Calnexin, More Than Just a Molecular Chaperone
Abstract
1. Introduction
2. Discovery of Calnexin
2.1. Calnexin-Deficient Animal Models
2.2. Calnexin as a Molecular Chaperone
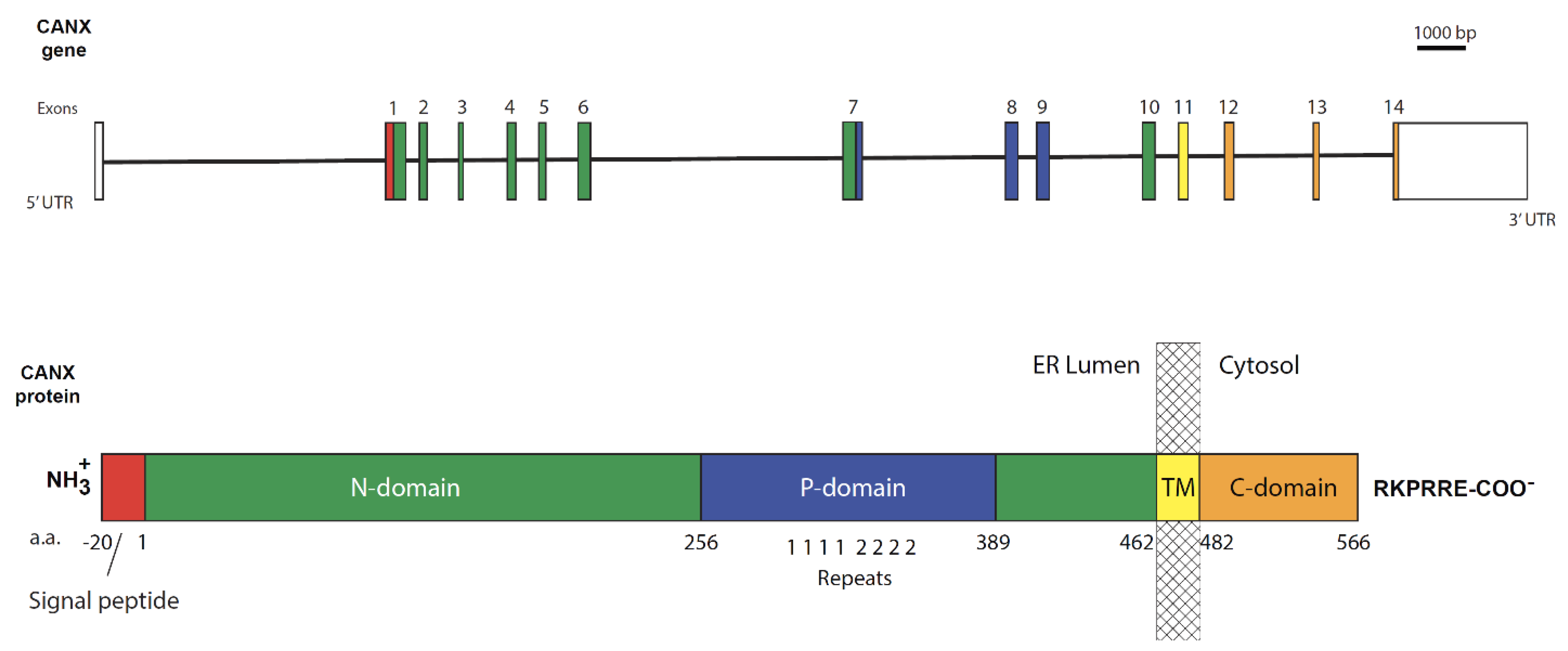
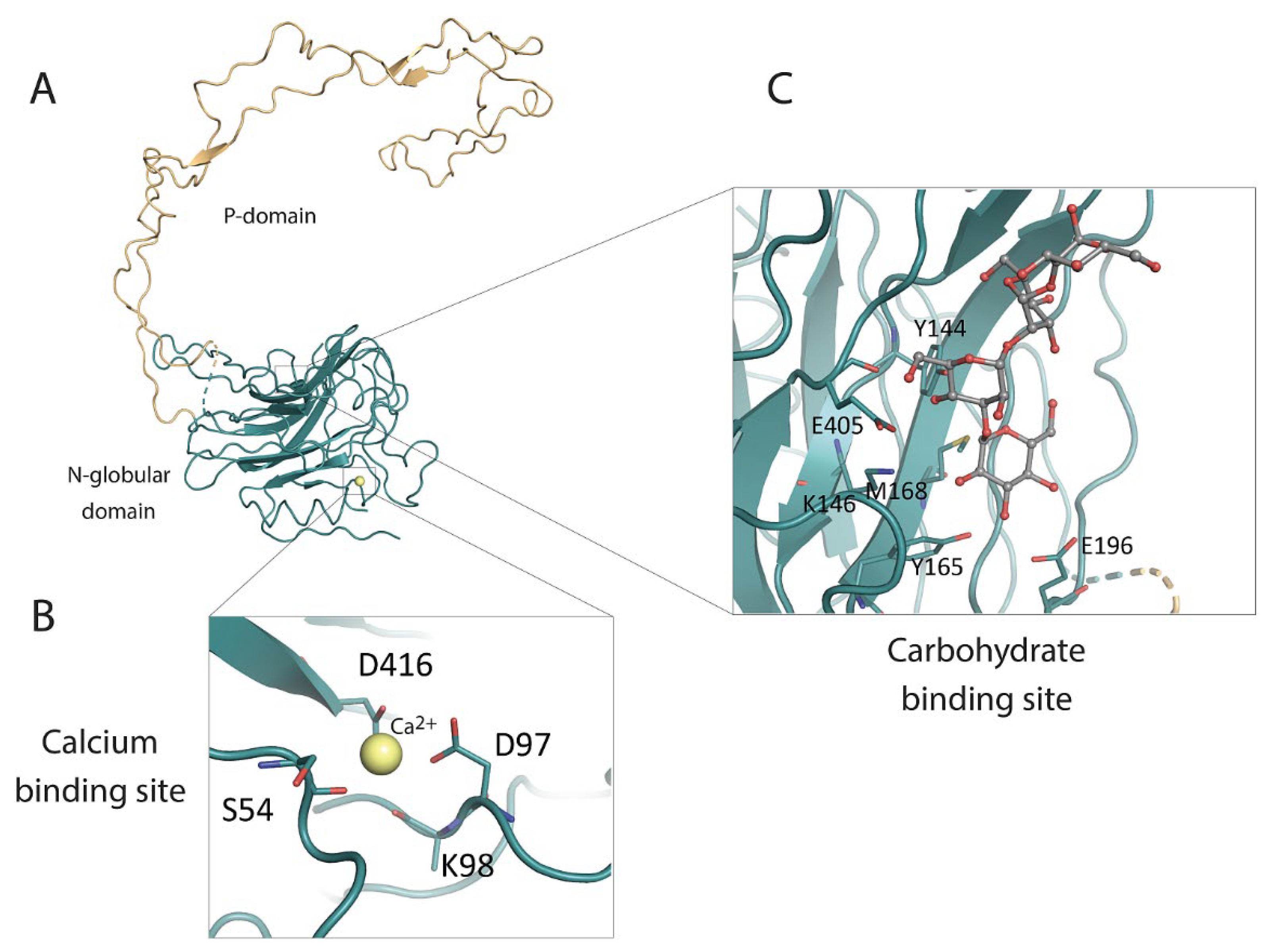
3. Structure of Calnexin
3.1. The ER Lumen-Localized N-Terminal Domain
3.2. The Transmembrane Domain
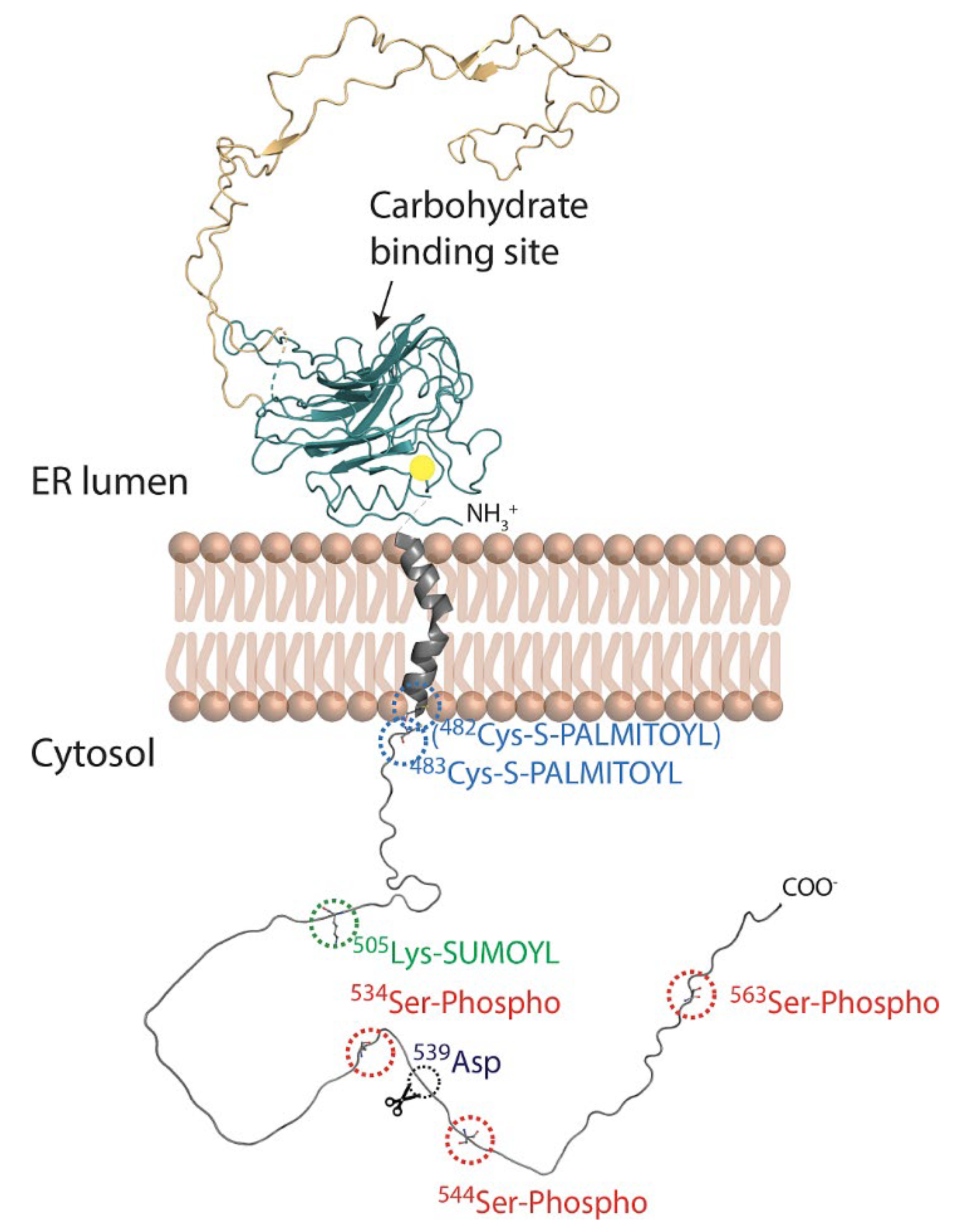
3.3. The Cytosolic C-Terminal Domain
4. Post-Translational Modifications of the Calnexin C-Terminal Domain
4.1. Palmitoylation
4.2. Phosphorylation
4.3. SUMOylation
4.4. Proteolytic Cleavage
5. The Calnexin C-Terminal Domain, a Cytosol-ER Regulatory Nexus
6. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fagone, P.; Jackowski, S. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 2009, 50, S311–S316. [Google Scholar] [CrossRef] [PubMed]
- Braakman, I.; Hebert, D.N. Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013201. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Kulak, N.A.; Geyer, P.E.; Mann, M. Loss-less Nano-fractionator for High Sensitivity, High Coverage Proteomics. Mol. Cell Proteom. 2017, 16, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Anelli, T.; Sitia, R. Protein quality control in the early secretory pathway. EMBO J. 2008, 27, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In vivo aspects of protein folding and quality control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef] [PubMed]
- Ellgaard, L.; McCaul, N.; Chatsisvili, A.; Braakman, I. Co- and Post-Translational Protein Folding in the ER. Traffic 2016, 17, 615–638. [Google Scholar] [CrossRef]
- Takizawa, T.; Tatematsu, C.; Watanabe, K.; Kato, K.; Nakanishi, Y. Cleavage of calnexin caused by apoptotic stimuli: Implica-tion for the regulation of apoptosis. J. Biochem. 2004, 136, 399–405. [Google Scholar] [CrossRef]
- Lakkaraju, A.K.; van der Goot, F.G. Calnexin controls the STAT3-mediated transcriptional response to EGF. Mol. Cell 2013, 51, 386–396. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Qi, H.; Yap, M.C.; Tahbaz, N.; Milburn, L.A.; Lucchinetti, E.; Lou, P.H.; Zaugg, M.; LaPointe, P.G.; Mercier, P.; et al. The ER chaperone calnexin controls mitochondrial positioning and respiration. Sci. Signal. 2020, 13, eaax6660. [Google Scholar] [CrossRef] [PubMed]
- Lynes, E.M.; Raturi, A.; Shenkman, M.; Ortiz Sandoval, C.; Yap, M.C.; Wu, J.; Janowicz, A.; Myhill, N.; Benson, M.D.; Camp-bell, R.E.; et al. Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J. Cell Sci. 2013, 126, 3893–3903. [Google Scholar] [CrossRef]
- Roderick, H.L.; Lechleiter, J.D.; Camacho, P. Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J. Cell Biol. 2000, 149, 1235–1248. [Google Scholar] [CrossRef]
- Li, H.D.; Liu, W.X.; Michalak, M. Enhanced clathrin-dependent endocytosis in the absence of calnexin. PLoS ONE 2011, 6, e21678. [Google Scholar] [CrossRef]
- Louvard, D.; Reggio, H.; Warren, G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J. Cell Biol. 1982, 92, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Wada, I.; Rindress, D.; Cameron, P.H.; Ou, W.J.; Doherty, J.J., 2nd; Louvard, D.; Bell, A.W.; Dignard, D.; Thomas, D.Y.; Ber-geron, J.J. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 1991, 266, 19599–19610. [Google Scholar] [CrossRef] [PubMed]
