell imaging. Eye 2015, 29, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.S.; Luo, X.; Ribas, V.T.; Petrs-Silva, H.; Koch, J.C. The Role of Axonal Transport in Glaucoma. Int. J. Mol. Sci. 2022, 23, 3935. [Google Scholar] [CrossRef] [PubMed]
- Dunaief, J.L.; Dentchev, T.; Ying, G.S.; Milam, A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Boia, R.; Ruzafa, N.; Aires, I.D.; Pereiro, X.; Ambrosio, A.F.; Vecino, E.; Santiago, A.R. Neuroprotective Strategies for Retinal Ganglion Cell Degeneration: Current Status and Challenges Ahead. Int. J. Mol. Sci. 2020, 21, 2262. [Google Scholar] [CrossRef]
- Vernazza, S.; Oddone, F.; Tirendi, S.; Bassi, A.M. Risk Factors for Retinal Ganglion Cell Distress in Glaucoma and Neuroprotective Potential Intervention. Int. J. Mol. Sci. 2021, 22, 7994. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The neuronal organization of the retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef]
- Martin, P.R. Colour processing in the primate retina: Recent progress. J. Physiol. 1998, 513 Pt 3, 631–638. [Google Scholar] [CrossRef]
- Manookin, M.B.; Patterson, S.S.; Linehan, C.M. Neural Mechanisms Mediating Motion Sensitivity in Parasol Ganglion Cells of the Primate Retina. Neuron 2018, 97, 1327–1340.e1324. [Google Scholar] [CrossRef]
- Weber, A.J.; Kaufman, P.L.; Hubbard, W.C. Morphology of single ganglion cells in the glaucomatous primate retina. Invest. Ophthalmol. Vis. Sci. 1998, 39, 2304–2320. [Google Scholar]
- Martin, P.R.; Grunert, U. Analysis of the short wavelength-sensitive (“blue”) cone mosaic in the primate retina: Comparison of New World and Old World monkeys. J. Comp. Neurol. 1999, 406, 1–14. [Google Scholar] [CrossRef]
- Crook, J.D.; Peterson, B.B.; Packer, O.S.; Robinson, F.R.; Gamlin, P.D.; Troy, J.B.; Dacey, D.M. The smooth monostratified ganglion cell: Evidence for spatial diversity in the Y-cell pathway to the lateral geniculate nucleus and superior colliculus in the macaque monkey. J. Neurosci. 2008, 28, 12654–12671. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.H. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron 2019, 104, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.R.; Masland, R.H. The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.C.; Saito, C.A.; Lee, B.B.; Kremers, J.; da Silva Filho, M.; Kilavik, B.E.; Yamada, E.S.; Perry, V.H. Morphology and physiology of primate M- and P-cells. Prog. Brain Res. 2004, 144, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, J.; Loizos, K.; Yue, L.; Humayun, M.S.; Lazzi, G. Color and cellular selectivity of retinal ganglion cell subtypes through frequency modulation of electrical stimulation. Sci. Rep. 2021, 11, 5177. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Mozaffarieh, M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv. Ophthalmol. 2007, 52 (Suppl. 2), S162–S173. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.B.; Coloma, F.M.; Metheetrairut, A.; Trope, G.E.; Heathcote, J.G.; Ethier, C.R. Deformation of the lamina cribrosa by elevated intraocular pressure. Br. J. Ophthalmol. 1994, 78, 643–648. [Google Scholar] [CrossRef]
- Downs, J.C.; Roberts, M.D.; Burgoyne, C.F. Mechanical environment of the optic nerve head in glaucoma. Optom. Vis. Sci. 2008, 85, 425–435. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Downs, J.C.; Bellezza, A.J.; Suh, J.K.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Kunikata, H.; Aizawa, N.; Kiyota, N.; Maiya, Y.; Yokoyama, Y.; Omodaka, K.; Takahashi, H.; Yasui, T.; Kato, K.; et al. Optic Nerve Head Blood Flow, as Measured by Laser Speckle Flowgraphy, Is Significantly Reduced in Preperimetric Glaucoma. Curr. Eye Res. 2016, 41, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; Coleman, A.L.; Blood Flow in Glaucoma, D. Blood pressure, perfusion pressure, and glaucoma. Am. J. Ophthalmol. 2010, 149, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Wu, S.Y.; Hennis, A.; Honkanen, R.; Nemesure, B.; Group, B.E.S. Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology 2008, 115, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, F.; Ying-Lai, M.; Chung, J.; Azen, S.P.; Varma, R.; Los Angeles Latino Eye Study, G. Blood pressure, perfusion pressure, and open-angle glaucoma: The Los Angeles Latino Eye Study. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wong, T.Y.; Mitchell, P.; Friedman, D.S.; He, M.; Aung, T. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: The singapore malay eye study. Invest. Ophthalmol. Vis. Sci. 2010, 51, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- Geijer, C.; Bill, A. Effects of raised intraocular pressure on retinal, prelaminar, laminar, and retrolaminar optic nerve blood flow in monkeys. Invest. Ophthalmol. Vis. Sci. 1979, 18, 1030–1042. [Google Scholar] [PubMed]
- Alm, A.; Bill, A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp. Eye Res. 1973, 15, 15–29. [Google Scholar] [CrossRef]
- Grieshaber, M.C.; Mozaffarieh, M.; Flammer, J. What is the link between vascular dysregulation and glaucoma? Surv. Ophthalmol. 2007, 52 (Suppl. 2), S144–S154. [Google Scholar] [CrossRef]
