PAX6 Expression Patterns in the Adult Human Limbal Stem Cell Niche
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Cell Isolation and Culturing
2.3. Western Blotting
3. Results:
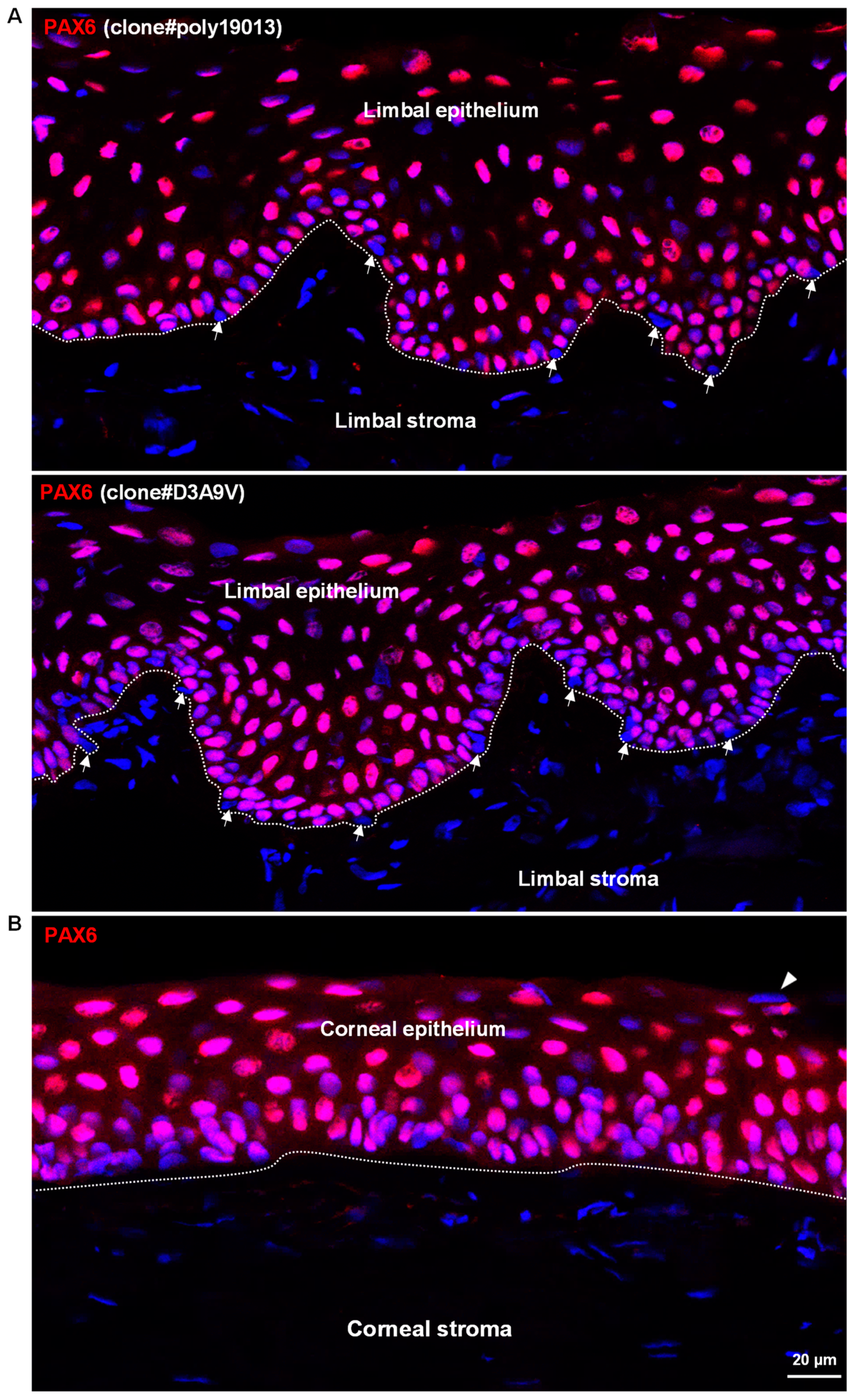
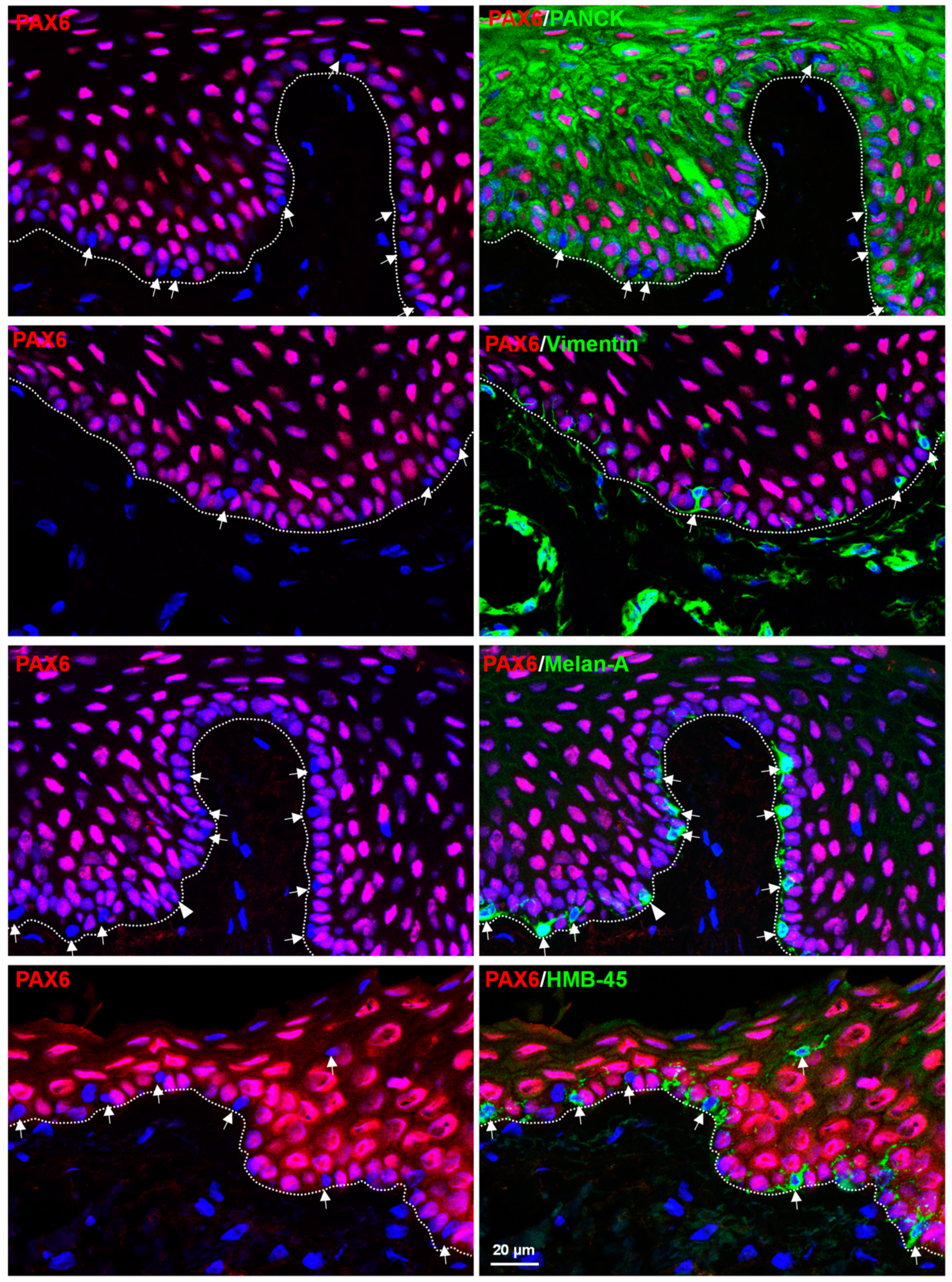
3.1. In Situ Localization of PAX6 in Non-Cultured Human Corneoscleral Tissues
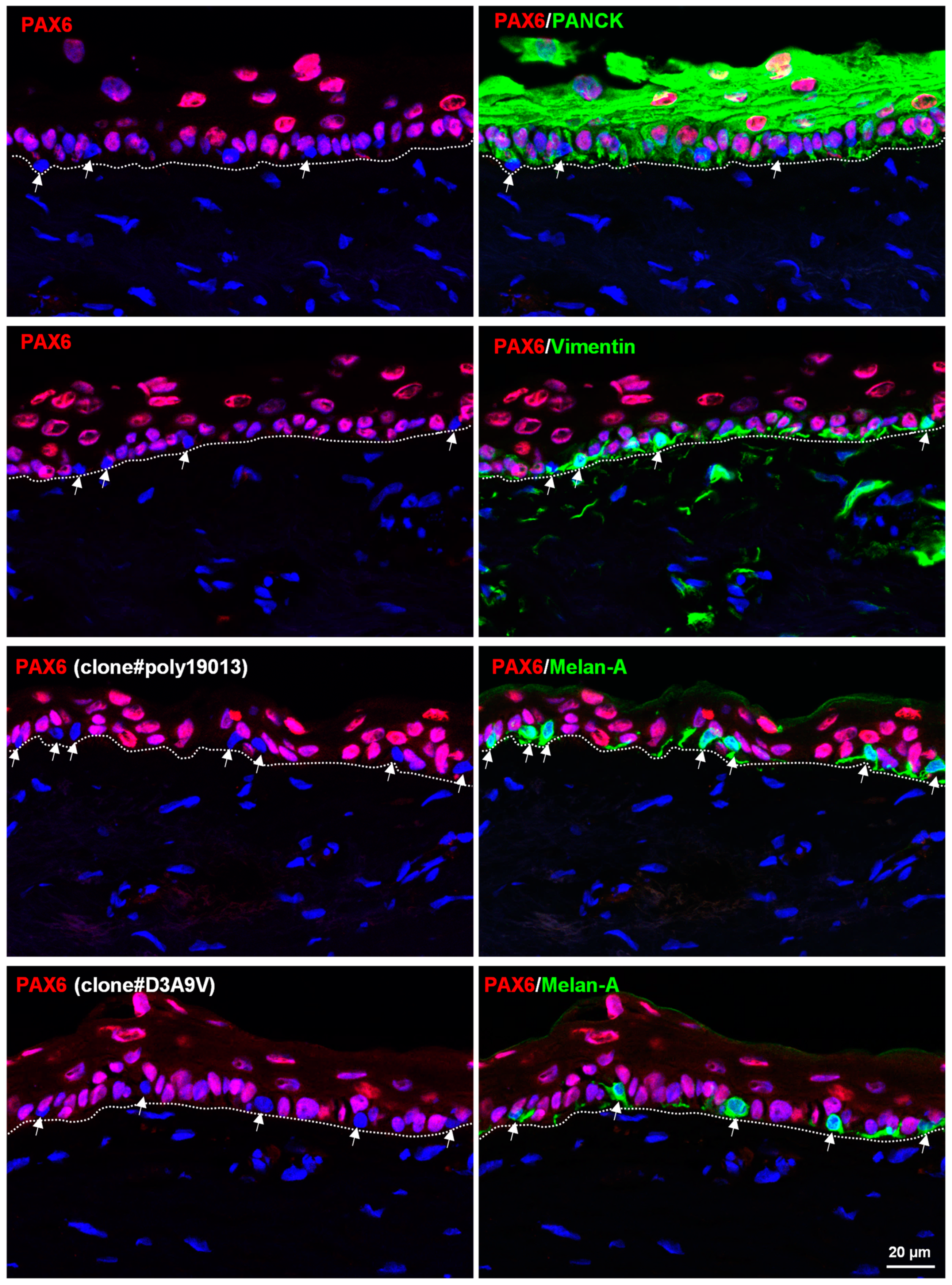
3.2. In Situ Localization of PAX6 in Organ-Cultured Human Corneoscleral Tissues
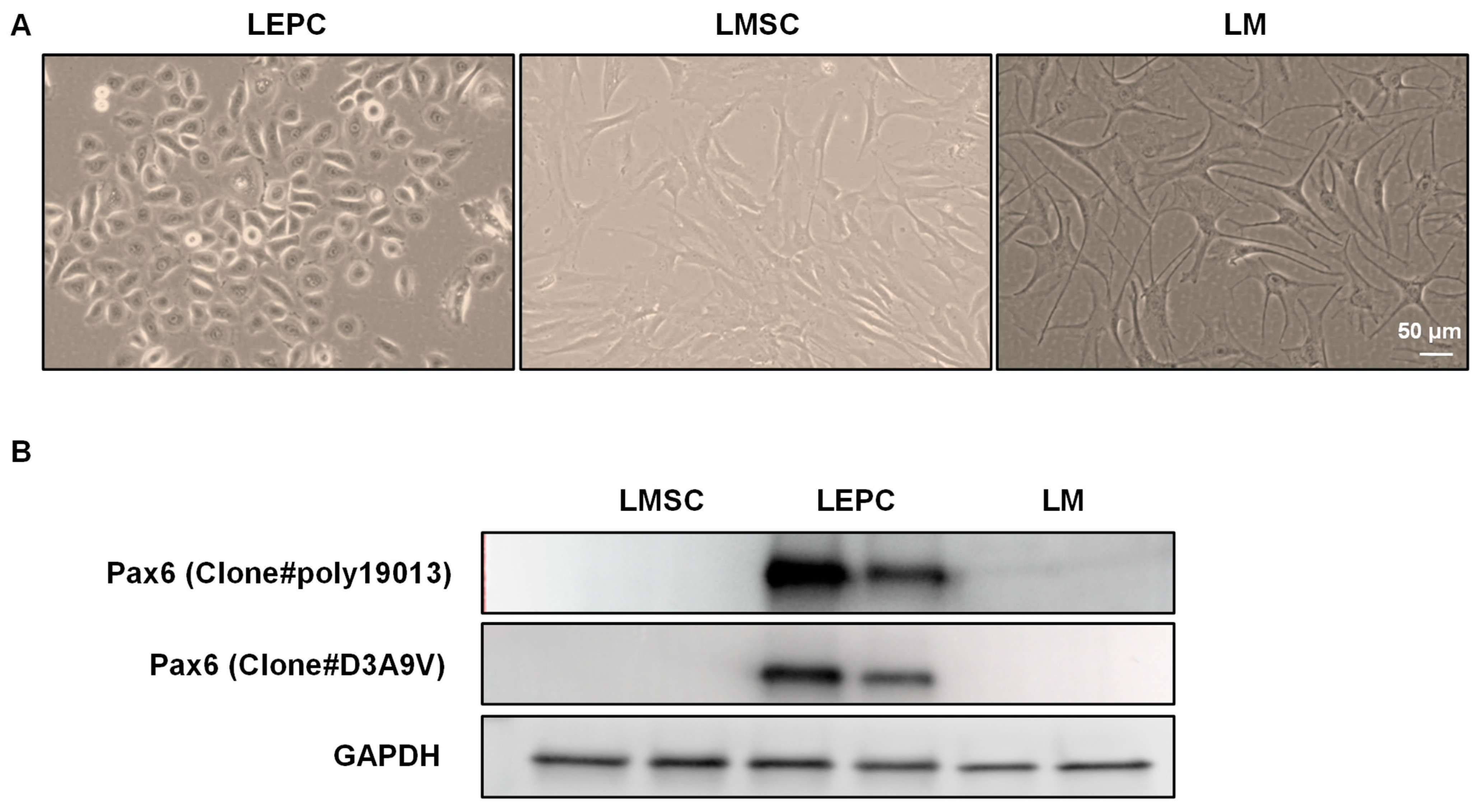
3.3. PAX6 Expression in Cultured Limbal Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of Slow-Cycling Limbal Epithelial Basal Cells That Can Be Preferentially Stimulated to Proliferate: Implications on Epithelial Stem Cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.F.; Bron, A.J. Limbal Palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar] [PubMed]
- Shortt, A.J.; Secker, G.A.; Munro, P.M.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Characterization of the Limbal Epithelial Stem Cell Niche: Novel Imaging Techniques Permit in vivo Observation and Targeted Biopsy of Limbal Epithelial Stem Cells. Stem Cells 2007, 25, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Dziasko, M.A.; Tuft, S.J.; Daniels, J.T. Limbal Melanocytes Support Limbal Epithelial Stem Cells in 2D and 3D Microenvironments. Exp. Eye Res. 2015, 138, 70–79. [Google Scholar] [CrossRef]
- Polisetti, N.; Gießl, A.; Zenkel, M.; Heger, L.; Dudziak, D.; Naschberger, E.; Stich, L.; Steinkasserer, A.; Kruse, F.E.; Schlötzer-Schrehardt, U. Melanocytes as Emerging Key Players in Niche Regulation of Limbal Epithelial Stem Cells. Ocul. Surf. 2021, 22, 172–189. [Google Scholar] [CrossRef]
