Early Neurobehavioral Characterization of the CD Mouse Model of Williams–Beuren Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
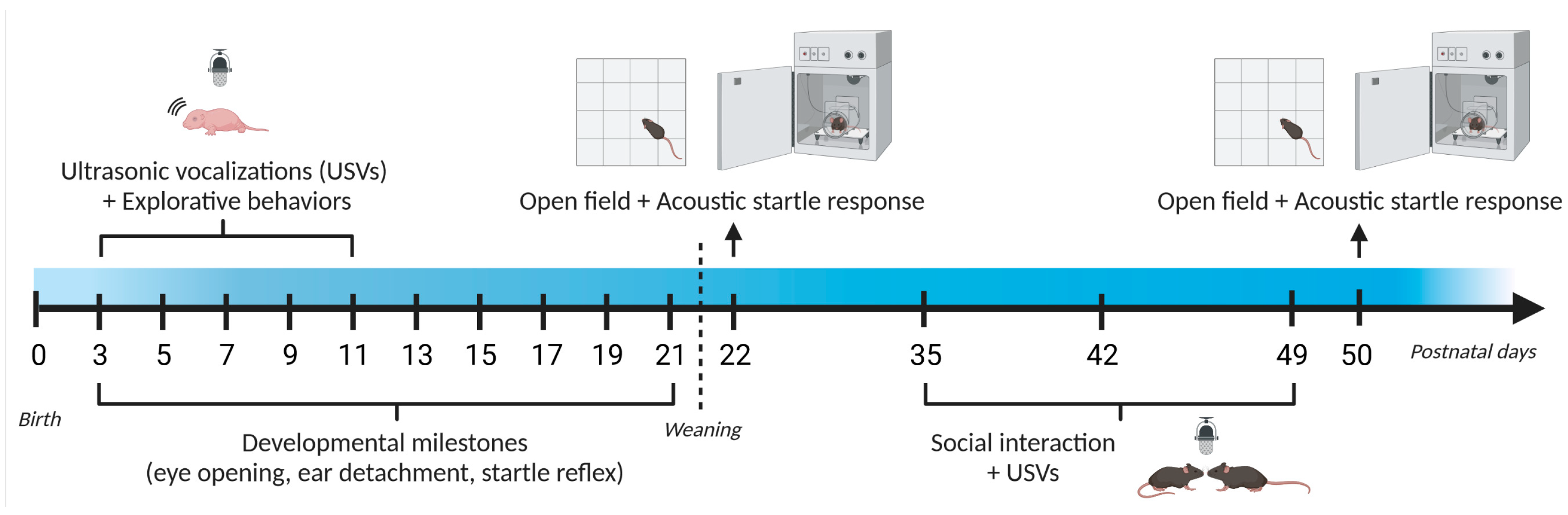
2.2. Study 1: Early Behavioral Characterization of the CD Mouse Model
2.2.1. Behavioral Assessment of Mouse Pups
2.2.2. Behavioral Assessment of Juvenile Mice
- Sensori-motor assessment
- Social interaction and USVs
- Assessment of female estrous cycle
2.3. Study 2: Early Brain Characterization of the CD Mouse Model
2.4. Statistical Analysis
3. Results
3.1. Study 1: Early Behavioral Characterization of the CD Mouse Model
3.1.1. Behavioral Assessment of Mouse Pups
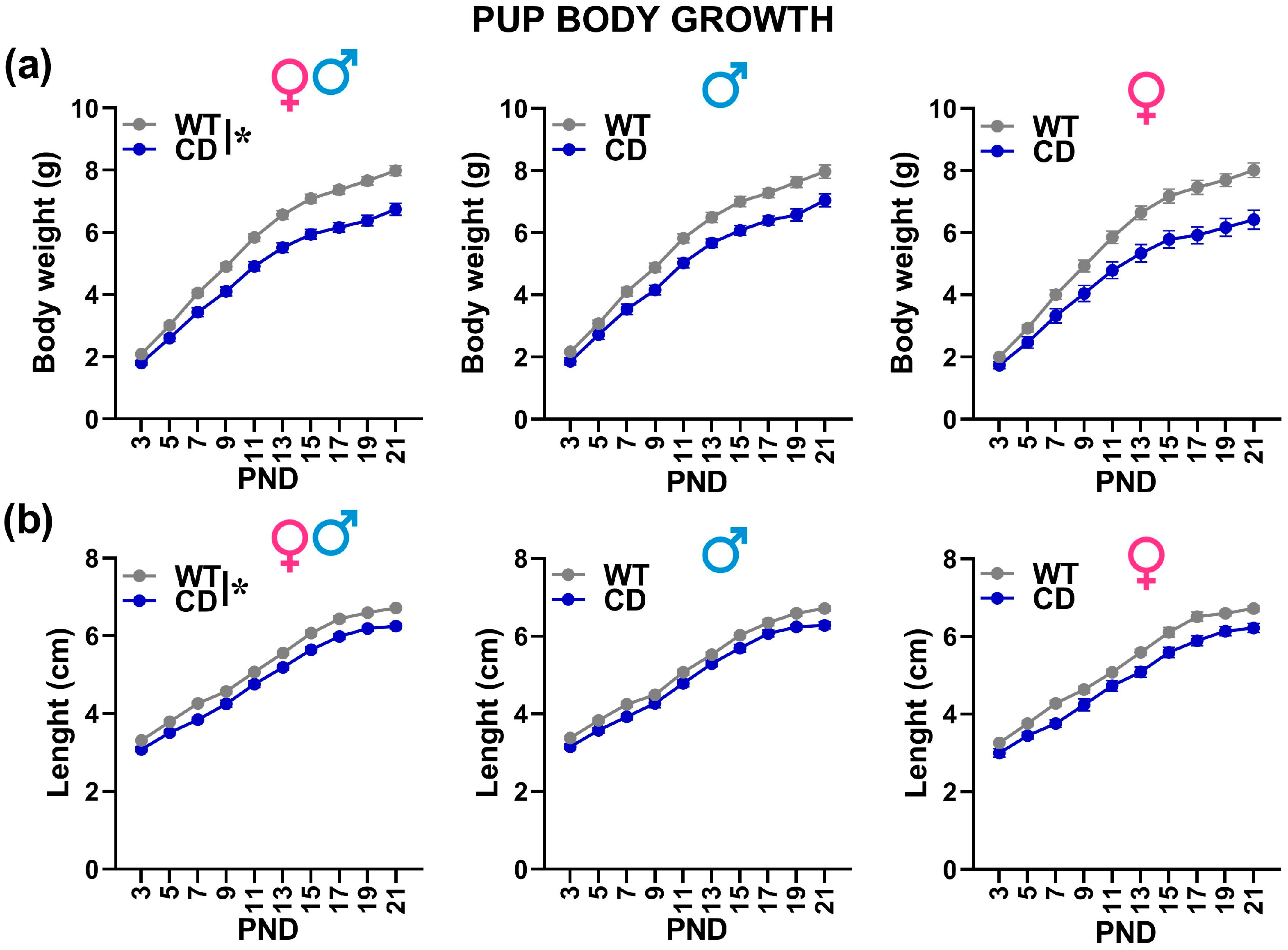
- Body weight/length and developmental milestones
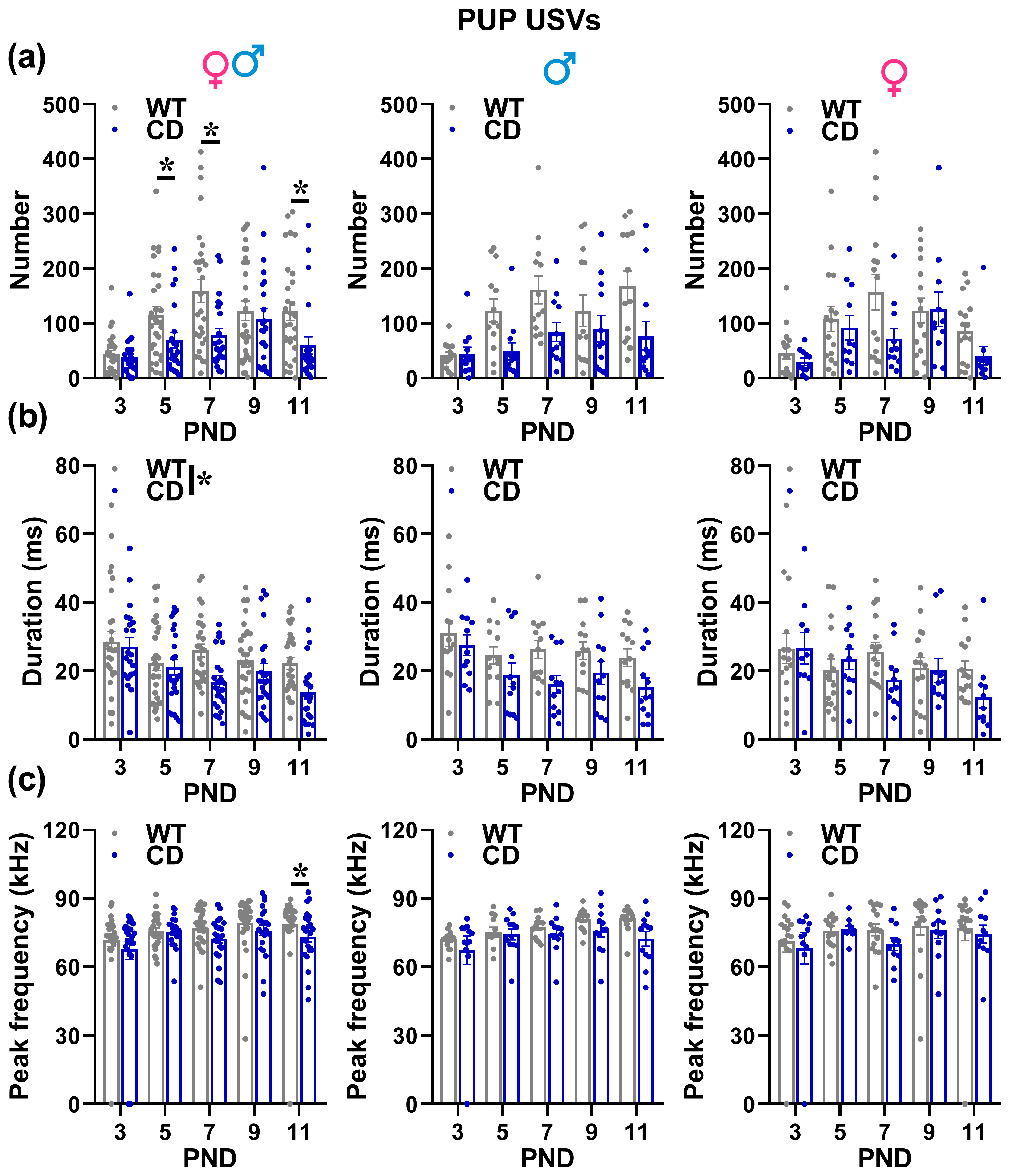
- USVs
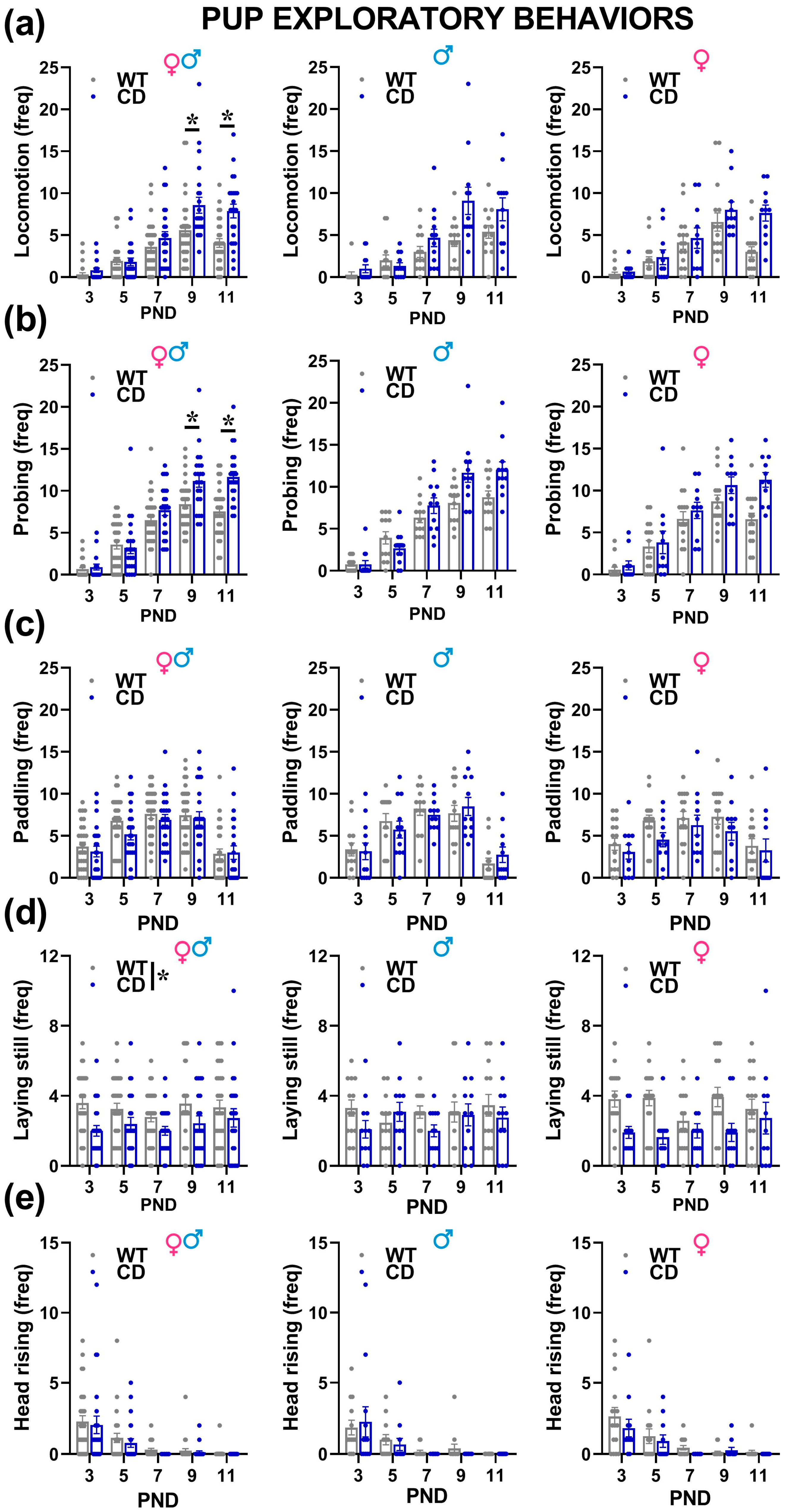
- Explorative behaviors
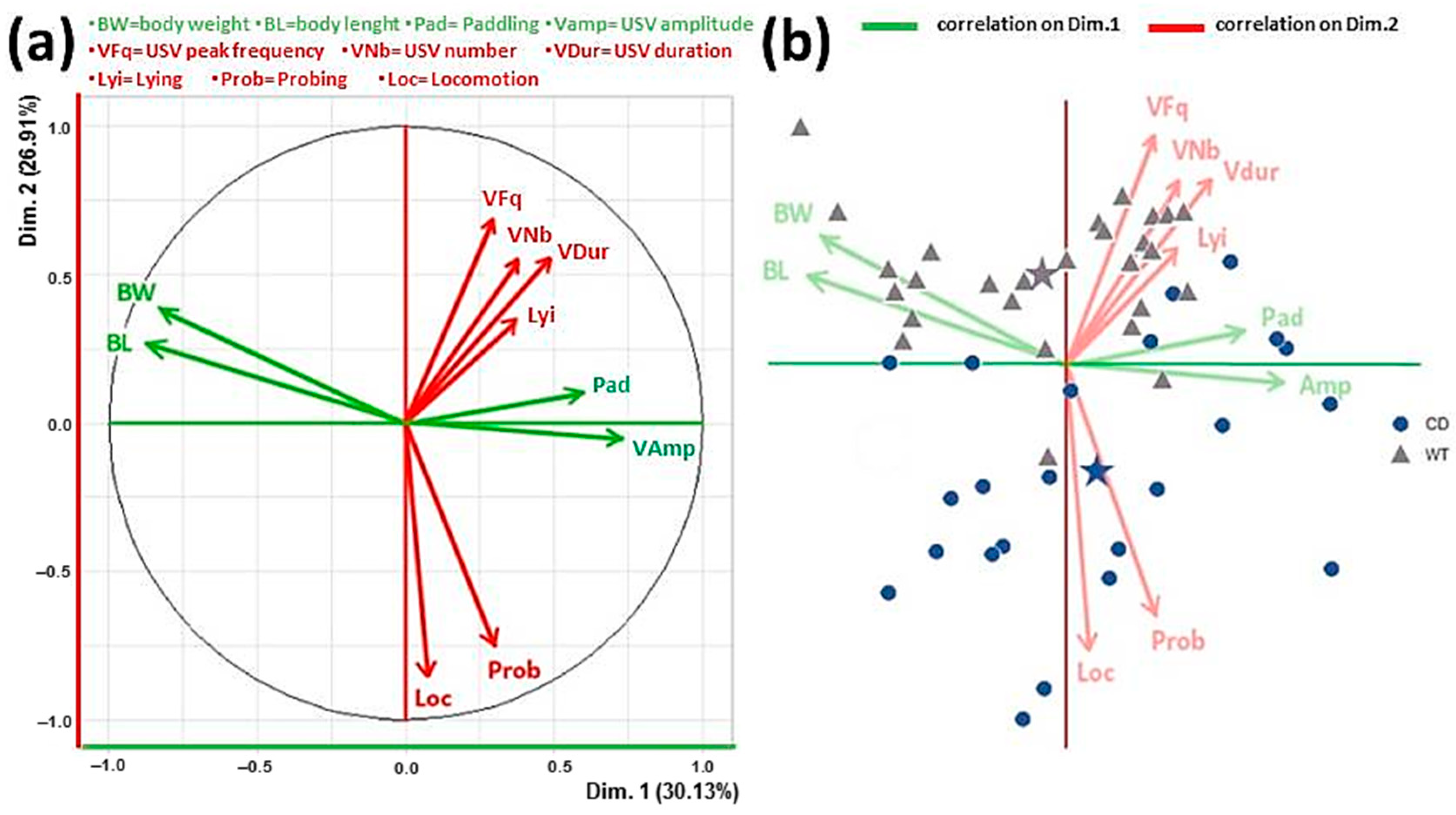
- PCA analysis
3.1.2. Behavioral Assessment of Juvenile Mice
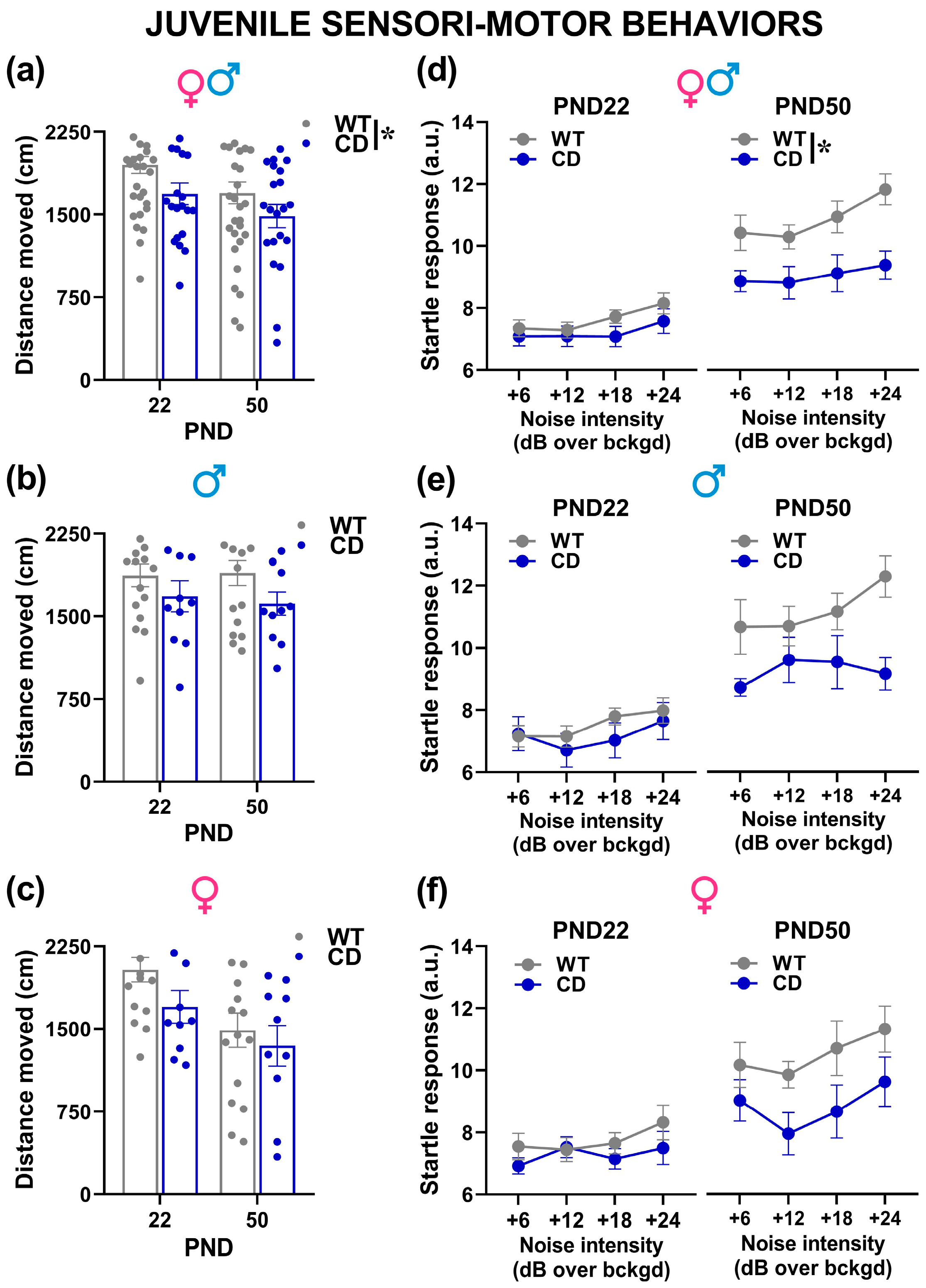
- Sensori-motor assessment
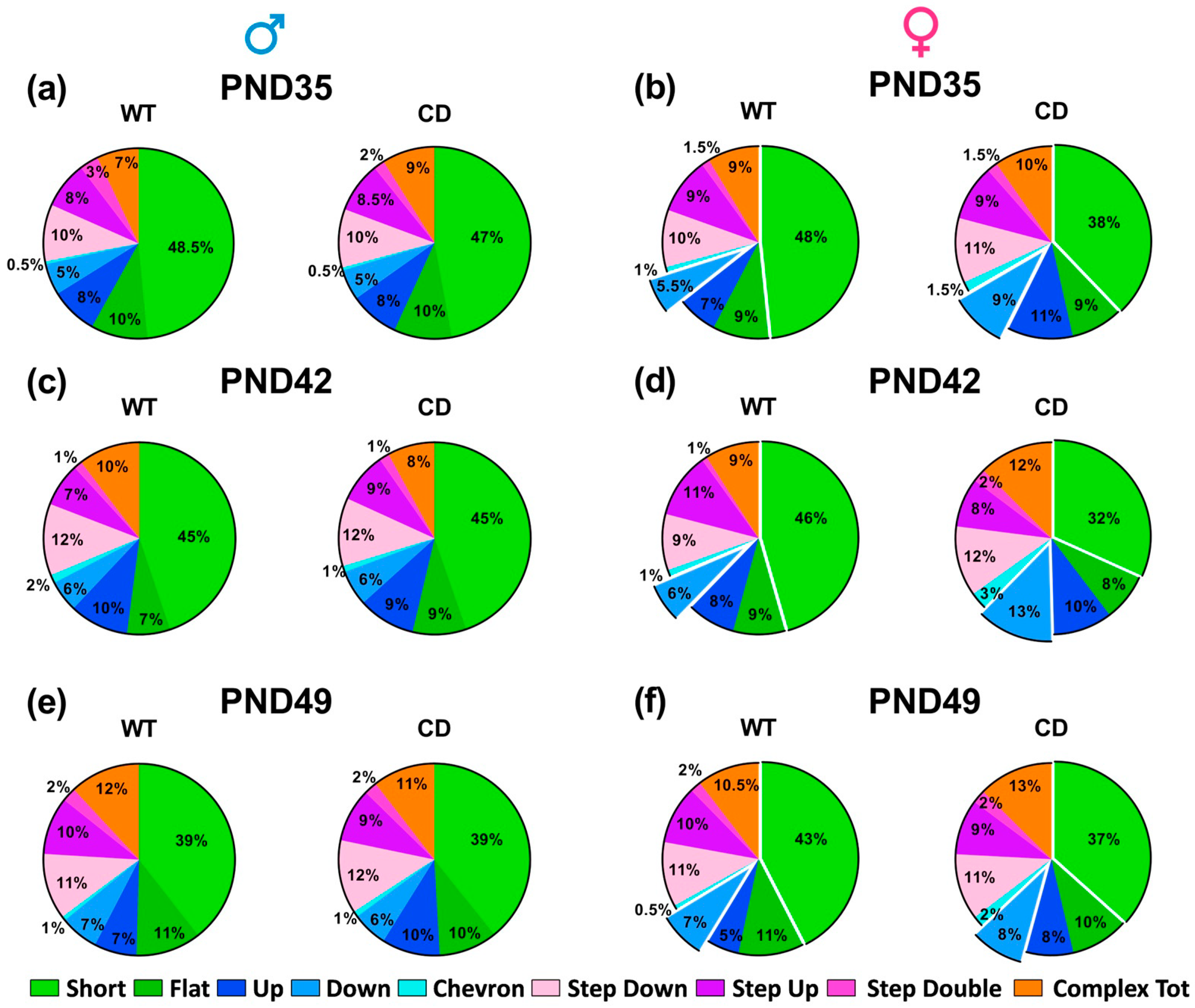
- Social interaction and USVs
- Assessment of female estrous cycle
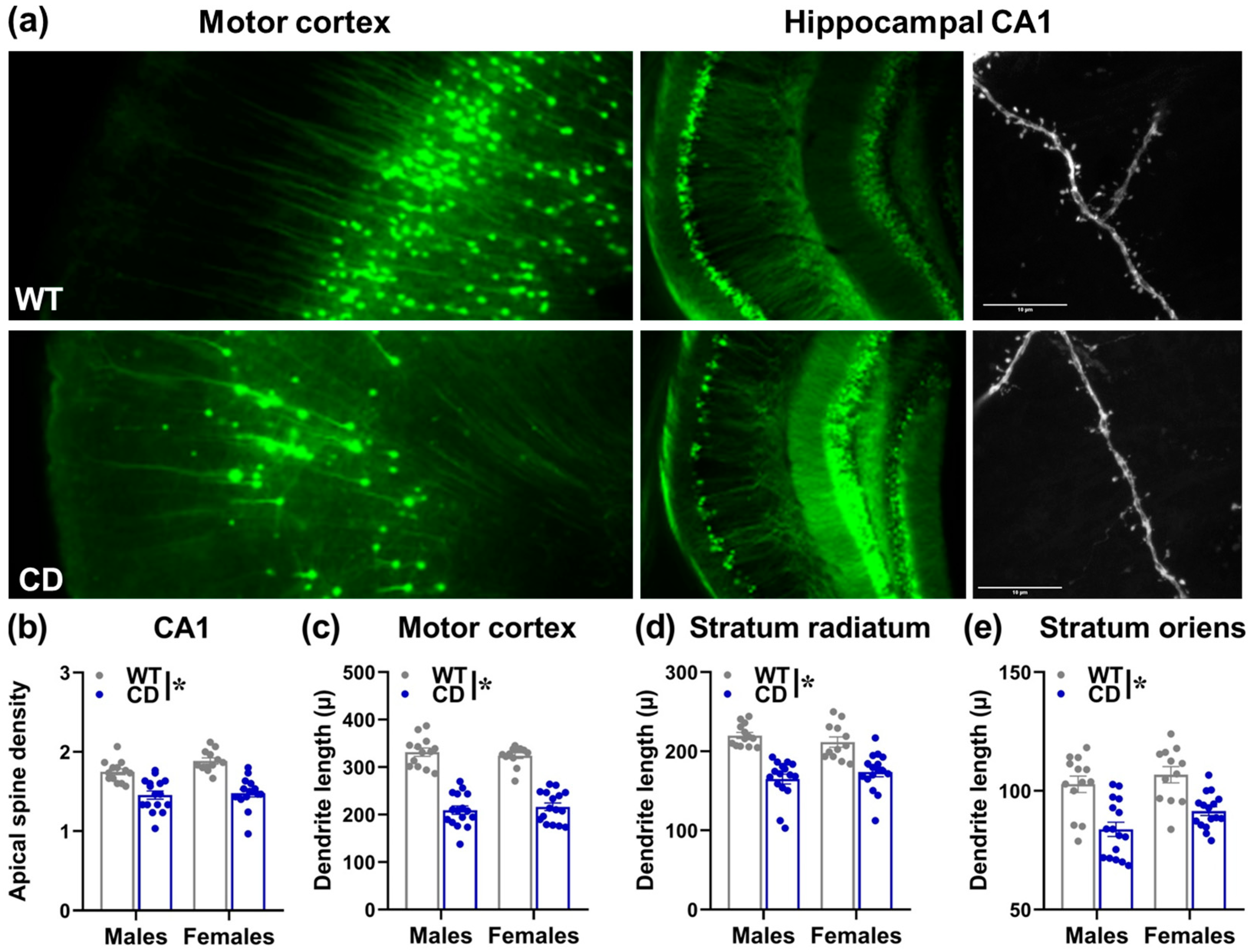
3.2. Study 2: Early Brain Characterization of the CD Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pankau, R.; Partsch, C.C.J.; Gosch, A.; Oppermann, H.C.; Wessel, A. Statural growth in Williams-Beuren syndrome. Eur. J. Pediatr. 1992, 151, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.A.; Dodd, H.; Cairns, D. Psychopathological and Behavior Impairments in Williams-Beuren Syndrome: The Influence of Gender, Chronological Age, and Cognition. Child. Neuropsychol. 2009, 15, 359–374. [Google Scholar] [CrossRef]
- Schubert, C. The Genomic Basis of the Williams-Beuren Syndrome. Cell. Mol. Life Sci. 2009, 66, 1178–1197. [Google Scholar] [CrossRef] [Green Version]