- Galambos, P.; Vafiadis, J.; Vilchez, S.E.; Wagenfeld, L.; Matthiessen, E.T.; Richard, G.; Klemm, M.; Zeitz, O. Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology 2006, 113, 1832–1836. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Liu, H.; Mercieca, K.; Prinz, J.; Feng, Y.; Prokosch, V. The Association between Vascular Abnormalities and Glaucoma-What Comes First? Int. J. Mol. Sci. 2023, 24, 13211. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, X.; Chen, W.; Wang, H.; Huang, C.; Li, T.; Wang, N.; Xian, J. Altered coupling of cerebral blood flow and functional connectivity strength in visual and higher order cognitive cortices in primary open angle glaucoma. J. Cereb. Blood Flow. Metab. 2021, 41, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.A.; Elewa, A.M.; Arafa, L.F. Endothelin-1 and nitric oxide levels in patients with glaucoma. Ophthalmic Res. 2011, 46, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, Y.Z.; Harris, A.; Shoja, M.M.; Rusia, D.; Siesky, B.; Arieli, Y.; Wirostko, B. Endothelin and its suspected role in the pathogenesis and possible treatment of glaucoma. Curr. Eye Res. 2012, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Emre, M.; Orgul, S.; Haufschild, T.; Shaw, S.G.; Flammer, J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br. J. Ophthalmol. 2005, 89, 60–63. [Google Scholar] [CrossRef] [PubMed]
- McGrady, N.R.; Minton, A.Z.; Stankowska, D.L.; He, S.; Jefferies, H.B.; Krishnamoorthy, R.R. Upregulation of the endothelin A (ETA) receptor and its association with neurodegeneration in a rodent model of glaucoma. BMC Neurosci. 2017, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, C.; Rothaus, K.; Schopmeyer, L.; Feldmann, M.; Bauer, D.; Grisanti, S.; Heinz, C.; Kasper, M. Elevated endothelin-1 levels as risk factor for an impaired ocular blood flow measured by OCT-A in glaucoma. Sci. Rep. 2022, 12, 11801. [Google Scholar] [CrossRef] [PubMed]
- Orgul, S.; Cioffi, G.A.; Bacon, D.R.; Van Buskirk, E.M. An endothelin-1-induced model of chronic optic nerve ischemia in rhesus monkeys. J. Glaucoma 1996, 5, 135–138. [Google Scholar] [PubMed]
- Wang, L.; Fortune, B.; Cull, G.; Dong, J.; Cioffi, G.A. Endothelin B receptor in human glaucoma and experimentally induced optic nerve damage. Arch. Ophthalmol. 2006, 124, 717–724. [Google Scholar] [CrossRef]
- Haefliger, I.O.; Dettmann, E.; Liu, R.; Meyer, P.; Prunte, C.; Messerli, J.; Flammer, J. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv. Ophthalmol. 1999, 43 (Suppl. 1), S51–S58. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, I.; Fairless, R. Retinal Glutamate Neurotransmission: From Physiology to Pathophysiological Mechanisms of Retinal Ganglion Cell Degeneration. Life 2022, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Lotery, A.J. Glutamate excitotoxicity in glaucoma: Truth or fiction? Eye 2005, 19, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, Y.; Shimazawa, M.; Nakajima, Y.; Komuro, I.; Matsuda, T.; Baba, A.; Araie, M.; Kita, S.; Iwamoto, T.; Hara, H. A Na+/Ca2+ exchanger isoform, NCX1, is involved in retinal cell death after N-methyl-D-aspartate injection and ischemia-reperfusion. J. Neurosci. Res. 2009, 87, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Brittain, M.K.; Brustovetsky, T.; Sheets, P.L.; Brittain, J.M.; Khanna, R.; Cummins, T.R.; Brustovetsky, N. Delayed calcium dysregulation in neurons requires both the NMDA receptor and the reverse Na+/Ca2+ exchanger. Neurobiol. Dis. 2012, 46, 109–117. [Google Scholar] [CrossRef]
- Brandt, S.K.; Weatherly, M.E.; Ware, L.; Linn, D.M.; Linn, C.L. Calcium preconditioning triggers neuroprotection in retinal ganglion cells. Neuroscience 2011, 172, 387–397. [Google Scholar] [CrossRef]
- Hartwick, A.T.; Hamilton, C.M.; Baldridge, W.H. Glutamatergic calcium dynamics and deregulation of rat retinal ganglion cells. J. Physiol. 2008, 586, 3425–3446. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Martin, K.R.; Levkovitch-Verbin, H.; Valenta, D.; Baumrind, L.; Pease, M.E.; Quigley, H.A. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest. Ophthalmol. Vis. Sci. 2002, 43, 2236–2243. [Google Scholar] [PubMed]
- Schuettauf, F.; Thaler, S.; Bolz, S.; Fries, J.; Kalbacher, H.; Mankowska, A.; Zurakowski, D.; Zrenner, E.; Rejdak, R. Alterations of amino acids and glutamate transport in the DBA/2J mouse retina; possible clues to degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Harada, C.; Nakamura, K.; Quah, H.M.; Okumura, A.; Namekata, K.; Saeki, T.; Aihara, M.; Yoshida, H.; Mitani, A.; et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Investig. 2007, 117, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Barde, Y.A.; Schwab, M.; Thoenen, H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J. Neurosci. 1986, 6, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Dekeyster, E.; Geeraerts, E.; Buyens, T.; Van den Haute, C.; Baekelandt, V.; De Groef, L.; Salinas-Navarro, M.; Moons, L. Tackling Glaucoma from within the Brain: An Unfortunate Interplay of BDNF and TrkB. PLoS ONE 2015, 10, e0142067. [Google Scholar] [CrossRef]
