Microstructural Changes in the Corpus Callosum in Systemic Lupus Erythematous
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Controls
2.3. Ethics Approval
2.4. Clinical Evaluation
2.5. Disease Activity/Cumulative Damage Evaluation
2.6. Neuropsychiatric (NP) Evaluation
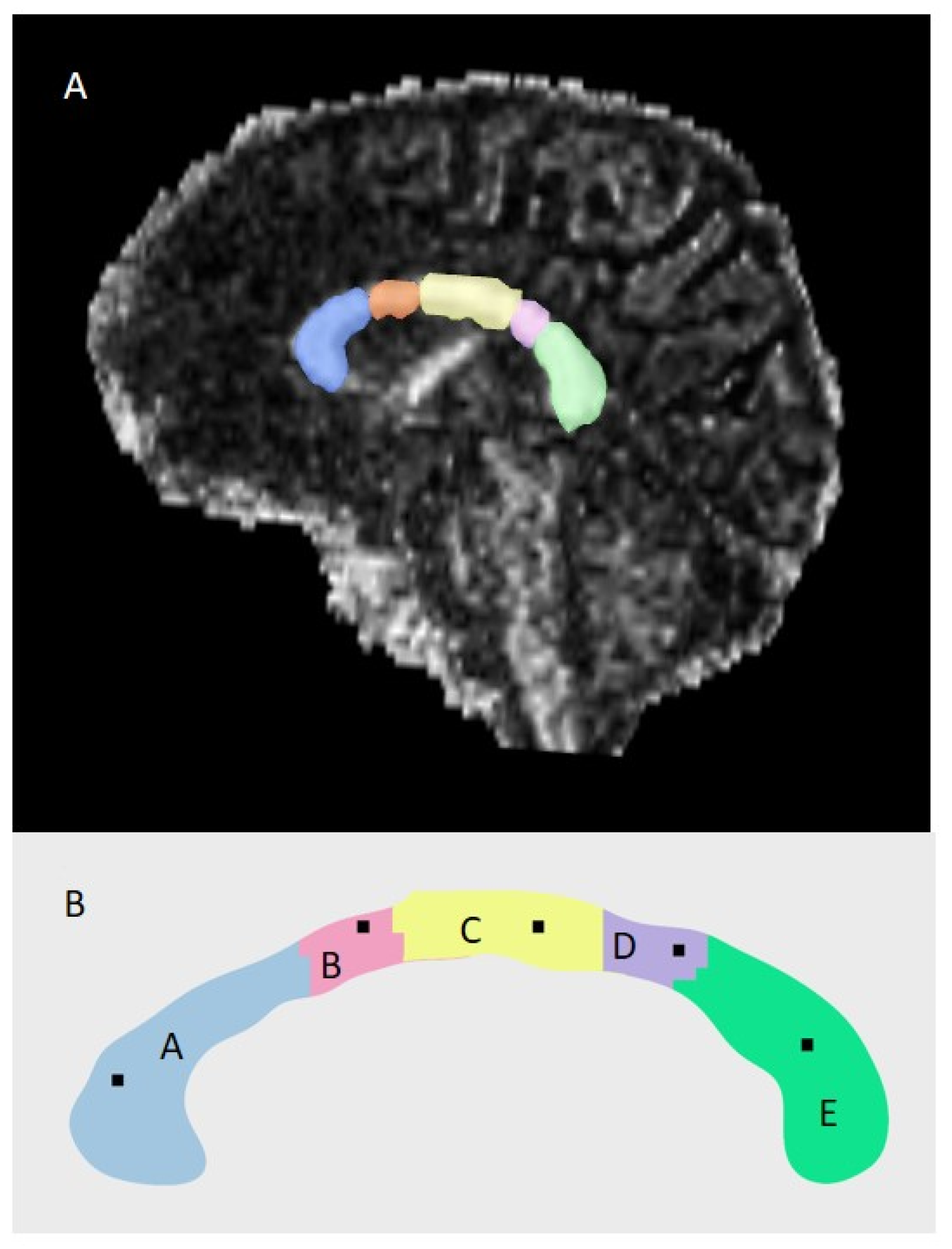
2.7. MRI Acquisition
2.8. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lateef, A.; Petri, M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14 (Suppl. 4), 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarukitsopa, S.; Hoganson, D.D.; Crowson, C.S.; Sokumbi, O.; Davis, M.D.; Michet, C.J.; Matteson, E.L.; Kremers, H.M.; Chowdhary, V.R. Epidemiology of systemic lupus erythematosus and cutaneous lupus in a predominantly white population in the United States. Arthritis Care Res. 2015, 67, 817–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postal, M.; Lapa, A.T.; Reis, F.; Rittner, L.; Appenzeller, S. Magnetic resonance imaging in neuropsychiatric systemic lupus erythematosus: Current state of the art and novel approaches. Lupus 2017, 26, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.; Brito, I. Juvenile Systemic Lupus Erythematosus: Neuropsychiatric manifestations. Acta Reumatol Port. 2012, 37, 117–125. [Google Scholar] [PubMed]
- Silva, C.A.; Avčin, T.; Brunner, H.I. Taxonomy for systemic lupus erythematosus with onset before adulthood. Arthritis Care Res. 2012, 64, 1787–1793. [Google Scholar] [CrossRef] [Green Version]
- Tucker, L.; Uribe, A.; Fernández, M.; Vilá, L.; McGwin, G.; Apte, M.; Fessler, B.; Bastian, H.; Reveille, J.; Alarcón, G. Adolescent onset of lupus results in more aggressive disease and worse outcomes: Results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 2008, 17, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Kozora, E.; Filley, C.M. Cognitive dysfunction and white matter abnormalities in systemic lupus erythematosus. J. Int. Neuropsychol. Soc. 2011, 17, 385–392. [Google Scholar] [CrossRef]
- Frittoli, R.B.; de Oliveira, P.K.; Bellini, B.S.; Marini, R.; Fernandes, P.T.; Appenzeller, S. Association between academic performance and cognitive dysfunction in patients with juvenile systemic lupus erythematosus. Revista Brasileira Reumatologia 2016, 56, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Klein-Gitelman, M.; Brunner, H.I. The impact and implications of neuropsychiatric systemic lupus erythematosus in adolescents. Curr. Rheumatol. Rep. 2009, 11, 212–217. [Google Scholar] [CrossRef]
- Appenzeller, S.; Bonilha, L.; Rio, P.A.; Min, L.L.; Costallat, L.T.; Cendes, F. Longitudinal analysis of gray and white matter loss in patients with systemic lupus erythematosus. NeuroImage 2007, 34, 694–701. [Google Scholar] [CrossRef]