- Polisetti, N.; Sharaf, L.; Schlötzer-Schrehardt, U.; Schlunck, G.; Reinhard, T. Efficient Isolation and Functional Characterization of Niche Cells from Human Corneal Limbus. Int. J. Mol. Sci. 2022, 23, 2750. [Google Scholar] [CrossRef]
- Al-Jaibaji, O.; Swioklo, S.; Connon, C.J. Mesenchymal Stromal Cells for Ocular Surface Repair. Expert Opin. Biol. Ther. 2019, 19, 643–653. [Google Scholar] [CrossRef]
- Polisetti, N.; Gießl, A.; Li, S.; Sorokin, L.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511-E8 Promotes Efficient in Vitro Expansion of Human Limbal Melanocytes. Sci. Rep. 2020, 10, 11074. [Google Scholar] [CrossRef]
- Deng, S.X.; Kruse, F.; Gomes, J.A.P.; Chan, C.C.; Daya, S.; Dana, R.; Figueiredo, F.C.; Kinoshita, S.; Rama, P.; Sangwan, V.; et al. Global Consensus on the Management of Limbal Stem Cell Deficiency. Cornea 2020, 39, 1291–1302. [Google Scholar] [CrossRef]
- Reinhard, T.; Spelsberg, H.; Henke, L.; Kontopoulos, T.; Enczmann, J.; Wernet, P.; Berschick, P.; Sundmacher, R.; Böhringer, D. Long-Term Results of Allogeneic Penetrating Limbo-Keratoplasty in Total Limbal Stem Cell Deficiency. Ophthalmology 2004, 111, 775–782. [Google Scholar] [CrossRef]
- Lagali, N.; Wowra, B.; Fries, F.N.; Latta, L.; Moslemani, K.; Utheim, T.P.; Wylegala, E.; Seitz, B.; Käsmann-Kellner, B. PAX6 Mutational Status Determines Aniridia-Associated Keratopathy Phenotype. Ophthalmology 2020, 127, 273–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlötzer-Schrehardt, U.; Latta, L.; Gießl, A.; Zenkel, M.; Fries, F.N.; Käsmann-Kellner, B.; Kruse, F.E.; Seitz, B. Dysfunction of the Limbal Epithelial Stem Cell Niche in Aniridia-Associated