- Gonzalez-Hoyuela, M.; Barbas, J.A.; Rodriguez-Tebar, A. The autoregulation of retinal ganglion cell number. Development 2001, 128, 117–124. [Google Scholar] [CrossRef]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef]
- Lambuk, L.; Mohd Lazaldin, M.A.; Ahmad, S.; Iezhitsa, I.; Agarwal, R.; Uskokovic, V.; Mohamud, R. Brain-Derived Neurotrophic Factor-Mediated Neuroprotection in Glaucoma: A Review of Current State of the Art. Front. Pharmacol. 2022, 13, 875662. [Google Scholar] [CrossRef]
- Pease, M.E.; McKinnon, S.J.; Quigley, H.A.; Kerrigan-Baumrind, L.A.; Zack, D.J. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2000, 41, 764–774. [Google Scholar]
- Quigley, H.A.; McKinnon, S.J.; Zack, D.J.; Pease, M.E.; Kerrigan-Baumrind, L.A.; Kerrigan, D.F.; Mitchell, R.S. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3460–3466. [Google Scholar]
- Osborne, A.; Khatib, T.Z.; Songra, L.; Barber, A.C.; Hall, K.; Kong, G.Y.X.; Widdowson, P.S.; Martin, K.R. Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin-related kinase receptor-B signaling. Cell Death Dis. 2018, 9, 1007. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Nakamoto, K.; Kobayashi, M.; Suzuki, H.; Arima, T.; Tobita, Y.; Takao, K.; Igarashi, T.; Okuda, T.; Okada, T.; et al. Brain-derived Neurotrophic Factor in the Aqueous Humor of Glaucoma Patients. J. Nippon. Med. Sch. 2021, 88, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Shpak, A.A.; Guekht, A.B.; Druzhkova, T.A.; Kozlova, K.I.; Gulyaeva, N.V. Brain-Derived Neurotrophic Factor in Patients with Primary Open-Angle Glaucoma and Age-related Cataract. Curr. Eye Res. 2018, 43, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects. Cells 2021, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best. Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Izzotti, A.; Sacca, S.C.; Longobardi, M.; Cartiglia, C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest. Ophthalmol. Vis. Sci. 2009, 50, 5251–5258. [Google Scholar] [CrossRef]
- Clopton, D.A.; Saltman, P. Low-level oxidative stress causes cell-cycle specific arrest in cultured cells. Biochem. Biophys. Res. Commun. 1995, 210, 189–196. [Google Scholar] [CrossRef]
- Giancotti, F.G. Integrin signaling: Specificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 1997, 9, 691–700. [Google Scholar] [CrossRef]
- Knepper, P.A.; Goossens, W.; Hvizd, M.; Palmberg, P.F. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1360–1367. [Google Scholar]
- Zhou, L.; Li, Y.; Yue, B.Y. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: The trabecular meshwork. J. Cell Physiol. 1999, 180, 182–189. [Google Scholar] [CrossRef]
- Alvarado, J.A.; Alvarado, R.G.; Yeh, R.F.; Franse-Carman, L.; Marcellino, G.R.; Brownstein, M.J. A new insight into the cellular regulation of aqueous outflow: How trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm’s canal endothelial cells. Br. J. Ophthalmol. 2005, 89, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.Y.X.; Van Bergen, N.J.; Trounce, I.A.; Crowston, J.G. Mitochondrial Dysfunction and Glaucoma. J. Glaucoma 2009, 18, 93–100. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Leung, K.W.; Zhang, Y.H.; Duan, S.; Zhong, X.F.; Jiang, R.Z.; Peng, Z.; Tombran-Tink, J.; Ge, J. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: Protection by antioxidants. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.W.; Danias, J.; Pohorenec, G.; Yuan, H.M.; Burakgazi, E.; Chalmers-Redman, R.; Podos, S.M.; Tatton, W.G. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3451–3459. [Google Scholar] [PubMed]
- Shi, W.; Tan, C.; Liu, C.; Chen, D. Mitochondrial fission mediated by Drp1-Fis1 pathway and neurodegenerative diseases. Rev. Neurosci. 2023, 34, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; You, M.; Fan, C.; Rong, R.; Li, H.; Xia, X. Pathologically high intraocular pressure induces mitochondrial dysfunction through Drp1 and leads to retinal ganglion cell PANoptosis in glaucoma. Redox Biol. 2023, 62, 102687. [Google Scholar] [CrossRef]
- Tezel, G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res. 2022, 87, 100998. [Google Scholar] [CrossRef]
- Kaur, C.; Ling, E.A. Blood brain barrier in hypoxic-ischemic conditions. Curr. Neurovasc Res. 2008, 5, 71–81. [Google Scholar] [CrossRef]
- Chua, J.; Vania, M.; Cheung, C.M.; Ang, M.; Chee, S.P.; Yang, H.; Li, J.; Wong, T.T. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol. Vis. 2012, 18, 431–438. [Google Scholar]
- Wei, X.; Cho, K.S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Investig. 2011, 121, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cho, K.S.; Vu, T.H.K.; Shen, C.H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- Von Thun Und Hohenstein-Blaul, N.; Bell, K.; Pfeiffer, N.; Grus, F.H. Autoimmune aspects in glaucoma. Eur. J. Pharmacol. 2016, 787, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Neufeld, A.H. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res. 2001, 64, 523–532. [Google Scholar] [CrossRef] [PubMed]