- Degen, E.; Williams, D.B. Participation of a novel 88-kD protein in the biogenesis of murine class I histocompatibility mole-cules. J. Cell Biol. 1991, 112, 1099–1115. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbach, F.; David, V.; Watkins, S.; Brenner, M.B. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc. Natl. Acad. Sci. USA 1992, 89, 4734–4738. [Google Scholar] [CrossRef]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Bergeron, J.J.; Wada, I.; Degen, E.; Williams, D.B. The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J. Biol. Chem. 1992, 267, 10914–10918. [Google Scholar]
- David, V.; Hochstenbach, F.; Rajagopalan, S.; Brenner, M.B. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin). J. Biol. Chem. 1993, 268, 9585–9592. [Google Scholar] [CrossRef]
- Cala, S.E.; Ulbright, C.; Kelley, J.S.; Jones, L.R. Purification of a 90-kDa protein (Band VII) from cardiac sarcoplasmic reticu-lum. Identification as calnexin and localization of casein kinase II phosphorylation sites. J. Biol. Chem. 1993, 268, 2969–2975. [Google Scholar] [CrossRef] [PubMed]
- Coe, H.; Michalak, M. ERp57, a multifunctional endoplasmic reticulum resident oxidoreductase. Int. J. Biochem. Cell Biol. 2010, 42, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Braakman, I.; Helenius, A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc. Natl. Acad. Sci. USA 1994, 91, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol. Biol. Cell 1994, 5, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Gehring, K. Calnexin cycle-structural features of the ER chaperone system. FEBS J. 2020, 287, 4322–4340. [Google Scholar] [CrossRef]
- Parodi, A.J. Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 2000, 69, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, J.J.; Parodi, A.J. Getting in and out from calnexin/calreticulin cycles. J. Biol. Chem. 2008, 283, 10221–10225. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004, 73, 1019–1049. [Google Scholar] [CrossRef]
- Lamriben, L.; Graham, J.B.; Adams, B.M.; Hebert, D.N. N-Glycan-based ER Molecular Chaperone and Protein Quality Con-trol System: The Calnexin Binding Cycle. Traffic 2016, 17, 308–326. [Google Scholar] [CrossRef]
- Hebert, D.N.; Molinari, M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2007, 87, 1377–1408. [Google Scholar] [CrossRef] [PubMed]
- Agellon, L.B.; Michalak, M. The Endoplasmic Reticulum and the Cellular Reticular Network. Adv. Exp. Med. Biol. 2017, 981, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Chevet, E.; Wong, H.N.; Gerber, D.; Cochet, C.; Fazel, A.; Cameron, P.H.; Gushue, J.N.; Thomas, D.Y.; Bergeron, J.J. Phos-phorylation by CK2 and MAPK enhances calnexin association with ribosomes. EMBO J. 1999, 18, 3655–3666. [Google Scholar] [CrossRef] [PubMed]
- Lakkaraju, A.K.; Abrami, L.; Lemmin, T.; Blaskovic, S.; Kunz, B.; Kihara, A.; Dal Peraro, M.; van der Goot, F.G. Palmitoylated calnexin is a key component of the ribosome-translocon complex. EMBO J. 2012, 31, 1823–1835. [Google Scholar] [CrossRef]
- Lynes, E.M.; Bui, M.; Yap, M.C.; Benson, M.D.; Schneider, B.; Ellgaard, L.; Berthiaume, L.G.; Simmen, T. Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J. 2012, 31, 457–470. [Google Scholar] [CrossRef]
- Wiest, D.L.; Burgess, W.H.; McKean, D.; Kearse, K.P.; Singer, A. The molecular chaperone calnexin is expressed on the sur-face of immature thymocytes in association with clonotype-independent CD3 complexes. EMBO J. 1995, 14, 3425–3433. [Google Scholar] [CrossRef]