- Petri, M.; Naqibuddin, M.; Carson, K.A.; Wallace, D.J.; Weisman, M.H.; Holliday, S.L.; Sampedro, M.; Narayana, S.; Fox, P.; Franklin, C.; et al. Brain magnetic resonance imaging in newly diagnosed systemic lupus erythematosus. J. Rheumatol. 2008, 35, 2348–2354. [Google Scholar] [CrossRef]
- Park, J.-S.; Yoon, U.; Kwak, K.-C.; Seo, S.W.; Kim, S.I.; Na, D.L.; Lee, J.-M. The relationships between extent and microstructural properties of the midsagittal corpus callosum in human brain. NeuroImage 2011, 56, 174–184. [Google Scholar] [CrossRef]
- Bourekas, E.C.; Varakis, K.; Bruns, D.; Christoforidis, G.A.; Baujan, M.; Slone, H.W.; Kehagias, D. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am. J. Roentgenol. 2002, 179, 251–257. [Google Scholar] [CrossRef]
- Kazi, A.Z.; Joshi, P.C.; Kelkar, A.B.; Mahajan, M.S.; Ghawate, A.S. MRI evaluation of pathologies affecting the corpus callosum: A pictorial essay. Indian J. Radiol. Imaging. 2013, 23, 321–332. [Google Scholar] [CrossRef]
- Engelhardt, E.; Moreira, D.M. A substância branca cerebral: Localização dos principais feixes com anisotropia fracionada direcional. Rev. Bras. Neurol. 2008, 44, 19–34. [Google Scholar]
- Lapa, A.T.; Postal, M.; Sinicato, N.A.; Ferreira, W.G.; Bellini, B.S.; Fernandes, P.T.; Rittner, L.; Marini, R.; Cendes, F.; Appenzeller, S. Reduction of cerebral and corpus callosum volumes in childhood-onset systemic lupus erythematosus: A volumetric magnetic resonance imaging analysis. Arthritis Rheumatol. 2016, 68, 2193–2199. [Google Scholar] [CrossRef]
- Gyori, N.G.; Clark, C.A.; Alexander, D.C.; Kaden, E. On the potential for mapping apparent neural soma density via a clinically viable diffusion MRI protocol. Neuroimage 2021, 239, 118303. [Google Scholar] [CrossRef]
- Pinheiro, G.R.; Cover, G.S.; Bento, M.P.; Rittner, L. Automatic callosal fiber convergence plane computation through DTI-based divergence map. In Medical Imaging 2018: Biomedical Applications in Molecular, Structural, and Functional Imaging; SPIE: Bellingham, WA, USA, 2018; Volume 10578, pp. 256–264. [Google Scholar] [CrossRef]
- Costallat, B.L.; Ferreira, D.M.; Lapa, A.T.; Rittner, L.; Costallat, L.T.L.; Appenzeller, S. Brain diffusion tensor MRI in systematic lupus erythematosus: A systematic review. Autoimmun. Rev. 2018, 17, 36–43. [Google Scholar] [CrossRef]
- Cover, G.; Pereira, M.E.C.; Bento, M.P.; Appenzeler, S.; Rittner, L. Data-Driven Corpus Callosum Parcellation Method through Diffusion Tensor Imaging. IEEE Access 2017, 5, 22421–22432. [Google Scholar] [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; Mcshane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- Borba, F.E.; Latorre, C.L.; Brenol, T.C.J.; Kayser, C.; Silva, A.N.; Zimmermann, F.A.; de Pádua, P.M.; Costallat, L.T.L.; Bonfá, E.; Sato, E.I.; et al. Consenso de Lúpus Eritematoso Sistêmico. Revista Brasileira Reumatologia 2008, 48, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.N.; Gharavi, A.E.; Patel, S.P.; Hughes, G.R. Evaluation of the anticardiolipin antibody test: Report of an international workshop held 4 April 1986. Clin. Exp. Immunol. 1987, 68, 215–222. [Google Scholar] [PubMed]