- de Hoz, R.; Gallego, B.I.; Ramirez, A.I.; Rojas, B.; Salazar, J.J.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Trivino, A.; et al. Rod-like microglia are restricted to eyes with laser-induced ocular hypertension but absent from the microglial changes in the contralateral untreated eye. PLoS ONE 2013, 8, e83733. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Drexler, W.; Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008, 27, 45–88. [Google Scholar] [CrossRef]
- Geevarghese, A.; Wollstein, G.; Ishikawa, H.; Schuman, J.S. Optical Coherence Tomography and Glaucoma. Annu. Rev. Vis. Sci. 2021, 7, 693–726. [Google Scholar] [CrossRef]
- Sommer, A.; Katz, J.; Quigley, H.A.; Miller, N.R.; Robin, A.L.; Richter, R.C.; Witt, K.A. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch. Ophthalmol. 1991, 109, 77–83. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Cherecheanu, A.P.; Garhofer, G.; Schmidl, D.; Schmetterer, L. Imaging of retinal ganglion cells in glaucoma: Pitfalls and challenges. Cell Tissue Res. 2013, 353, 261–268. [Google Scholar] [CrossRef]
- Dong, Z.M.; Wollstein, G.; Wang, B.; Schuman, J.S. Adaptive optics optical coherence tomography in glaucoma. Prog. Retin. Eye Res. 2017, 57, 76–88. [Google Scholar] [CrossRef]
- Kocaoglu, O.P.; Cense, B.; Jonnal, R.S.; Wang, Q.; Lee, S.; Gao, W.; Miller, D.T. Imaging retinal nerve fiber bundles using optical coherence tomography with adaptive optics. Vision. Res. 2011, 51, 1835–1844. [Google Scholar] [CrossRef]
- Sarossy, M.; Crowston, J.; Kumar, D.; Weymouth, A.; Wu, Z. Prediction of glaucoma severity using parameters from the electroretinogram. Sci. Rep. 2021, 11, 23886. [Google Scholar] [CrossRef]
- Banitt, M.R.; Ventura, L.M.; Feuer, W.J.; Savatovsky, E.; Luna, G.; Shif, O.; Bosse, B.; Porciatti, V. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2346–2352. [Google Scholar] [CrossRef]
- Bode, S.F.; Jehle, T.; Bach, M. Pattern electroretinogram in glaucoma suspects: New findings from a longitudinal study. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4300–4306. [Google Scholar] [CrossRef]
- Jung, K.I.; Jeon, S.; Shin, D.Y.; Lee, J.; Park, C.K. Pattern Electroretinograms in Preperimetric and Perimetric Glaucoma. Am. J. Ophthalmol. 2020, 215, 118–126. [Google Scholar] [CrossRef]
- Lee, T.; Seo, D.R.; Kim, J.Y.; Choi, W.; Lee, S.Y.; Lee, J.M.; Seong, G.J.; Kim, C.Y.; Bae, H.W. Relationship between N95 Amplitude of Pattern Electroretinogram and Optical Coherence Tomography Angiography in Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3854. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G.; Harwerth, R.S.; Smith, E.L., 3rd. The photopic negative response of the macaque electroretinogram: Reduction by experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1124–1136. [Google Scholar]
- Machida, S.; Gotoh, Y.; Toba, Y.; Ohtaki, A.; Kaneko, M.; Kurosaka, D. Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 2008, 49, 2201–2207. [Google Scholar] [CrossRef]
- Wilsey, L.J.; Fortune, B. Electroretinography in glaucoma diagnosis. Curr. Opin. Ophthalmol. 2016, 27, 118–124. [Google Scholar] [CrossRef]
- Chan, H.L.; Brown, B. Multifocal ERG changes in glaucoma. Ophthalmic Physiol. Opt. 1999, 19, 306–316. [Google Scholar] [CrossRef]
- Hasegawa, S.; Takagi, M.; Usui, T.; Takada, R.; Abe, H. Waveform changes of the first-order multifocal electroretinogram in patients with glaucoma. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1597–1603. [Google Scholar]
- Fortune, B.; Bearse, M.A., Jr.; Cioffi, G.A.; Johnson, C.A. Selective loss of an oscillatory component from temporal retinal multifocal ERG responses in glaucoma. Invest. Ophthalmol. Vis. Sci. 2002, 43, 2638–2647. [Google Scholar]
- Palmowski, A.M.; Allgayer, R.; Heinemann-Vemaleken, B. The multifocal ERG in open angle glaucoma--a comparison of high and low contrast recordings in high- and low-tension open angle glaucoma. Doc. Ophthalmol. 2000, 101, 35–49. [Google Scholar] [CrossRef]
- Gardiner, S.K.; Swanson, W.H.; Demirel, S.; McKendrick, A.M.; Turpin, A.; Johnson, C.A. A two-stage neural spiking model of visual contrast detection in perimetry. Vision. Res. 2008, 48, 1859–1869. [Google Scholar] [CrossRef]
- Dacey, D.M.; Lee, B.B. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature 1994, 367, 731–735. [Google Scholar] [CrossRef]
- Sample, P.A.; Bosworth, C.F.; Blumenthal, E.Z.; Girkin, C.; Weinreb, R.N. Visual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucoma. Invest. Ophthalmol. Vis. Sci. 2000, 41, 1783–1790. [Google Scholar]
- Johnson, C.A.; Adams, A.J.; Casson, E.J.; Brandt, J.D. Progression of early glaucomatous visual field loss as detected by blue-on-yellow and standard white-on-white automated perimetry. Arch. Ophthalmol. 1993, 111, 651–656. [Google Scholar] [CrossRef]