- Wiest, D.L.; Bhandoola, A.; Punt, J.; Kreibich, G.; McKean, D.; Singer, A. Incomplete endoplasmic reticulum (ER) retention in immature thymocytes as revealed by surface expression of "ER-resident" molecular chaperones. Proc. Natl. Acad. Sci. USA 1997, 94, 1884–1889. [Google Scholar] [CrossRef]
- Okazaki, Y.; Ohno, H.; Takase, K.; Ochiai, T.; Saito, T. Cell surface expression of calnexin, a molecular chaperone in the endo-plasmic reticulum. J. Biol. Chem. 2000, 275, 35751–35758. [Google Scholar] [CrossRef]
- Myhill, N.; Lynes, E.M.; Nanji, J.A.; Blagoveshchenskaya, A.D.; Fei, H.; Carmine Simmen, K.; Cooper, T.J.; Thomas, G.; Sim-men, T. The subcellular distribution of calnexin is mediated by PACS-2. Mol. Biol. Cell 2008, 19, 2777–2788. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, D.; Wang, X.; Fang, J.; Liu, X.; Song, J.; Li, X.; Ren, X.; Li, Q.; Li, Q.; et al. Calnexin Impairs the Antitumor Im-munity of CD4+ and CD8+ T Cells. Cancer Immunol. Res. 2019, 7, 123–135. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nagashio, R.; Jiang, S.X.; Saito, K.; Tsuchiya, B.; Ryuge, S.; Katono, K.; Nakashima, H.; Fukuda, E.; Goshima, N.; et al. Calnexin is a novel sero-diagnostic marker for lung cancer. Lung Cancer 2015, 90, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strat-egies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment edi-tor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, E.E.; Hardie, R.C.; Colley, N.J. Calnexin is essential for rhodopsin maturation, Ca2+ regulation, and photorecep-tor cell survival. Neuron 2006, 49, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, T.H.; Park, B.J.; Chang, J.W.; Yu, J.R.; Koo, H.S.; Park, H.; Yoo, Y.J.; Ahnn, J. Caenorhabditis elegans calnexin is N-glycosylated and required for stress response. Biochem. Biophys. Res. Commun. 2005, 338, 1018–1030. [Google Scholar] [CrossRef]
- Xu, K.; Tavernarakis, N.; Driscoll, M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron 2001, 31, 957–971. [Google Scholar] [CrossRef]
- Hung, I.C.; Cherng, B.W.; Hsu, W.M.; Lee, S.J. Calnexin is required for zebrafish posterior lateral line development. Int. J. Dev. Biol. 2013, 57, 427–438. [Google Scholar] [CrossRef]
- Kraus, A.; Groenendyk, J.; Bedard, K.; Baldwin, T.A.; Krause, K.-H.; Dubois-Dauphin, M.; Dyck, J.; Rosenbaum, E.E.; Korn-gut, L.; Colley, N.J.; et al. Calnexin deficiency leads to dysmyelination. J. Biol. Chem. 2010, 285, 18928–18938. [Google Scholar] [CrossRef]
- Denzel, A.; Molinari, M.; Trigueros, C.; Martin, J.E.; Velmurgan, S.; Brown, S.; Stamp, G.; Owen, M.J. Early postnatal death and motor disorders in mice congenitally deficient in calnexin expression. Mol. Cell Biol. 2002, 22, 7398–7404. [Google Scholar] [CrossRef]
- Mesaeli, N.; Nakamura, K.; Zvaritch, E.; Dickie, P.; Dziak, E.; Krause, K.H.; Opas, M.; MacLennan, D.H.; Michalak, M. Calre-ticulin is essential for cardiac development. J. Cell Biol. 1999, 144, 857–868. [Google Scholar] [CrossRef]
- Paskevicius, T.; Jung, J.; Pujol, M.; Eggleton, P.; Qin, W.; Robinson, A.; Gutowski, N.; Holley, J.; Smallwood, M.; Newcombe, J.; et al. The Fabp5/calnexin complex is a prerequisite for sensitization of mice to experimental autoimmune encephalomyeli-tis. FASEB J. 2020, 34, 16662–16675. [Google Scholar] [CrossRef]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, R.; Kornfeld, S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985, 54, 631–664. [Google Scholar] [CrossRef] [PubMed]