- Brandt, J.T.; Triplett, D.A.; Alving, B.; Scharrer, I. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Criteria for the diagnosis of lupus anticoagulants: An update. Thromb. Haemost. 1995, 74, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. The Committee on Prognosis Studies in SLE. Derivation of the SLEDAI: A disease activity index for lupus patients. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef]
- Yee, C.-S.; Farewell, V.; Isenberg, D.; Griffiths, B.; Teh, L.-S.; Bruce, I.N.; Ahmad, Y.; Rahman, A.; Prabu, A.; Akil, M.; et al. The use of Systemic Lupus Erythematosus Disease Activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology 2011, 50, 982–988. [Google Scholar] [CrossRef] [Green Version]
- Gladman, D.D.; Urowitz, M.B.; Goldsmith, C.H.; Fortin, P.; Ginzler, E.; Gordon, C.; Hanly, J.G.; Isenberg, D.A.; Kalunian, K.; Nived, O.; et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 809–813. [Google Scholar] [CrossRef]
- ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999, 42, 599–608. [Google Scholar] [CrossRef]
- Ross, G.S.; Zelko, F.; Klein-Gitelman, M.; Levy, D.M.; Muscal, E.; Schanberg, L.E.; Anthony, K.; Brunner, H.I. A proposed framework to standardize the neurocognitive assessment of patients with pediatric systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010, 62, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Memória, C.M.; Yassuda, M.S.; Nakano, E.Y.; Forlenza, O.V. Brief screening for mild cognitive impairment: Validation of the Brazilian version of the Montreal cognitive assessment. Int. J. Geriatr. Psychiatry 2013, 28, 34–40. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Oliveira, M.H.; Gorenstein, C.; Lotufo Neto, F.; Andrade, L.H.; Wang, Y.P. Validation of the Brazilian Portuguese version of the Beck Depression Inventory-II in a community sample. Braz. J. Psychiatry. 2012, 34, 389–394. [Google Scholar] [CrossRef]
- Quintão, S.; Delgado, A.R.; Prieto, G. Validity study of the Beck Anxiety Inventory (Portuguese version) by the Rasch Rating Scale model. Psicol. Reflex Crit. 2013, 26, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Aryanto, K.Y.E.; Oudkerk, M.; van Ooijen, P.M.A. Free DICOM de-identification tools in clinical research: Functioning and safety of patient privacy. Eur. Radiol. 2015, 25, 3685–3695. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Rittner, L.; Freitas, P.; Appenzeller, A.; Lotufo, R. Automatic DTI-based parcellation of the corpus callosum through the watershed transform. Braz. J. Biomed. Eng. 2014, 30, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Caldeira, T.; Julio, P.R.; Appenzeller, S.; Rittner, L. inCCsight: A software for exploration and visualization of DT-MRI data of the Corpus Callosum. Comput. Graph. 2021, 99, 259–271. [Google Scholar] [CrossRef]