- Demirel, S.; Johnson, C.A. Incidence and prevalence of short wavelength automated perimetry deficits in ocular hypertensive patients. Am. J. Ophthalmol. 2001, 131, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Sample, P.A.; Weinreb, R.N. Corneal thickness measurements and visual function abnormalities in ocular hypertensive patients. Am. J. Ophthalmol. 2003, 135, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Bowd, C.; Medeiros, F.A.; Weinreb, R.N.; Sample, P.A.; Hoffmann, E.M.; Zangwill, L.M. Combining structural and functional testing for detection of glaucoma. Ophthalmology 2006, 113, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Sample, P.A.; Weinreb, R.N. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am. J. Ophthalmol. 2004, 137, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Samuels, S.J. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest. Ophthalmol. Vis. Sci. 1997, 38, 413–425. [Google Scholar]
- Chauhan, B.C.; Johnson, C.A. Test-retest variability of frequency-doubling perimetry and conventional perimetry in glaucoma patients and normal subjects. Invest. Ophthalmol. Vis. Sci. 1999, 40, 648–656. [Google Scholar]
- Cordeiro, M.F.; Hill, D.; Patel, R.; Corazza, P.; Maddison, J.; Younis, S. Detecting retinal cell stress and apoptosis with DARC: Progression from lab to clinic. Prog. Retin. Eye Res. 2022, 86, 100976. [Google Scholar] [CrossRef]
- Cordeiro, M.F.; Guo, L.; Luong, V.; Harding, G.; Wang, W.; Jones, H.E.; Moss, S.E.; Sillito, A.M.; Fitzke, F.W. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 13352–13356. [Google Scholar] [CrossRef]
- Guo, L.; Moss, S.E.; Alexander, R.A.; Ali, R.R.; Fitzke, F.W.; Cordeiro, M.F. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest. Ophthalmol. Vis. Sci. 2005, 46, 175–182. [Google Scholar] [CrossRef]
- Yoles, E.; Wheeler, L.A.; Schwartz, M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Invest. Ophthalmol. Vis. Sci. 1999, 40, 65–73. [Google Scholar]
- Kalapesi, F.B.; Coroneo, M.T.; Hill, M.A. Human ganglion cells express the alpha-2 adrenergic receptor: Relevance to neuroprotection. Br. J. Ophthalmol. 2005, 89, 758–763. [Google Scholar] [CrossRef]
- Prokosch, V.; Panagis, L.; Volk, G.F.; Dermon, C.; Thanos, S. Alpha2-adrenergic receptors and their core involvement in the process of axonal growth in retinal explants. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6688–6699. [Google Scholar] [CrossRef]
- Lambert, W.S.; Ruiz, L.; Crish, S.D.; Wheeler, L.A.; Calkins, D.J. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol. Neurodegener. 2011, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Krupin, T.; Liebmann, J.M.; Greenfield, D.S.; Ritch, R.; Gardiner, S.; Low-Pressure Glaucoma Study, G. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the Low-Pressure Glaucoma Treatment Study. Am. J. Ophthalmol. 2011, 151, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Chang, H.W. Comparison of the effects of brimonidine 0.2% and timolol 0.5% on retinal nerve fiber layer thickness in ocular hypertensive patients: A prospective, unmasked study. J. Ocul. Pharmacol. Ther. 2005, 21, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Salt, T.E.; Maass, A.; Luong, V.; Moss, S.E.; Fitzke, F.W.; Cordeiro, M.F. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest. Ophthalmol. Vis. Sci. 2006, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Labruyere, J.; Wang, G.; Wozniak, D.F.; Price, M.T.; Sesma, M.A. NMDA antagonist neurotoxicity: Mechanism and prevention. Science 1991, 254, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Hare, W.A.; WoldeMussie, E.; Lai, R.K.; Ton, H.; Ruiz, G.; Chun, T.; Wheeler, L. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Vorwerk, C.K.; Lipton, S.A.; Zurakowski, D.; Hyman, B.T.; Sabel, B.A.; Dreyer, E.B. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1618–1624. [Google Scholar]
- Danesh-Meyer, H.V.; Levin, L.A. Neuroprotection: Extrapolating from neurologic diseases to the eye. Am. J. Ophthalmol. 2009, 148, 186–191.e182. [Google Scholar] [CrossRef]
- Osborne, N.N.; Wood, J.P.; Cupido, A.; Melena, J.; Chidlow, G. Topical flunarizine reduces IOP and protects the retina against ischemia-excitotoxicity. Invest. Ophthalmol. Vis. Sci. 2002, 43, 1456–1464. [Google Scholar]
- Koseki, N.; Araie, M.; Tomidokoro, A.; Nagahara, M.; Hasegawa, T.; Tamaki, Y.; Yamamoto, S. A placebo-controlled 3-year study of a calcium blocker on visual field and ocular circulation in glaucoma with low-normal pressure. Ophthalmology 2008, 115, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Kitazawa, Y.; Yamamoto, T.; Okabe, I.; Ichien, K. Prevention of visual field defect progression with brovincamine in eyes with normal-tension glaucoma. Ophthalmology 1996, 103, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Tartaglione, R.; Cerulli, A.; Mancino, R.; Spano, A.; Cavaliere, F.; Rombola, L.; Bagetta, G.; Corasaniti, M.T.; Morrone, L.A. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int. Rev. Neurobiol. 