- Hebert, D.N.; Foellmer, B.; Helenius, A. Glucose trimming and reglucosylation determine glycoprotein association with cal-nexin in the endoplasmic reticulum. Cell 1995, 81, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Rodan, A.R.; Simons, J.F.; Trombetta, E.S.; Helenius, A. N-linked oligosaccharides are necessary and sufficient for association of glycosylated forms of bovine RNase with calnexin and calreticulin. EMBO J. 1996, 15, 6921–6930. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Darby, N.J.; Tessier, D.C.; Michalak, M.; Bergeron, J.J.; Thomas, D.Y. Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J. Biol. Chem. 1998, 273, 6009–6012. [Google Scholar] [CrossRef] [PubMed]
- Frickel, E.M.; Riek, R.; Jelesarov, I.; Helenius, A.; Wuthrich, K.; Ellgaard, L. TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc. Natl. Acad. Sci. USA 2002, 99, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Bastos-Aristizabal, S.; Määttänen, P.; Rosenauer, A.; Zheng, F.; Killikelly, A.; Trempe, J.F.; Thomas, D.Y.; Gehring, K. Structural basis of cyclophilin B binding by the calnexin/calreticulin P-domain. J. Biol. Chem. 2010, 285, 35551–35557. [Google Scholar] [CrossRef]
- Kozlov, G.; Muñoz-Escobar, J.; Castro, K.; Gehring, K. Mapping the ER Interactome: The P Domains of Calnexin and Calre-ticulin as Plurivalent Adapters for Foldases and Chaperones. Structure 2017, 25, 1415–1422.e1413. [Google Scholar] [CrossRef]
- Sakono, M.; Seko, A.; Takeda, Y.; Ito, Y. PDI family protein ERp29 forms 1:1 complex with lectin chaperone calreticulin. Biochem. Biophys. Res. Commun. 2014, 452, 27–31. [Google Scholar] [CrossRef]
- Tannous, A.; Patel, N.; Tamura, T.; Hebert, D.N. Reglucosylation by UDP-glucose:glycoprotein glucosyltransferase 1 delays glycoprotein secretion but not degradation. Mol. Biol. Cell 2015, 26, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Plemper, R.K.; Wolf, D.H. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem. Sci. 1999, 24, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.M.; Choudhury, P.; Liu, Y.; Sifers, R.N. Processing by endoplasmic reticulum mannosidases partitions a secretion-impaired glycoprotein into distinct disposal pathways. J. Biol. Chem. 2000, 275, 25015–25022. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, N.; Wada, I.; Hasegawa, K.; Yorihuzi, T.; Tremblay, L.O.; Herscovics, A.; Nagata, K. A novel ER alpha-mannosidase-like protein accelerates ER-associated degradation. EMBO Rep. 2001, 2, 415–422. [Google Scholar] [CrossRef]
- Tremblay, L.O.; Herscovics, A. Cloning and expression of a specific human alpha 1,2-mannosidase that trims Man9GlcNAc2 to Man8GlcNAc2 isomer B during N-glycan biosynthesis. Glycobiology 1999, 9, 1073–1078. [Google Scholar] [CrossRef]
- Meusser, B.; Hirsch, C.; Jarosch, E.; Sommer, T. ERAD: The long road to destruction. Nat. Cell Biol. 2005, 7, 766–772. [Google Scholar] [CrossRef]
- Jakob, C.A.; Burda, P.; Roth, J.; Aebi, M. Degradation of misfolded endoplasmic reticulum glycoproteins in Saccharomyces cerevisiae is determined by a specific oligosaccharide structure. J. Cell Biol. 1998, 142, 1223–1233. [Google Scholar] [CrossRef]