- Herrera, W.G.; Pereira, M.; Bento, M.; Lapa, A.T.; Appenzeller, S.; Rittner, L. A framework for quality control of corpus callosum segmentation in large-scale studies. J. Neurosci. Methods 2020, 334, 108593. [Google Scholar] [CrossRef]
- Jones, J.T.; DiFrancesco, M.; Zaal, A.I.; Klein-Gitelman, M.S.; Gitelman, D.; Ying, J.; Brunner, H.I. Childhood-onset lupus with clinical neurocognitive dysfunction shows lower streamline density and pairwise connectivity on diffusion tensor imaging. Lupus 2015, 24, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Tan, X.; Wang, J.; Han, K.; Niu, M.; Xu, J.; Liu, X.; Zhao, X.; Zhong, M.; Huang, Q.; et al. Brain white matter structural networks in patients with non-neuropsychiatric systemic lupus erythematosus. Brain Imaging Behav. 2018, 12, 142–155. [Google Scholar] [CrossRef]
- Wiseman, S.J.; Bastin, M.E.; Hamilton, I.F.; Hunt, D.; Ritchie, S.J.; Amft, E.N.; Thomson, S.; Belch, J.F.; Ralston, S.H.; Wardlaw, J.M. Fatigue and cognitive function in systemic lupus erythematosus: Associations with white matter microstructural damage. A diffusion tensor MRI study and meta-analysis. Lupus 2017, 26, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesar, B.; Dwyer, M.; Shucard, J.; Polak, P.; Bergsland, N.; Benedict, R.; Weinstock-Guttman, B.; Shucard, D.; Zivadinov, R. Cognitive and White Matter Tract Differences in MS and Diffuse Neuropsychiatric Systemic Lupus Erythematosus. AJNR Am. J. Neuroradiol. 2015, 36, 1874–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercan, E.; Ingo, C.; Tritanon, O.; Magro-Checa, C.; Smith, A.; Smith, S.; Huizinga, T.; van Buchem, M.A.; Ronen, I. A multimodal MRI approach to identify and characterize microstructural brain changes in neuropsychiatric systemic lupus erythematosus. Neuroimage Clin. 2015, 8, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-P.; Wu, C.-S.; Cheng, J.-Z.; Chen, C.-M.; Chen, Y.-C.; Chou, M.-C. Automatic Segmentation of the Corpus Callosum Using a Cell-Competition Algorithm: Diffusion Tensor Imaging-Based Evaluation of Callosal Atrophy and Tissue Alterations in Patients With Systemic Lupus Erythematosus. J. Comput. Assist. Tomogr. 2015, 39, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wilcke, T.; Cagnoli, P.; Wang, P.; Schultz, T.; Lotz, A.; Mccune, W.J.; Sundgren, P.C. Diminished white matter integrity in patients with systemic lupus erythematosus. Neuroimage Clin. 2014, 5, 291–297. [Google Scholar] [CrossRef]