- Pober, B.R. Williams-Beuren Syndrome. N. Engl. J. Med. 2010, 362, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.A.; Wilson, S.J.; Reutens, D.C. Research Review: Williams Syndrome: A Critical Review of the Cognitive, Behavioral, and Neuroanatomical Phenotype. J. Child Psychol. Psychiatry 2008, 49, 576–608. [Google Scholar] [CrossRef] [PubMed]
- Segura-Puimedon, M.; Sahún, I.; Velot, E.; Dubus, P.; Borralleras, C.; Rodrigues, A.J.; Valero, M.C.; Valverde, O.; Sousa, N.; Herault, Y.; et al. Heterozygous deletion of the Williams–Beuren syndrome critical interval in mice recapitulates most features of the human disorder. Hum. Mol. Genet. 2014, 23, 6481–6494. [Google Scholar] [CrossRef] [Green Version]
- Borralleras, C.; Mato, S.; Amédée, T.; Matute, C.; Mulle, C.; Pérez-Jurado, L.A.; Campuzano, V. Synaptic plasticity and spatial working memory are impaired in the CD mouse model of Williams-Beuren syndrome. Mol. Brain 2016, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Borralleras, C.; Sahún, I.; Pérez-Jurado, L.A.; Campuzano, V. Intracisternal Gtf2i Gene Therapy Ameliorates Deficits in Cognition and Synaptic Plasticity of a Mouse Model of Williams–Beuren Syndrome. Mol. Ther. 2015, 23, 1691–1699. [Google Scholar] [CrossRef]
- Dasilva, M.; Navarro-Guzman, A.; Ortiz-Romero, P.; Camassa, A.; Muñoz-Cespedes, A.; Campuzano, V.; Sanchez-Vives, M.V. Altered Neocortical Dynamics in a Mouse Model of Williams–Beuren Syndrome. Mol. Neurobiol. 2020, 57, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Altayo, F.P.; Ortiz-Romero, P.; Puertas-Umbert, L.; Dantas, A.P.; Perez, B.; Vila, E.; D’Ocon, P.; Campuzano, V. Stenosis Coexists with Compromised Alpha1-Adrenergic Contractions in the Ascending Aorta of a Mouse Model of Williams-Beuren Syndrome. Sci. Rep. 2020, 10, 889. [Google Scholar] [CrossRef]