- Oda, Y.; Hosokawa, N.; Wada, I.; Nagata, K. EDEM as an acceptor of terminally misfolded glycoproteins released from cal-nexin. Science 2003, 299, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M.; Calanca, V.; Galli, C.; Lucca, P.; Paganetti, P. Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science 2003, 299, 1397–1400. [Google Scholar] [CrossRef]
- Christianson, J.C.; Ye, Y. Cleaning up in the endoplasmic reticulum: Ubiquitin in charge. Nat. Struct. Mol. Biol. 2014, 21, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, S.; Wang, X.; Li, C.; Yang, J.; Zhu, X.; Xiao, L.; Sun, L. ER-Phagy: A New Regulator of ER Homeostasis. Front. Cell Dev. Biol. 2021, 9, 684526. [Google Scholar] [CrossRef] [PubMed]
- Forrester, A.; De Leonibus, C.; Grumati, P.; Fasana, E.; Piemontese, M.; Staiano, L.; Fregno, I.; Raimondi, A.; Marazza, A.; Bruno, G.; et al. A selective ER-phagy exerts procollagen quality control via a Calnexin-FAM134B complex. EMBO J. 2019, 38, e99847. [Google Scholar] [CrossRef]
- Fregno, I.; Fasana, E.; Bergmann, T.J.; Raimondi, A.; Loi, M.; Soldà, T.; Galli, C.; D’Antuono, R.; Morone, D.; Danieli, A.; et al. ER-to-lysosome-associated degradation of proteasome-resistant ATZ polymers occurs via receptor-mediated vesicular transport. EMBO J. 2018, 37, e99259. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, G.; Bangia, N.; Pan, M.; Cresswell, P. A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. J. Immunol. 2001, 166, 1703–1709. [Google Scholar] [CrossRef]
- Jackson, M.R.; Cohen-Doyle, M.F.; Peterson, P.A.; Williams, D.B. Regulation of MHC class I transport by the molecular chap-erone, calnexin (p88, IP90). Science 1994, 263, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.K.; Mitchell, E.K.; Yang, Y.; Peterson, P.A.; Waneck, G.L.; Williams, D.B. MHC class I molecules form ternary com-plexes with calnexin and TAP and undergo peptide-regulated interaction with TAP via their extracellular domains. J. Exp. Med. 1996, 184, 337–348. [Google Scholar] [CrossRef]
- Zhang, Q.; Tector, M.; Salter, R.D. Calnexin recognizes carbohydrate and protein determinants of class I major histocompati-bility complex molecules. J. Biol. Chem. 1995, 270, 3944–3948. [Google Scholar] [CrossRef]
- Vassilakos, A.; Cohen-Doyle, M.F.; Peterson, P.A.; Jackson, M.R.; Williams, D.B. The molecular chaperone calnexin facilitates folding and assembly of class I histocompatibility molecules. EMBO J. 1996, 15, 1495–1506. [Google Scholar] [CrossRef]
- Prasad, S.A.; Yewdell, J.W.; Porgador, A.; Sadasivan, B.; Cresswell, P.; Bennink, J.R. Calnexin expression does not enhance the generation of MHC class I-peptide complexes. Eur. J. Immunol. 1998, 28, 907–913. [Google Scholar] [CrossRef]
- Sadasivan, B.K.; Cariappa, A.; Waneck, G.L.; Cresswell, P. Assembly, peptide loading, and transport of MHC class I mole-cules in a calnexin-negative cell line. Cold Spring Harb. Symp. Quant. Biol. 1995, 60, 267–275. [Google Scholar] [CrossRef]
- Anderson, K.S.; Cresswell, P. A role for calnexin (IP90) in the assembly of class II MHC molecules. EMBO J. 1994, 13, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Kearse, K.P.; Williams, D.B.; Singer, A. Persistence of glucose residues on core oligosaccharides prevents association of TCR alpha and TCR beta proteins with calnexin and results specifically in accelerated degradation of nascent TCR alpha proteins within the endoplasmic reticulum. EMBO J. 1994, 13, 3678–3686. [Google Scholar] [CrossRef]