- Zimny, A.; Szmyrka-Kaczmarek, M.; Szewczyk, P.; Bladowska, J.; Pokryszko-Dragan, A.; Gruszka, E.; Wiland, P.; Sasiadek, M. In vivo evaluation of brain damage in the course of systemic lupus erythematosus using magnetic resonance spectroscopy, perfusion-weighted and diffusiontensor imaging. Lupus 2014, 23, 10–19. [Google Scholar] [CrossRef]
- Hughes, M.; Sundgren, P.C.; Fan, X.; Foerster, B.; Nan, B.; Welsh, R.C.; Williamson, J.A.; Attwood, J.; Maly, P.V.; Chenevert, T.L.; et al. Diffusion tensor imaging in patients with acute onset of neuropsychiatric systemic lupus erythematosus: A prospective study of apparent diffusion coefficient, fractional anisotropy values, and eigenvalues in different regions of the brain. Acta Radiol. 2007, 48, 213–222. [Google Scholar] [CrossRef]
- Emmer, B.J.; Veer, I.M.; Steup-Beekman, G.M.; Huizinga, T.W.J.; van der Grond, J.; van Buchem, M.A. Tract-based spatial statistics on diffusion tensor imaging in systemic lupus erythematosus reveals localized involvement of white matter tracts. Arthritis Rheum. 2010, 62, 3716–3721. [Google Scholar] [CrossRef]
- Ercan, E.; Checa, C.M.; Valabregue, R.; Branzoli, F.; Wood, E.; Steup-Beekman, G.M.; Webb, A.G.; Huizinga, T.W.J.; Van Buchem, M.A.; Ronen, I. Glial and axonal changes in systemic lupus erythematosus measured with diffusion of intracellular metabolites. Brain 2016, 139, 1447–1457. [Google Scholar] [CrossRef] [Green Version]
- Shapira-Lichter, I.; Weinstein, M.; Lustgarten, N.; Ash, E.; Litinsky, I.; Aloush, V.; Anouk, M.; Caspi, D.; Hendler, T.; Paran, D. Impaired diffusion tensor imaging findings in the corpus callosum and cingulum may underlie impaired learning and memory abilities in systemic lupus erythematosus. Lupus 2016, 25, 1200–1208. [Google Scholar] [CrossRef]
- Shastri, R.; Shah, G.; Wang, P.; Cagnoli, P.; Schmidt-Wilcke, T.; McCune, J.; Harris, R.; Sundgren, P. MR Diffusion Tractography to Identify and Characterize Microstructural White Matter Tract Changes in Systemic Lupus Erythematosus Patients. Acad. Radiol. 2016, 23, 1431–1440. [Google Scholar] [CrossRef]
- Appenzeller, S.; Carnevalle, A.D.; Li, L.M.; Costallat, L.T.; Cendes, F. Hippocampal atrophy in systemic lupus erythematosus. Ann. Rheum. Dis. 2006, 65, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Appenzeller, S.; Rondina, J.M.; Li, L.M.; Costallat, L.T.; Cendes, F. Cerebral and corpus callosum atrophy in systemic lupus erythematosus. Arthritis Rheum. 2005, 52, 2783–2789. [Google Scholar] [CrossRef]
- Jeltsch-David, H.; Muller, S. Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: The MRL-lpr mouse strain as a model. Autoimmun. Rev. 2014, 13, 963–973. [Google Scholar] [CrossRef] [Green Version]