2007, 82, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cavaliere, F.; Rombola, L.; Gliozzi, M.; Cerulli, A.; Nucci, C.; Fazzi, E.; Bagetta, G.; Corasaniti, M.T.; Morrone, L.A. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. Prog. Brain Res. 2008, 173, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Inokuchi, Y.; Nishi, M.; Shimazawa, M.; Otsubo, K.; Hara, H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008, 1226, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest. Ophthalmol. Vis. Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Centofanti, M.; Gandolfi, S.; Marangoni, D.; Rossetti, L.; Tanga, L.; Tardini, M.; Traina, S.; Ungaro, N.; Vetrugno, M.; et al. Effects of coenzyme Q10 in conjunction with vitamin E on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J. Glaucoma 2014, 23, 391–404. [Google Scholar] [CrossRef]
- Sawai, H.; Clarke, D.B.; Kittlerova, P.; Bray, G.M.; Aguayo, A.J. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J. Neurosci. 1996, 16, 3887–3894. [Google Scholar] [CrossRef]
- Chen, H.; Weber, A.J. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest. Ophthalmol. Vis. Sci. 2001, 42, 966–974. [Google Scholar]
- Domenici, L.; Origlia, N.; Falsini, B.; Cerri, E.; Barloscio, D.; Fabiani, C.; Sansò, M.; Giovannini, L. Rescue of Retinal Function by BDNF in a Mouse Model of Glaucoma. PLoS ONE 2014, 9, e115579. [Google Scholar] [CrossRef]
- Ko, M.L.; Hu, D.N.; Ritch, R.; Sharma, S.C.; Chen, C.F. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci. Lett. 2001, 305, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Weber, A.J. Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Res. 2004, 1011, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Lee, M.Y.; Meyer, V.; Wiese, A.; Hofmann, H.D. Evidence for multiple, local functions of ciliary neurotrophic factor (CNTF) in retinal development: Expression of CNTF and its receptors and in vitro effects on target cells. J. Neurochem. 1997, 68, 979–990. [Google Scholar] [CrossRef]
- Wen, R.; Song, Y.; Liu, Y.; Li, Y.; Zhao, L.; Laties, A.M. CNTF negatively regulates the phototransduction machinery in rod photoreceptors: Implication for light-induced photostasis plasticity. Adv. Exp. Med. Biol. 2008, 613, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Mey, J.; Thanos, S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993, 602, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Takahata, K.; Katsuki, H.; Kume, T.; Nakata, D.; Ito, K.; Muraoka, S.; Yoneda, F.; Kashii, S.; Honda, Y.; Akaike, A. Retinal neuronal death induced by intraocular administration of a nitric oxide donor and its rescue by neurotrophic factors in rats. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1760–1766. [Google Scholar] [CrossRef]
- Colafrancesco, V.; Parisi, V.; Sposato, V.; Rossi, S.; Russo, M.A.; Coassin, M.; Lambiase, A.; Aloe, L. Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma. J. Glaucoma 2011, 20, 100–108. [Google Scholar] [CrossRef]
- Lambiase, A.; Aloe, L.; Centofanti, M.; Parisi, V.; Mantelli, F.; Colafrancesco, V.; Manni, G.L.; Bucci, M.G.; Bonini, S.; Levi-Montalcini, R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA 2009, 106, 13469–13474. [Google Scholar] [CrossRef]
- Guo, L.; Davis, B.M.; Ravindran, N.; Galvao, J.; Kapoor, N.; Haamedi, N.; Shamsher, E.; Luong, V.; Fico, E.; Cordeiro, M.F. Topical recombinant human Nerve growth factor (rh-NGF) is neuroprotective to retinal ganglion cells by targeting secondary degeneration. Sci. Rep. 2020, 10, 3375. [Google Scholar] [CrossRef]
- Jiang, C.; Moore, M.J.; Zhang, X.; Klassen, H.; Langer, R.; Young, M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol. Vis. 2007, 13, 1783–1792. [Google Scholar]
- Checa-Casalengua, P.; Jiang, C.; Bravo-Osuna, I.; Tucker, B.A.; Molina-Martínez, I.T.; Young, M.J.; Herrero-Vanrell, R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J. Control. Release 2011, 156, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Khoobehi, A.; Lavik, E.; Langer, R.; Young, M. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J. Pharm. Sci. 2007, 96, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, P.; Huang, H.; Li, L.; Liu, L.; Webber, H.C.; Dalal, R.; Siew, L.; Fligor, C.M.; Chang, K.C.; et al. Mouse γ-Synuclein Promoter-Mediated Gene Expression and Editing in Mammalian Retinal Ganglion Cells. J. Neurosci. 2020, 40, 3896–3914. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Anderson, S.R.; Breen, K.T.; Romero, C.O.; Steele, M.R.; Chiodo, V.A.; Boye, S.L.; Hauswirth, W.W.; Tomlinson, S.; Vetter, M.L. Complement C3-Targeted Gene Therapy Restricts Onset and Progression of Neurodegeneration in Chronic Mouse Glaucoma. Mol. Ther. 2018, 26, 2379–2396. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, J.; Starr, C.; Mohns, E.J.; Li, Y.; Chen, E.P.; Yoon, Y.; Kellner, C.P.; Tanaka, K.; Wang, H.; et al. Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell 2021, 184, 4299–4314.e4212. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhuang, P.; Feng, X.; Liu, P.; Liu, D.; Huang, H.; Li, L.; Chen, W.; Liu, L.; Sun, Y.; et al. NMNAT2 is downregulated in glaucomatous RGCs, and RGC-specific gene therapy rescues neurodegeneration and visual function. Mol. Ther. 2022, 30, 1421–1431. [Google Scholar] [CrossRef]