Keratopathy. Ocul. Surf. 2021, 21, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Stegmaier, J.; Kobitski, A.Y.; Schott, B.; Weger, B.D.; Margariti, D.; Cereceda Delgado, A.R.; Gourain, V.; Scherr, T.; Yang, L.; et al. Pax6 Organizes the Anterior Eye Segment by Guiding Two Distinct Neural Crest Waves. PLoS Genet. 2020, 16, e1008774. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Hikichi, T.; Nakamura, T.; Sotozono, C.; Kinoshita, S.; Masui, S. PAX6 Regulates Human Corneal Epithelium Cell Identity. Exp. Eye Res. 2017, 154, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunny, S.S.; Lachova, J.; Dupacova, N.; Kozmik, Z. Multiple Roles of Pax6 in Postnatal Cornea Development. Dev. Biol. 2022, 491, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yamada, Y.; Fan, M.; Bangaru, S.D.; Lin, B.; Yang, J. The β Subunit of Voltage-Gated Ca2+ Channels Interacts with and Regulates the Activity of a Novel Isoform of Pax6. J. Biol. Chem. 2010, 285, 2527–2536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Xu, F.; Zhu, J.; Krawczyk, M.; Zhang, Y.; Yuan, J.; Patel, S.; Wang, Y.; Lin, Y.; Zhang, M.; et al. Transcription Factor PAX6 (Paired Box 6) Controls Limbal Stem Cell Lineage in Development and Disease. J. Biol. Chem. 2015, 290, 20448–20454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvekl, A.; Tamm, E.R. Anterior Eye Development and Ocular Mesenchyme: New Insights from Mouse Models and Human Diseases. Bioessays 2004, 26, 374–386. [Google Scholar] [CrossRef] [Green Version]