- Luyendijk, J.; Steens, S.C.A.; Ouwendijk, W.J.N.; Steup-Beekman, G.M.; Bollen, E.L.E.M.; van der Grond, J.; Huizinga, T.W.J.; Emmer, B.J.; van Buchem, M.A. Neuropsychiatric systemic lupus erythematosus: Lessons learned from magnetic resonance imaging. Arthritis Rheum. 2011, 63, 722–732. [Google Scholar] [CrossRef]
- Barraclough, M.; Elliott, R.; McKie, S.; Parker, B.; Bruce, I.N. Cognitive dysfunction and functional magnetic resonance imaging in systemic lupus erythematosus. Lupus 2015, 24, 1239–1247. [Google Scholar] [CrossRef]

| Demographic Data | cSLE N = 71 | aSLE N = 49 | Controls N = 58 |
|---|---|---|---|
| Sex - female | 64 (90.1%) | 44 (89.8%) | 42 (72.4%) * |
| Current age (years) | 24.7 (SD ± 4.6) | 33.2 (SD ± 3.7) * | 29.9 (SD ± 4.1) |
| Disease duration (years) | 11.8 (SD ± 4.8) | 11.3 (SD ± 4.05) | |
| Clinical Data | |||
| Malar rash | 38 (53.5%) | 22 (44.9%) | |
| Discoid injury | 03 (4.2%) | 05 (7.0%) | |
| Photosensitivity | 24 (33.8%) * | 25 (51%) | |
| Oral injury | 15 (21.1%) * | 04 (8.2%) | |
| Arthritis | 54 (76.1%) | 35 (71.4%) | |
| Serositis | 17 (23.9%) | 17 (34.7%) | |
| Nephritis | 35 (49.3%) | 27 (55.1%) | |
| Hematological alteration | 50 (70.4%) | 35 (71.4%) | |
| Laboratorial Data | |||
| ↓C3 | 22 (31.4%) | 22 (45.8%) | |
| ↓C4 | 18 (25.3%) | 16 (32.6%) | |
| ANA | 69 (97.2%) | 47 (96%) | |
| dsDNA | 15 (21.4%) | 19 (38.8%) | |
| Anti-Ro | 16 (23.5%) | 19 (42.2%) | |
| Anti-Sm | 23 (33.8%) | 13 (28.9%) | |
| Anti-La | 2 (2.8%) | 8 (16.3%) * | |
| APS | 12 (16.9%) | 9 (18.8%) | |
| Lupus anticoagulant | 15 (21.1%) | 8 (16.3%) | |
| Anticardiolipin antibodies | 22 (31%) | 9 (18.8%) | |
| Leukopenia | 36 (50.7%) | 29 (59.2%) | |
| Hemolytic anemia | 9 (12.7%) | 12 (24.5%) | |
| Thrombocytopenia | 26 (36.6%) | 14 (28.6%) | |
| NPSLE | |||
| Overt NPSLE manifestations | 53 (74.6%) | 36 (73.5%) | |
| Anxiety | 22 (31%) | 26 (53.1%) * | |
| Depression | 18 (25.4%) | 14 (28.6%) | |
| Cognitive impairment | 31 (43.7%) | 16 (32.7%) | |
| Headache | 15 (21.1%) | 13 (26.5%) | |
| Seizure | 12 (16.9%) | 9 (18.4%) | |
| Treatment | |||
| Corticosteroids | 55 (77.5%) | 42 (85.7%) | |
| Immunosuppressive | 49 (69%) | 26 (53.1%) | |
| Azathioprine | 25 (35.2%) | 16 (32.7%) | |
| Mycophenolate | 19 (26.8%) | 6 (12.2%) | |
| Cyclosporine | 6 (8.5%) | 0 | |
| Methotrexate | 0 | 5 (10.2%) | |
| Hydroxychloroquine | 50 (70.4%) | 36 (73.4%) | |
| cSLE * | aSLE * | p Valor * | cSLE * | Controls * | p Valor * | aSLE * | Controls * | p Valor * | |
|---|---|---|---|---|---|---|---|---|---|
| FA total | 0.63603 (±0.0665) | 0.67624 (±0.08920) | 0.002 | 0.63603 (±0.0665) | 0.64599 (±0.0319) | 0.647 | 0.67624 (±0.08920) | 0.64599 (±0.0319) | 0.038 |
| MD total | 0.00096 (±0.00014) | 0.00087 (±0.00015) | 0.002 | 0.00096 (±0.00014) | 0.00093 (±0.00008) | 0.564 | 0.00087 (±0.00015) | 0.00093 (±0.00008) | 0.042 |