- Ortiz-Romero, P.; Borralleras, C.; Bosch-Morató, M.; Guivernau, B.; Albericio, G.; Muñoz, F.J.; Pérez-Jurado, L.A.; Campuzano, V. Epigallocatechin-3-gallate improves cardiac hypertrophy and short-term memory deficits in a Williams-Beuren syndrome mouse model. PLoS ONE 2018, 13, e0194476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Romero, A.; Galera-López, L.; Ortiz-Romero, P.; Llorente-Ovejero, A.; Reyes-Ramírez, L.D.L.; de Tena, I.B.; Garcia-Elias, A.; Mas-Stachurska, A.; Reixachs-Solé, M.; Pastor, A.; et al. Cannabinoid signaling modulation through JZL184 restores key phenotypes of a mouse model for Williams–Beuren syndrome. eLife 2022, 11, e72560. [Google Scholar] [CrossRef] [PubMed]
- Ricceri, L.; Moles, A.; Crawley, J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: Relevant social behavior patterns across the life span. Behav. Brain Res. 2007, 176, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M.; Silverman, J.L.; Scattoni, M.L.; Turner, S.M.; Harris, M.J.; Saxena, R.; Crawley, J.N. Developmental delays and reduced pup ultrasonic vocalizations but normal sociability in mice lacking the postsynaptic cell adhesion protein neuroligin2. Behav. Brain Res. 2013, 251, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Chadman, K.K.; Gong, S.; Scattoni, M.L.; Boltuck, S.E.; Gandhy, S.U.; Heintz, N.; Crawley, J.N. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008, 1, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Scattoni, M.L.; Gandhy, S.U.; Ricceri, L.; Crawley, J.N. Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism. PLoS ONE 2008, 3, e3067. [Google Scholar] [CrossRef] [Green Version]
- Branchi, I.; Ricceri, L. Transgenic and knock-out mouse pups: The growing need for behavioral analysis. Genes Brain Behav. 2002, 1, 135–141. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Crawley, J.; Ricceri, L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009, 33, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Caruso, A.; Ricceri, L.; Scattoni, M.L. Ultrasonic vocalizations as a fundamental tool for early and adult behavioral phenotyping of Autism Spectrum Disorder rodent models. Neurosci. Biobehav. Rev. 2020, 116, 31–43. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Jochman, K.A.; Kim, J.U.; Koy, J.J.; Wilson, E.D.; Chen, Q.; Wilson, C.R.; Lahvis, G.P. Affiliative Behavior, Ultrasonic Communication and Social Reward Are Influenced by Genetic Variation in Adolescent Mice. PLoS ONE 2007, 2, e351. [Google Scholar] [CrossRef]
- Lahvis, G.P.; Alleva, E.; Scattoni, M.L. Translating mouse vocalizations: Prosody and frequency modulation. Genes Brain Behav. 2010, 10, 4–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terranova, M.L.; Laviola, G.; Alleva, E. Ontogeny of amicable social behavior in the mouse: Gender differences and ongoing isolation outcomes. Dev. Psychobiol. 1993, 26, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Terranova, M.L.; Laviola, G.; de Acetis, L.; Alleva, E. A description of the ontogeny of mouse agonistic behavior. J. Comp. Psychol. 1998, 112, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Spear, L. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Yang, M.; Bozdagi, O.; Scattoni, M.L.; Wöhr, M.; Roullet, F.I.; Katz, A.M.; Abrams, D.N.; Kalikhman, D.; Simon, H.; Woldeyohannes, L.; et al. Reduced Excitatory Neurotransmission and Mild Autism-Relevant Phenotypes in Adolescent Shank3 Null Mutant Mice. J. Neurosci. 2012, 32, 6525–6541. [Google Scholar] [CrossRef] [Green Version]
- Gaudissard, J.; Ginger, M.; Premoli, M.; Memo, M.; Frick, A.; Pietropaolo, S. Behavioral abnormalities in the Fmr1-KO2 mouse model of fragile X syndrome: The relevance of early life phases. Autism Res. 2017, 10, 1584–1596. [Google Scholar] [CrossRef]
- Gauducheau, M.V.; Lemaire-Mayo, V.; D’Amato, F.R.; Oddi, D.; Crusio, W.E.; Pietropaolo, S. Age-Specific Autistic-Like Behaviors in Heterozygous Fmr1-Ko Female Mice. Autism Res. 2017, 10, 1067–1078. [Google Scholar] [CrossRef]