- Van Leeuwen, J.E.; Kearse, K.P. Calnexin associates exclusively with individual CD3 delta and T cell antigen receptor (TCR) alpha proteins containing incompletely trimmed glycans that are not assembled into multisubunit TCR complexes. J. Biol. Chem. 1996, 271, 9660–9665. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.G.; Franklin, R.A.; Robinson, P.J.; Pederson, N.E.; Howe, C.; Kearse, K.P. T cell receptor assembly and expression in the absence of calnexin. Arch. Biochem. Biophys. 2000, 378, 182–189. [Google Scholar] [CrossRef]
- Van Leeuwen, J.E.; Kearse, K.P. The related molecular chaperones calnexin and calreticulin differentially associate with nas-cent T cell antigen receptor proteins within the endoplasmic reticulum. J. Biol. Chem. 1996, 271, 25345–25349. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Mitchell, R.N.; Schreiber, K.L.; McKean, D.J.; Abbas, A.K. Molecular mechanisms that control expression of the B lymphocyte antigen receptor complex. J. Exp. Med. 1995, 181, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pun, C.; Hozumi, N. Roles of calnexin and Ig-alpha beta interactions with membrane Igs in the surface expression of the B cell antigen receptor of the IgM and IgD classes. J. Immunol. 1997, 158, 2762–2770. [Google Scholar] [CrossRef]
- Foy, S.P.; Matsuuchi, L. Association of B lymphocyte antigen receptor polypeptides with multiple chaperone proteins. Immunol. Lett. 2001, 78, 149–160. [Google Scholar] [CrossRef]
- Tjoelker, L.W.; Seyfried, C.E.; Eddy, R.L., Jr.; Byers, M.G.; Shows, T.B.; Calderon, J.; Schreiber, R.B.; Gray, P.W. Human, mouse, and rat calnexin cDNA cloning: Identification of potential calcium binding motifs and gene localization to human chromosome 5. Biochemistry 1994, 33, 3229–3236. [Google Scholar] [CrossRef]
- Oliver, J.D.; Roderick, H.L.; Llewellyn, D.H.; High, S. ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol. Biol. Cell 1999, 10, 2573–2582. [Google Scholar] [CrossRef]
- Ho, S.C.; Rajagopalan, S.; Chaudhuri, S.; Shieh, C.C.; Brenner, M.B.; Pillai, S. Membrane anchoring of calnexin facilitates its interaction with its targets. Mol. Immunol. 1999, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dowal, L.; Yang, W.; Freeman, M.R.; Steen, H.; Flaumenhaft, R. Proteomic analysis of palmitoylated platelet proteins. Blood 2011, 118, e62–e73. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Wan, J.; Arstikaitis, P.; Takahashi, H.; Huang, K.; Bailey, A.O.; Thompson, J.X.; Roth, A.F.; Drisdel, R.C.; Mastro, R.; et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 2008, 456, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Yount, J.S.; Moltedo, B.; Yang, Y.Y.; Charron, G.; Moran, T.M.; López, C.B.; Hang, H.C. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat. Chem. Biol. 2010, 6, 610–614. [Google Scholar] [CrossRef]
- Cameron, P.H.; Chevet, E.; Pluquet, O.; Thomas, D.Y.; Bergeron, J.J. Calnexin phosphorylation attenuates the release of par-tially misfolded alpha1-antitrypsin to the secretory pathway. J. Biol. Chem. 2009, 284, 34570–34579. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kraus, A.; Prins, D.; Groenendyk, J.; Aubry, I.; Liu, W.-X.; Li, H.-D.; Julien, O.; Touret, N.; Sykes, B.D.; et al. UBC9-dependent association between calnexin and protein tyrosine phosphatase 1B (PTP1B) at the endoplasmic reticulum. J. Biol. Chem. 2015, 290, 5725–5738. [Google Scholar] [CrossRef]