- Funderburgh, M.L.; Du, Y.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. PAX6 Expression Identifies Progenitor Cells for Corneal Keratocytes. FASEB J. 2005, 19, 1371–1373. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Y.; Cheng, A.M.S.; Zhang, Y.; Zhu, Y.-T.; He, H.; Mahabole, M.; Tseng, S.C.G. Pax 6 Controls Neural Crest Potential of Limbal Niche Cells to Support Self-Renewal of Limbal Epithelial Stem Cells. Sci. Rep. 2019, 9, 9763. [Google Scholar] [CrossRef]
- Nishina, S.; Kohsaka, S.; Yamaguchi, Y.; Handa, H.; Kawakami, A.; Fujisawa, H.; Azuma, N. PAX6 Expression in the Developing Human Eye. Br. J. Ophthalmol. 1999, 83, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Chen, Y.-T.; Hayashida, Y.; Blanco, G.; Kheirkah, A.; He, H.; Chen, S.-Y.; Liu, C.-Y.; Tseng, S.C.G. Down-Regulation of Pax6 Is Associated with Abnormal Differentiation of Corneal Epithelial Cells in Severe Ocular Surface Diseases. J. Pathol. 2008, 214, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polisetty, N.; Fatima, A.; Madhira, S.L.; Sangwan, V.S.; Vemuganti, G.K. Mesenchymal Cells from Limbal Stroma of Human Eye. Mol. Vis. 2008, 14, 431–442. [Google Scholar] [PubMed]
- Polisetti, N.; Sharaf, L.; Martin, G.; Schlunck, G.; Reinhard, T. P-Cadherin Is Expressed by Epithelial Progenitor Cells and Melanocytes in the Human Corneal Limbus. Cells 2022, 11, 1975. [Google Scholar] [CrossRef]
- Polisetti, N.; Roschinski, B.; Schlötzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int. J. Mol. Sci. 2021, 22, 10067. [Google Scholar] [CrossRef]
- Polisetti, N.; Schlötzer-Schrehardt, U.; Reinhard, T.; Schlunck, G. Isolation and Enrichment of Melanocytes from Human Corneal Limbus Using CD117 (c-Kit) as Selection Marker. Sci. Rep. 2020, 10, 17588. [Google Scholar] [CrossRef]