- Hanson, J.L.; Hurley, L.M. Female Presence and Estrous State Influence Mouse Ultrasonic Courtship Vocalizations. PLoS ONE 2012, 7, e40782. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Son, J.; Yoo, H.; Kim, H.; Oh, J.; Han, D.; Hwang, Y.; Kaang, Y.H.A.B.-K. Effects of the Female Estrous Cycle on the Sexual Behaviors and Ultrasonic Vocalizations of Male C57BL/6 and Autistic BTBR T+ tf/J Mice. Exp. Neurobiol. 2016, 25, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Mellor, R.H.; Bernstein, M.; Keller-Peck, C.; Nguyen, Q.T.; Wallace, M.; Nerbonne, J.M.; Lichtman, J.W.; Sanes, J.R. Imaging Neuronal Subsets in Transgenic Mice Expressing Multiple Spectral Variants of GFP. Neuron 2000, 28, 41–51. [Google Scholar] [CrossRef]
- Premoli, M.; Petroni, V.; Bulthuis, R.; Bonini, S.A.; Pietropaolo, S. Ultrasonic Vocalizations in Adult C57BL/6J Mice: The Role of Sex Differences and Repeated Testing. Front. Behav. Neurosci. 2022, 16, 883353. [Google Scholar] [CrossRef]
- Branchi, I.; Santucci, D.; Puopolo, M.; Alleva, E. Neonatal behaviors associated with ultrasonic vocalizations in mice (mus musculus): A slow-motion analysis. Dev. Psychobiol. 2004, 44, 37–44. [Google Scholar] [CrossRef]
- Maggio, J.C.; Whitney, G. Ultrasonic Vocalizing by Adult Female Mice (Mus Musculus). J. Comp. Psychol. 1985, 99, 420–436. [Google Scholar] [CrossRef]
- Moles, A.F.; Costantini, F.; Garbugino, L.; Zanettini, C.; D’Amato, F.R. Ultrasonic Vocalizations Emitted During Dyadic Interactions in Female Mice: A Possible Index of Sociability? Behav. Brain. Res. 2007, 182, 223–230. [Google Scholar] [CrossRef]
- Oddi, D.E.; Subashi, E.; Middei, S.; Bellocchio, L.; Lemaire-Mayo, V.; Guzman, M.; Crusio, W.E.; D’Amato, F.R.; Pietropaolo, S. Early Social Enrichment Rescues Adult Behavioral and Brain Abnormalities in a Mouse Model of Fragile X Syndrome. Neuropsychopharmacology 2015, 40, 1113–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scattoni, M.L.; Ricceri, L.; Crawley, J.N. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011, 10, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petroni, V.; Subashi, E.; Premoli, M.; Memo, M.; Lemaire, V.; Pietropaolo, S. Long-term behavioral effects of prenatal stress in the Fmr1-knock-out mouse model for fragile X syndrome. Front. Cell. Neurosci. 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Petroni, V.; Subashi, E.; Premoli, M.; Wöhr, M.; Crusio, W.E.; Lemaire, V.; Pietropaolo, S. Autistic-like behavioral effects of prenatal stress in juvenile Fmr1 mice: The relevance of sex differences and gene–environment interactions. Sci. Rep. 2022, 12, 7269. [Google Scholar] [CrossRef]
- Caligioni, C.S. Assessing Reproductive Status/Stages in Mice. Curr. Protoc. Neurosci. 2009, 48, A-4I. [Google Scholar] [CrossRef] [Green Version]
- Mervis, C.B. Williams Syndrome: 15 Years of Psychological Research. Dev. Neuropsychol. 2003, 23, 1–12. [Google Scholar]
- Masataka, N. Why early linguistic milestones are delayed in children with Williams syndrome: Late onset of hand banging as a possible rate–limiting constraint on the emergence of canonical babbling. Dev. Sci. 2001, 4, 158–164. [Google Scholar] [CrossRef]
- Wöhr, M.; Roullet, F.I.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Communication Impairments in Mice Lacking Shank1: Reduced Levels of Ultrasonic Vocalizations and Scent Marking Behavior. PLoS ONE 2011, 6, e20631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyke, W.; Premoli, M.; Alzate, V.E.; López-Moreno, J.A.; Lemaire-Mayo, V.; Crusio, W.E.; Marsicano, G.; Wöhr, M.; Pietropaolo, S. Communication and social interaction in the cannabinoid-type 1 receptor null mouse: Implications for autism spectrum disorder. Autism Res. 2021, 14, 1854–1872. [Google Scholar] [CrossRef] [PubMed]
- Strafacci, A.D.S.L.; Camargo, J.F.; Bertapelli, F.; Júnior, G.G. Growth assessment in children with Williams-Beuren syndrome: A systematic review. J. Appl. Genet. 2020, 61, 205–212. [Google Scholar] [CrossRef]