- Visuvanathan, S.; Baker, A.N.; Lagali, P.S.; Coupland, S.G.; Miller, G.; Hauswirth, W.W.; Tsilfidis, C. XIAP gene therapy effects on retinal ganglion cell structure and function in a mouse model of glaucoma. Gene Ther. 2022, 29, 147–156. [Google Scholar] [CrossRef]
- Donahue, R.J.; Fehrman, R.L.; Gustafson, J.R.; Nickells, R.W. BCLXL gene therapy moderates neuropathology in the DBA/2J mouse model of inherited glaucoma. Cell Death Dis. 2021, 12, 781. [Google Scholar] [CrossRef]
- Nishida, A.; Takahashi, M.; Tanihara, H.; Nakano, I.; Takahashi, J.B.; Mizoguchi, A.; Ide, C.; Honda, Y. Incorporation and differentiation of hippocampus-derived neural stem cells transplanted in injured adult rat retina. Invest. Ophthalmol. Vis. Sci. 2000, 41, 4268–4274. [Google Scholar]
- Singhal, S.; Bhatia, B.; Jayaram, H.; Becker, S.; Jones, M.F.; Cottrill, P.B.; Khaw, P.T.; Salt, T.E.; Limb, G.A. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl. Med. 2012, 1, 188–199. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Gil, D.W.; WoldeMussie, E. Role of alpha-2 adrenergic receptors in neuroprotection and glaucoma. Surv. Ophthalmol. 2001, 45 (Suppl. 3), S290–S294; discussion S295–S296. [Google Scholar] [CrossRef]
- Bylund, D.B.; Chacko, D.M. Characterization of alpha2 adrenergic receptor subtypes in human ocular tissue homogenates. Invest. Ophthalmol. Vis. Sci. 1999, 40, 2299–2306. [Google Scholar] [PubMed]
- Takayama, J.; Tomidokoro, A.; Ishii, K.; Tamaki, Y.; Fukaya, Y.; Hosokawa, T.; Araie, M. Time course of the change in optic nerve head circulation after an acute increase in intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.; Harada, T.; Quah, H.-M.; Maekawa, F.; Yoshida, K.; Ohno, S.; Wada, K.; Parada, L.; Tanaka, K. Potential role of glial cell line-derived neurotrophic factor receptors in Müller glial cells during light-induced retinal degeneration. Neuroscience 2003, 122, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ratican, S.E.; Osborne, A.; Martin, K.R. Progress in Gene Therapy to Prevent Retinal Ganglion Cell Loss in Glaucoma and Leber’s Hereditary Optic Neuropathy. Neural Plast. 2018, 2018, 7108948. [Google Scholar] [CrossRef] [PubMed]
- Conlon, R.; Saheb, H.; Ahmed, I.I.K. Glaucoma treatment trends: A review. Can. J. Ophthalmol. 2017, 52, 114–124. [Google Scholar] [CrossRef]
- Kang, J.M.; Lin, S. Ginkgo biloba and its potential role in glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 116–120. [Google Scholar] [CrossRef]
- Hirooka, K.; Tokuda, M.; Miyamoto, O.; Itano, T.; Baba, T.; Shiraga, F. The Ginkgo biloba extract (EGb 761) provides a neuroprotective effect on retinal ganglion cells in a rat model of chronic glaucoma. Curr. Eye Res. 2004, 28, 153–157. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Luis, J.; Eastlake, K.; Lamb, W.D.B.; Limb, G.A.; Jayaram, H.; Khaw, P.T. Cell-Based Therapies for Glaucoma. Transl. Vision. Sci. Technol. 2023, 12, 23. [Google Scholar] [CrossRef]
- Sahle, F.F.; Kim, S.; Niloy, K.K.; Tahia, F.; Fili, C.V.; Cooper, E.; Hamilton, D.J.; Lowe, T.L. Nanotechnology in regenerative ophthalmology. Adv. Drug Deliv. Rev. 2019, 148, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S. Clinical Applications of Artificial Intelligence in Glaucoma. J. Ophthalmic Vis. Res. 2023, 18, 97–112. [Google Scholar] [CrossRef] [PubMed]
| Theories | Key Points |
|---|---|
| Mechanical theory |
|
| Vascular theory |
|
| Excitotoxicity theory |
|
| Neurotrophic factor deprivation theory |
|
| Oxidative Stress and Inflammation |
|
| Retinal Imaging | Description | Applications | Advantages | Disadvantages |
|---|---|---|---|---|
| Optical coherence tomography (OCT) | A non-invasive imaging technique that uses light waves to take high-resolution cross-sectional pictures of the retina. | Measurement of RNFL 1 thickness, optic nerve, and macular ganglion cell complex | High-resolution images and non-invasive | Affected by media opacities (e.g., cataract), poor visualization of RGCs 2 |
| Confocal scanning laser ophthalmoscopy (cSLO) | cSLO utilizes a narrow laser beam to scan the retina point by point through a small aperture. | Visualization of optic nerve head | High contrast image, excellent lateral resolution, and monitoring of RNFL defects and glaucomatous changes | Affected by media opacities (e.g., cataract) and poor axial resolution |
| Adaptive optics imaging | Adaptive optics imaging employs deformable mirrors and wavefront sensors to correct optical aberrations. | Visualization of RGC | High-resolution, allowing visualization of individual cells, and can correct optical aberrations | Pupil dilation, longer time needed, and costly |