- Joseph, A.; Powell-Richards, A.O.R.; Shanmuganathan, V.A.; Dua, H.S. Epithelial Cell Characteristics of Cultured Human Limbal Explants. Br. J. Ophthalmol. 2004, 88, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Ordonez, P.; Di Girolamo, N. Limbal Epithelial Stem Cells: Role of the Niche Microenvironment. Stem Cells 2012, 30, 100–107. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Cai, S.; Sun, M.; Wang, J.; Li, S.; Li, X.; Tighe, S.; Chen, S.; Xie, H.; et al. Human Limbal Niche Cells Are a Powerful Regenerative Source for the Prevention of Limbal Stem Cell Deficiency in a Rabbit Model. Sci. Rep. 2018, 8, 6566. [Google Scholar] [CrossRef] [Green Version]
- Lagali, N.; Edén, U.; Utheim, T.P.; Chen, X.; Riise, R.; Dellby, A.; Fagerholm, P. In Vivo Morphology of the Limbal Palisades of Vogt Correlates with Progressive Stem Cell Deficiency in Aniridia-Related Keratopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5333–5342. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; González, S.; Mei, H.; Deng, S.X. Human Limbal Mesenchymal Cells Support the Growth of Human Corneal Epithelial Stem/Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6953–6959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veréb, Z.; Póliska, S.; Albert, R.; Olstad, O.K.; Boratkó, A.; Csortos, C.; Moe, M.C.; Facskó, A.; Petrovski, G. Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing. Sci. Rep. 2016, 6, 26227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo, S.; de la Mata, A.; López-Paniagua, M.; Herreras, J.M.; Pérez, I.; Calonge, M.; Nieto-Miguel, T. Subconjunctival Injection of Mesenchymal Stem Cells for Corneal Failure Due to Limbal Stem Cell Deficiency: State of the Art. Stem Cell Res. Ther. 