- Piquemal, M.; Abdulkarim-Abdalla, N.; Ortiz-Romero, P.; Lemaire-Mayo, V.; Crusio, W.E.; Louette, E.; Campuzano, V.; Pietropaolo, S. Chlorzoxazone, a Bkca Channel Agonist, Rescues the Pathological Phenotypes of Williams-Beuren Syndrome in a Preclinical Model. bioRxiv 2020. [Google Scholar] [CrossRef]
- Marler, J.A.; Elfenbein, J.L.; Ryals, B.M.; Urban, Z.; Netzloff, M.L. Sensorineural hearing loss in children and adults with Williams syndrome. Am. J. Med Genet. Part A 2005, 138A, 318–327. [Google Scholar] [CrossRef]
- Marler, J.A.; Sitcovsky, J.L.; Mervis, C.B.; Kistler, D.J.; Wightman, F.L. Auditory function and hearing loss in children and adults with Williams syndrome: Cochlear impairment in individuals with otherwise normal hearing. Am. J. Med Genet. Part C Semin. Med. Genet. 2010, 154C, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.A.F.; Kawahira, R.S.H.; Kim, C.; Matas, C.G. Audiological Profile and Cochlear Functionality in Williams Syndrome. Codas 2022, 34, e20210041. [Google Scholar] [CrossRef]
- Davenport, C.M.; Teubner, B.J.; Han, S.B.; Patton, M.H.; Eom, T.-Y.; Garic, D.; Lansdell, B.J.; Shirinifard, A.; Chang, T.-C.; Klein, J.; et al. Innate frequency-discrimination hyperacuity in Williams-Beuren syndrome mice. Cell 2022, 185, 3877–3895.e21. [Google Scholar] [CrossRef]
- Clayton, J.A. Studying both sexes: A guiding principle for biomedicine. FASEB J. 2016, 30, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Beery, A.K. Inclusion of females does not increase variability in rodent research studies. Curr. Opin. Behav. Sci. 2018, 23, 143–149. [Google Scholar] [CrossRef] [PubMed]

| GT | Sex | Eye Opening | Ear Detachment | Acoustic Startle | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PND 15 | PND 17 | PND 19 | PND 21 | PND 3 | PND 5 | PND 7 | PND 15 | PND 17 | PND 19 | |||||