| Electroretinography (ERG) | ERG measures the electrical responses of various cell types in the retina after visual stimulation. | Assesses the functional status of RGCs using PERG 3, PhNR 4, and mfERG 5 | Functional assessment of RGCs, objective measurement of retinal activity, may detect early glaucomatous changes | Affected by media opacities, interpretation based on test parameters, utilization still under evaluation |
| Standard automated perimetry (SAP) | SAP assesses the visual field by presenting light stimuli of varying intensities in a standardized pattern to detect visual deficits. | The central tool in glaucoma diagnosis, staging, and monitoring. Visual field defects correlate with RGC loss and damage to the optic nerve. | Widely accepted and utilized, objective measures of visual function, able to monitor progression, offering other specific techniques in assessing specific RGC types, such as SWAP 6 and FDT 7 | Dependent on patient’s cooperation and understanding, can be affected by media opacities and ptosis, test–retest variability |
| Detection of apoptosing retinal cells (DARC) | DARC utilizes fluorescently tagged annexin-V intravenously to mark apoptotic RGCs, which are then identified with cSLO. | To assess efficacy of therapeutic agents | Identification of individual apoptosis RGC cells and early detection of glaucomatous damage | Invasive, long-term effects not yet known |
| Category | Agent/Method | Effect/Outcome | Reference |
|---|---|---|---|
| Neuroprotective agents | Brimonidine | Alpha-2 adrenergic receptor agonist; reduces extracellular glutamate, blocks NMDA 1 receptor, and supports RGC 2 survival under high IOP 3 | [119,120,121,122,123,124] |
| MK801 (dizocilpine maleate) | Strong glutamate inhibitor; uncompetitive NMDA antagonist with neuroprotective effects; can be neurotoxic due to long half-life | [125,126] | |
| Memantine | Selective and non-competitive NMDA receptor inhibitor; showed partial RGC protection in preclinical studies but no significant benefit in Phase 3 clinical trial | [127,128,129] | |
| Calcium channel blockers | Topical 2% flunarizine | Reduced RGC injury in rabbits under high IOP conditions | [130] |
| Brovincamine and nilvadipine | Showed improved visual field progression and increased posterior choroidal circulation | [131,132] | |
| Anti-oxidative agents | Coenzyme Q10 (CoQ10) | Protects RGCs from oxidative stress, supports ATP 4 synthesis, inhibits ROS production; promotes RGC survival in oxidative stress models | [45,133,134,135,136,137] |
| Neurotrophic factors | Brain-derived neurotrophic factor (BDNF) | Intravitreal injection protects RGCs in animal models; restores PERG and VEP damage; increases RGC survival in hypertensive rat eyes with intraocular injections | [138,139,140,141,142] |
| Ciliary neurotrophic factor (CNTF) | Expressed in Muller cells; offers protection against NO-induced 5 cell death and optic nerve axotomy in rats | [143,144,145,146] | |
| Nerve growth factor (NGF) | NGF eye drops can mitigate glaucoma-associated optic nerve damage in rats, with higher RGC survival; topical rh-NGF 6 results in improved RGC survival, decreased apoptosis, and reduced astrocyte activity in the optic nerve in a rat model | [147,148,149] | |
| Glial cell line–derived neurotrophic factor (GDNF) | Enhances GLAST gene expression in Müller glia, essential for RGC protection; GDNF microspheres increase RGC survival and density in rat models | [150,151,152] | |
| Gene therapy | γ-synuclein (mSncg) promoter | Preserves the acutely injured RGC somata and axons | [153] |
| Complement C3 | Neuroprotection of RGC axons and somata | [154] | |
| Calcium/calmodulin-stimulated protein kinase II (CaMKII) | Protection of RGCs and their axons | [155] | |
| Nicotinamide mononucleotide adenylyl transferase (NMNAT) | Significant neuroprotection of both RGC soma and axon and preservation of visual function | [156] | |
| X-linked inhibitor of apoptosis (XIAP) | Provide both functional and structural protection of RGC | [157] | |
| BCLXL | Robustly attenuate both RGC soma pathology and axonal degeneration in the optic nerve | [158] | |
| Stem cell Therapy | Brain-derived, hippocampal-derived stem cells | Differentiate into amacrine and horizontal cells | [159] |
| Muller glia stem cells | Differentiate into RGCs, restore connectivity to other neurons, and address negative scotopic threshold response | [160] | |
| IOP management | Trabeculectomy and Pharmacological Medications | Essential for reducing mechanical stress on RGCs, preventing further degeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, K.M.; Tsung, T.-H.; Chen, Y.-H.; Lu, D.-W. The Role of Retinal Ganglion Cell Structure and Function in Glaucoma. Cells 2023, 12, 2797. https://doi.org/10.3390/cells12242797
Feng KM, Tsung T-H, Chen Y-H, Lu D-W. The Role of Retinal Ganglion Cell Structure and Function in Glaucoma. Cells. 2023; 12(24):2797. https://doi.org/10.3390/cells12242797
Chicago/Turabian StyleFeng, Kathy Ming, Ta-Hsin Tsung, Yi-Hao Chen, and Da-Wen Lu. 2023. "The Role of Retinal Ganglion Cell Structure and Function in Glaucoma" Cells 12, no. 24: 2797. https://doi.org/10.3390/cells12242797