2021, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Ramaesh, T.; Collinson, J.M.; Ramaesh, K.; Kaufman, M.H.; West, J.D.; Dhillon, B. Corneal Abnormalities in Pax6+/- Small Eye Mice Mimic Human Aniridia-Related Keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Mort, R.L.; Bentley, A.J.; Martin, F.L.; Collinson, J.M.; Douvaras, P.; Hill, R.E.; Morley, S.D.; Fullwood, N.J.; West, J.D. Effects of Aberrant Pax6 Gene Dosage on Mouse Corneal Pathophysiology and Corneal Epithelial Homeostasis. PLoS ONE 2011, 6, e28895. [Google Scholar] [CrossRef] [Green Version]
- Tavakkoli, F.; Damala, M.; Koduri, M.A.; Gangadharan, A.; Rai, A.K.; Dash, D.; Basu, S.; Singh, V. Transcriptomic Profiling of Human Limbus-Derived Stromal/Mesenchymal Stem Cells-Novel Mechanistic Insights into the Pathways Involved in Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 8226. [Google Scholar] [CrossRef]
- Matsuo, T.; Osumi-Yamashita, N.; Noji, S.; Ohuchi, H.; Koyama, E.; Myokai, F.; Matsuo, N.; Taniguchi, S.; Doi, H.; Iseki, S. A Mutation in the Pax-6 Gene in Rat Small Eye Is Associated with Impaired Migration of Midbrain Crest Cells. Nat. Genet. 1993, 3, 299–304. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hu, F.-R.; Wang, I.-J.; Hou, Y.-C.; Chen, W.-L. Migration of Limbal Melanocytes onto the Central Cornea after Ocular Surface Reconstruction: An in Vivo Confocal Microscopic Case Report. Cornea 2010, 29, 204–206. [Google Scholar] [CrossRef]
- Smith-Thomas, L.; Richardson, P.; Thody, A.J.; Graham, A.; Palmer, I.; Flemming, L.; Parsons, M.A.; Rennie, I.G.; MacNeil, S. Human Ocular Melanocytes and Retinal Pigment Epithelial Cells Differ in Their Melanogenic Properties in Vivo and in Vitro. Curr. Eye Res. 1996, 15, 1079–1091. [Google Scholar] [CrossRef]