| WT | M | 23.5 | 88.2 | 94.1 | 100 | 35.3 | 100 | 100 | 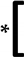 | 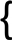 | 76.5 | 100 | 100 | |
| F | 37.5 | 93.8 | 100 | 100 | 25 | 93.8 | 100 | 62.5 | 100 | 100 | ||||
| CD | M | 33.3 | 83.3 | 91.7 | 100 | 25 | 100 | 100 | 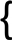 | 50 | 100 | 100 | ||
| F | 18.2 | 54.5 | 81.8 | 100 | 9.09 | 90.9 | 100 | 54.5 | 90.9 | 100 | ||||
| Cycle | PND 22 | PND 35 | PND 42 | PND 49 | PND 50 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | CD | WT | CD | WT | CD | WT | CD | WT | CD | ||||||
| PROESTRUS | - | - | 6.3 | 0 | 0 | 0 | 19 | 0 | 44 | 40 | |||||
| ESTRUS | - | - | 31 | 20 | 13 | 10 | 19 | 40 | 25 | 10 | |||||
| METAESTRUS | - | - | 13 | 30 | 31 | 0 | 6.3 | 0 | 0 | 0 | |||||
| DIESTRUS | - | - | 50 | 50 | 56 | 90 | 56 | 60 | 31 | 50 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannoccaro, S.; Ferraguto, C.; Petroni, V.; Marcelly, C.; Nogues, X.; Campuzano, V.; Pietropaolo, S. Early Neurobehavioral Characterization of the CD Mouse Model of Williams–Beuren Syndrome. Cells 2023, 12, 391. https://doi.org/10.3390/cells12030391
Giannoccaro S, Ferraguto C, Petroni V, Marcelly C, Nogues X, Campuzano V, Pietropaolo S. Early Neurobehavioral Characterization of the CD Mouse Model of Williams–Beuren Syndrome. Cells. 2023; 12(3):391. https://doi.org/10.3390/cells12030391
Chicago/Turabian StyleGiannoccaro, Silvia, Celeste Ferraguto, Valeria Petroni, Coline Marcelly, Xavier Nogues, Victoria Campuzano, and Susanna Pietropaolo. 2023. "Early Neurobehavioral Characterization of the CD Mouse Model of Williams–Beuren Syndrome" Cells 12, no. 3: 391. https://doi.org/10.3390/cells12030391
APA StyleGiannoccaro, S., Ferraguto, C., Petroni, V., Marcelly, C., Nogues, X., Campuzano, V., & Pietropaolo, S. (2023). Early Neurobehavioral Characterization of the CD Mouse Model of Williams–Beuren Syndrome. Cells, 12(3), 391. https://doi.org/10.3390/cells12030391