| RD total | 0.00057 (±0.00015) | 0.00048 (±0.00019) | 0.002 | 0.00057 (±0.00015) | 0.00055 (±0.0001) | 0.712 | 0.00048 (±0.00019) | 0.00055 (±0.0001) | 0.026 |
| AD total | 0.0017 (±0.00014) | 0.0016 (±0.00011) | 0.024 | 0.0017 (±0.00014) | 0.0016 (±0.0001) | 0.387 | 0.0016 (±0.00011) | 0.0016 (±0.0001) | 0.383 |
| Parcel A | |||||||||
| FA—A | 0.66445 (±0.0744) | 0.68242 (±0.1104) | 0.438 | 0.66445 (±0.0744) | 0.66942 (±0.4413) | 0.932 | 0.68242 (±0.1104) | 0.66942 (±0.4413) | 0.672 |
| MD—A | 0.00089 (±0.0001) | 0.00087 (±0.00027) | 0.819 | 0.00089 (±0.0001) | 0.00088 (±0.00007) | 0.918 | 0.00087 (±0.00027) | 0.00088 (±0.00007) | 0.974 |
| RD—A | 0.00050 (±0.00013) | 0.00047 (±0.00031) | 0.737 | 0.00050 (±0.00013) | 0.00049 (±0.00007) | 0.942 | 0.00047 (±0.00031) | 0.00049 (±0.00007) | 0.910 |
| AD—A | 0.00168 (±0.00015) | 0.00168 (±0.00022) | 0.993 | 0.00168 (±0.00015) | 0.00167 (±0.00016) | 0.899 | 0.00168 (±0.00022) | 0.00167 (±0.00016) | 0.955 |
| Parcel B | |||||||||
| FA—B | 0.59203 (±0.10095) | 0.64464 (±0.10792) | 0.005 | 0.59203 (±0.10095) | 0.60636 (±0.04704) | 0.637 | 0.64464 (±0.10792) | 0.60636 (±0.04704) | 0.073 |
| MD—B | 0.00093 (±0.00025) | 0.00081 (±0.00019) | 0.005 | 0.00093 (±0.00025) | 0.00090 (±0.00013) | 0.737 | 0.00081 (±0.00019) | 0.00090 (±0.00013) | 0.054 |
| RD—B | 0.00060 (±0.00027) | 0.00047 (±0.00023) | 0.004 | 0.00060 (±0.00027) | 0.00056 (±0.00022) | 0.779 | 0.00047 (±0.00023) | 0.00056 (±0.00022) | 0.039 |
| AD—B | 0.00158 (±0.00025) | 0.00148 (±0.00016) | 0.035 | 0.00158 (±0.00025) | 0.00155 (±0.00016) | 0.718 | 0.00148 (±0.00016) | 0.00155 (±0.00016) | 0.212 |
| Parcel C | |||||||||
| FA—C | 0.58574 (±0.09617) | 0.65456 (±0.09985) | <0.001 | 0.58574 (±0.09617) | 0.60965 (±0.04811) | 0.251 | 0.65456 (±0.09985) | 0.60965 (±0.04811) | 0.019 |
| MD—C | 0.00095 (±0.00019) | 0.00083 (±0.00021) | 0.001 | 0.00095 (±0.00019) | 0.00091 (±0.00013) | 0.376 | 0.00083 (±0.00021) | 0.00091 (±0.00013) | 0.078 |
| RD—C | 0.00062 (±0.00020) | 0.00048 (±0.00023) | <0.001 | 0.00062 (±0.00020) | 0.00058 (±0.00012) | 0.402 | 0.00048 (±0.00023) | 0.00058 (±0.00012) | 0.027 |
| AD—C | 0.00161 (±0.00019) | 0.00153 (±0.00019) | 0.126 | 0.00161 (±0.00019) | 0.00157 (±0.00018) | 0.493 | 0.00153 (±0.00019) | 0.00157 (±0.00018) | 0.681 |
| Parcel D | |||||||||
| FA—D | 0.58105 (±0.09756) | 0.63433 (±0.10684) | 0.006 | 0.58105 (±0.09756) | 0.59873 (±0.06512) | 0.518 | 0.63433 (±0.10684) | 0.59873 (±0.06512) | 0.112 |
| MD—D | 0.00108 (±0.00030) | 0.00090 (±0.00016) | <0.001 | 0.00108 (±0.00030) | 0.00102 (±0.00014) | 0.340 | 0.00090 (±0.00016) | 0.00102 (±0.00014) | 0.020 |
| RD—D | 0.00072 (±0.00032) | 0.00054 (±0.00020) | <0.001 | 0.00072 (±0.00032) | 0.00067 (±0.00015) | 0.463 | 0.00054 (±0.00020) | 0.00067 (±0.00015) | 0.020 |