- Kubic, J.D.; Young, K.P.; Plummer, R.S.; Ludvik, A.E.; Lang, D. Pigmentation PAX-Ways: The Role of Pax3 in Melanogenesis, Melanocyte Stem Cell Maintenance, and Disease. Pigment Cell Melanoma Res. 2008, 21, 627–645. [Google Scholar] [CrossRef]
- van Zyl, T.; Yan, W.; McAdams, A.M.; Monavarfeshani, A.; Hageman, G.S.; Sanes, J.R. Cell Atlas of the Human Ocular Anterior Segment: Tissue-Specific and Shared Cell Types. Proc. Natl. Acad. Sci. USA 2022, 119, e2200914119. [Google Scholar] [CrossRef] [PubMed]
| Cornea | Limbus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Epithelial | Stromal | Epithelial | Stromal | Melanocytes | |||||
| Basal | Intermediate | Superficial | Basal | Intermediate | Superficial | ||||
| PAX6 | + | + | +/− | − | + | + | +/− | − | − |
| Epithelial Keratins (PCK) | + | + | + | − | + | + | + | − | − |
| Vimentin | − | − | − | + | − | − | − | + | + |
| Melan-A | − | − | − | − | − | − | − | − | + |
| HMB45 | − | − | − | − | − | − | − | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polisetti, N.; Schlunck, G.; Reinhard, T. PAX6 Expression Patterns in the Adult Human Limbal Stem Cell Niche. Cells 2023, 12, 400. https://doi.org/10.3390/cells12030400
Polisetti N, Schlunck G, Reinhard T. PAX6 Expression Patterns in the Adult Human Limbal Stem Cell Niche. Cells. 2023; 12(3):400. https://doi.org/10.3390/cells12030400
Chicago/Turabian StylePolisetti, Naresh, Günther Schlunck, and Thomas Reinhard. 2023. "PAX6 Expression Patterns in the Adult Human Limbal Stem Cell Niche" Cells 12, no. 3: 400. https://doi.org/10.3390/cells12030400