- Wong, H.N.; Ward, M.A.; Bell, A.W.; Chevet, E.; Bains, S.; Blackstock, W.P.; Solari, R.; Thomas, D.Y.; Bergeron, J.J. Conserved in vivo phosphorylation of calnexin at casein kinase II sites as well as a protein kinase C/proline-directed kinase site. J. Biol. Chem. 1998, 273, 17227–17235. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Schrag, J.D.; Bergeron, J.J.; Li, Y.; Borisova, S.; Hahn, M.; Thomas, D.Y.; Cygler, M. The Structure of calnexin, an ER chaper-one involved in quality control of protein folding. Mol. Cell 2001, 8, 633–644. [Google Scholar] [CrossRef]
- Trombetta, E.S.; Helenius, A. Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol. 1998, 8, 587–592. [Google Scholar] [CrossRef]
- Baksh, S.; Michalak, M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J. Biol. Chem. 1991, 266, 21458–21465. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.J.; Bergeron, J.J.; Li, Y.; Kang, C.Y.; Thomas, D.Y. Conformational changes induced in the endoplasmic reticulum lu-minal domain of calnexin by Mg-ATP and Ca2+. J. Biol. Chem. 1995, 270, 18051–18059. [Google Scholar] [CrossRef]
- Ihara, Y.; Cohen-Doyle, M.F.; Saito, Y.; Williams, D.B. Calnexin discriminates between protein conformational states and functions as a molecular chaperone in vitro. Mol. Cell 1999, 4, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.R.; Cohen-Doyle, M.F.; Thomas, D.Y.; Williams, D.B. Localization of the lectin, ERp57 binding, and polypeptide binding sites of calnexin and calreticulin. J. Biol. Chem. 2002, 277, 29686–29697. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Xu, Y.; Brenner, M.B. Retention of unassembled components of integral membrane proteins by calnexin. Science 1994, 263, 387–390. [Google Scholar] [CrossRef]
- Dallavilla, T.; Abrami, L.; Sandoz, P.A.; Savoglidis, G.; Hatzimanikatis, V.; van der Goot, F.G. Model-Driven Understanding of Palmitoylation Dynamics: Regulated Acylation of the Endoplasmic Reticulum Chaperone Calnexin. PLoS Comput. Biol. 2016, 12, e1004774. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- De Brito, O.M.; Scorrano, L. An intimate liaison: Spatial organization of the endoplasmic reticulum-mitochondria relation-ship. EMBO J. 2010, 29, 2715–2723. [Google Scholar] [CrossRef]
- Ou, W.J.; Thomas, D.Y.; Bell, A.W.; Bergeron, J.J. Casein kinase II phosphorylation of signal sequence receptor alpha and the associated membrane chaperone calnexin. J. Biol. Chem. 1992, 267, 23789–23796. [Google Scholar] [CrossRef]
- Nguyên, D.T.; Kebache, S.; Fazel, A.; Wong, H.N.; Jenna, S.; Emadali, A.; Lee, E.H.; Bergeron, J.J.; Kaufman, R.J.; Larose, L.; et al. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol. Biol. Cell 2004, 15, 4248–4260. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Yang, M.; Zeng, L.; Sun, L. PACS-2: A key regulator of mitochondria-associated membranes (MAMs). Pharmacol. Res. 2020, 160, 105080. [Google Scholar] [CrossRef] [PubMed]
- Bollo, M.; Paredes, R.M.; Holstein, D.; Zheleznova, N.; Camacho, P.; Lechleiter, J.D. Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS ONE 2010, 5, e11925. [Google Scholar] [CrossRef]