| AD—D | 0.00179 (±0.00028) | 0.00164 (±0.00015) | <0.001 | 0.00179 (±0.00028) | 0.00173 (±0.00015) | 0.221 | 0.00164 (±0.00015) | 0.00173 (±0.00015) | 0.068 |
| Parcel E | |||||||||
| FA—E | 0.68225 (±0.06640) | 0.72460 (±0.04514) | <0.001 | 0.68225 (±0.06640) | 0.69112 (±0.04791) | 0.638 | 0.72460 (±0.04514) | 0.69112 (±0.04791) | 0.006 |
| MD—E | 0.00099 (±0.00013) | 0.00090 (±0.00007) | <0.001 | 0.00099 (±0.00013) | 0.00096 (±0.00010) | 0.341 | 0.00090 (±0.00007) | 0.00096 (±0.00010) | 0.005 |
| RD—E | 0.00055 (±0.00013) | 0.00044 (±0.00008) | <0.001 | 0.00055 (±0.00013) | 0.00053 (±0.00010) | 0.600 | 0.00044 (±0.00008) | 0.00053 (±0.00010) | <0.001 |
| AD—E | 0.00188 (±0.00019) | 0.00180 (±0.00014) | 0.042 | 0.00188 (±0.00019) | 0.00184 (±0.00016) | 0309 | 0.00180 (±0.00014) | 0.00184 (±0.00016) | 0.582 |
| DTI Scalar Maps | Reduced C4 | Positive Anticardiolipin Antibodies | Antiphospholipid Syndrome |
|---|---|---|---|
| FA total | 0.016 | 0.003 | 0.065 |
| MD total | 0.003 | 0.019 | 0.102 |
| RD total | 0.003 | 0.008 | 0.093 |
| AD total | 0.035 | 0.334 | 0.259 |
| FA—A | 0.059 | 0.000 | 0.001 |
| MD—A | 0.004 | 0.005 | 0.007 |
| RD—A | 0.005 | 0.001 | 0.002 |
| AD—A | 0.025 | 0.290 | 0.196 |
| FA—B | 0.026 | 0.017 | 0.058 |
| MD—B | 0.161 | 0.242 | 0.283 |
| RD—B | 0.086 | 0.122 | 0.196 |
| AD—B | 0.500 | 0.893 | 0.681 |
| FA—C | 0.037 | 0.222 | 0.519 |
| MD—C | 0.006 | 0.033 | 0.208 |
| RD—C | 0.007 | 0.051 | 0.292 |
| AD—C | 0.037 | 0.039 | 0.146 |
| FA—D | 0.006 | 0.036 | 0.073 |
| MD—D | 0.007 | 0.039 | 0.123 |
| RD—D | 0.022 | 0.028 | 0.081 |
| AD—D | 0.002 | 0.146 | 0.393 |
| FA—E | 0.578 | 0.058 | 0.958 |
| MD—E | 0.159 | 0.048 | 0.461 |
| RD—E | 0.159 | 0.034 | 0.841 |
| AD—E | 0.159 | 0.305 | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julio, P.R.; Caldeira, T.; Pinheiro, G.R.; Capello, C.H.; Fritolli, R.B.; Marini, R.; Cendes, F.; Fernandes, P.T.; Costallat, L.T.L.; Rittner, L.; et al. Microstructural Changes in the Corpus Callosum in Systemic Lupus Erythematous. Cells 2023, 12, 355. https://doi.org/10.3390/cells12030355
Julio PR, Caldeira T, Pinheiro GR, Capello CH, Fritolli RB, Marini R, Cendes F, Fernandes PT, Costallat LTL, Rittner L, et al. Microstructural Changes in the Corpus Callosum in Systemic Lupus Erythematous. Cells. 2023; 12(3):355. https://doi.org/10.3390/cells12030355
Chicago/Turabian StyleJulio, Paulo Rogério, Thais Caldeira, Gustavo Retuci Pinheiro, Carla Helena Capello, Renan Bazuco Fritolli, Roberto Marini, Fernando Cendes, Paula Teixeira Fernandes, Lilian T. L. Costallat, Leticia Rittner, and et al. 2023. "Microstructural Changes in the Corpus Callosum in Systemic Lupus Erythematous" Cells 12, no. 3: 355. https://doi.org/10.3390/cells12030355