- Geiss-Friedlander, R.; Melchior, F. Concepts in sumoylation: A decade on. Nat. Rev. Mol. Cell Biol. 2007, 8, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Feldhammer, M.; Uetani, N.; Miranda-Saavedra, D.; Tremblay, M.L. PTP1B: A simple enzyme for a complex world. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 430–445. [Google Scholar] [CrossRef]
- Dudek, E.; Millott, R.; Liu, W.X.; Beauchamp, E.; Berthiaume, L.G.; Michalak, M. N-Myristoyltransferase 1 interacts with cal-nexin at the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2015, 468, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Hunegnaw, R.; Vassylyeva, M.; Dubrovsky, L.; Pushkarsky, T.; Sviridov, D.; Anashkina, A.A.; Üren, A.; Brichacek, B.; Vassylyev, D.G.; Adzhubei, A.A.; et al. Interaction Between HIV-1 Nef and Calnexin: From Modeling to Small Molecule In-hibitors Reversing HIV-Induced Lipid Accumulation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1758–1771. [Google Scholar] [CrossRef]
- Myrum, C.; Soulé, J.; Bittins, M.; Cavagnini, K.; Goff, K.; Ziemek, S.K.; Eriksen, M.S.; Patil, S.; Szum, A.; Nair, R.R.; et al. Arc Interacts with the Integral Endoplasmic Reticulum Protein, Calnexin. Front. Cell Neurosci. 2017, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Wang, J.; Groenendyk, J.; Lee, D.; Michalak, M.; Agellon, L.B. Fatty acid binding protein (Fabp) 5 interacts with the calnexin cytoplasmic domain at the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2017, 493, 202–206. [Google Scholar] [CrossRef]
- Jennelle, L.; Hunegnaw, R.; Dubrovsky, L.; Pushkarsky, T.; Fitzgerald, M.L.; Sviridov, D.; Popratiloff, A.; Brichacek, B.; Buk-rinsky, M. HIV-1 protein Nef inhibits activity of ATP-binding cassette transporter A1 by targeting endoplasmic reticulum chaperone calnexin. J. Biol. Chem. 2014, 289, 28870–28884. [Google Scholar] [CrossRef]
- Waheed, A.A.; Freed, E.O. Lipids and membrane microdomains in HIV-1 replication. Virus Res. 2009, 143, 162–176. [Google Scholar] [CrossRef]
- Jung, J.; Eggleton, P.; Robinson, A.; Wang, J.; Gutowski, N.; Holley, J.; Newcombe, J.; Dudek, E.; Paul, A.M.; Zochodne, D.; et al. Calnexin is necessary for T cell transmigration into the central nervous system. JCI Insight 2018, 3, 98410. [Google Scholar] [CrossRef] [PubMed]
- Rao, E.; Singh, P.; Li, Y.; Zhang, Y.; Chi, Y.I.; Suttles, J.; Li, B. Targeting epidermal fatty acid binding protein for treatment of experimental autoimmune encephalomyelitis. BMC Immunol. 2015, 16, 28. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskevicius, T.; Farraj, R.A.; Michalak, M.; Agellon, L.B. Calnexin, More Than Just a Molecular Chaperone. Cells 2023, 12, 403. https://doi.org/10.3390/cells12030403
Paskevicius T, Farraj RA, Michalak M, Agellon LB. Calnexin, More Than Just a Molecular Chaperone. Cells. 2023; 12(3):403. https://doi.org/10.3390/cells12030403
Chicago/Turabian StylePaskevicius, Tautvydas, Rabih Abou Farraj, Marek Michalak, and Luis B. Agellon. 2023. "Calnexin, More Than Just a Molecular Chaperone" Cells 12, no. 3: 403. https://doi.org/10.3390/cells12030403
APA StylePaskevicius, T., Farraj, R. A., Michalak, M., & Agellon, L. B. (2023). Calnexin, More Than Just a Molecular Chaperone. Cells, 12(3), 403. https://doi.org/10.3390/cells12030403






