Role of tPA in Corticosterone-Induced Apoptosis of Mouse Mural Granulosa and Oviductal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Recovery of Ovaries and Oviducts
2.2. Culture of MGCs and OECs
2.3. Hoechst 33342 Staining for Assessment of Cell Apoptosis
2.4. Flow Cytometry Assessment of Cell Apoptosis
2.5. Real-Time PCR
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) for tPA Protein
2.7. Cell Transfection with siRNAs
2.8. Data Analysis
3. Results
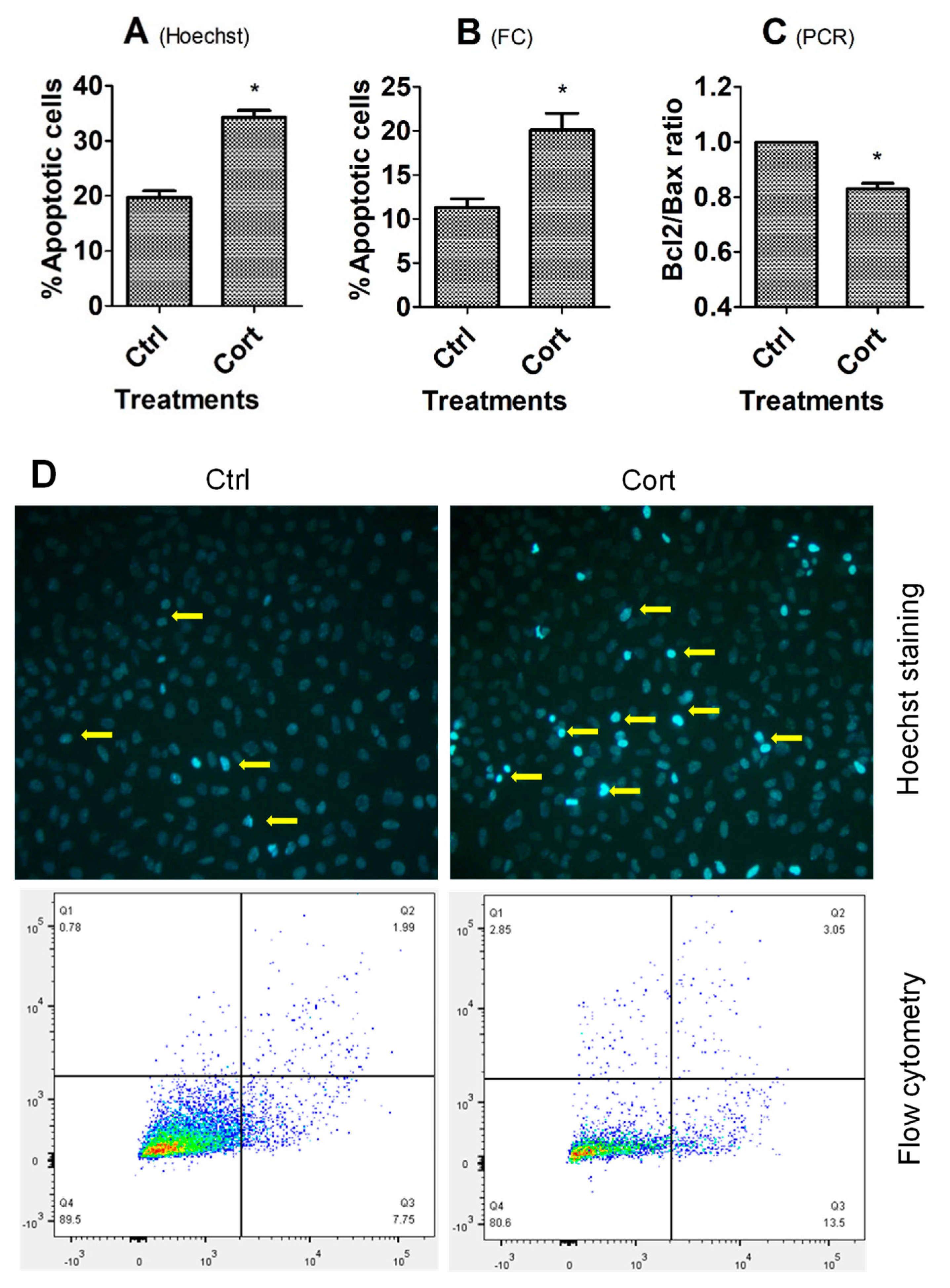
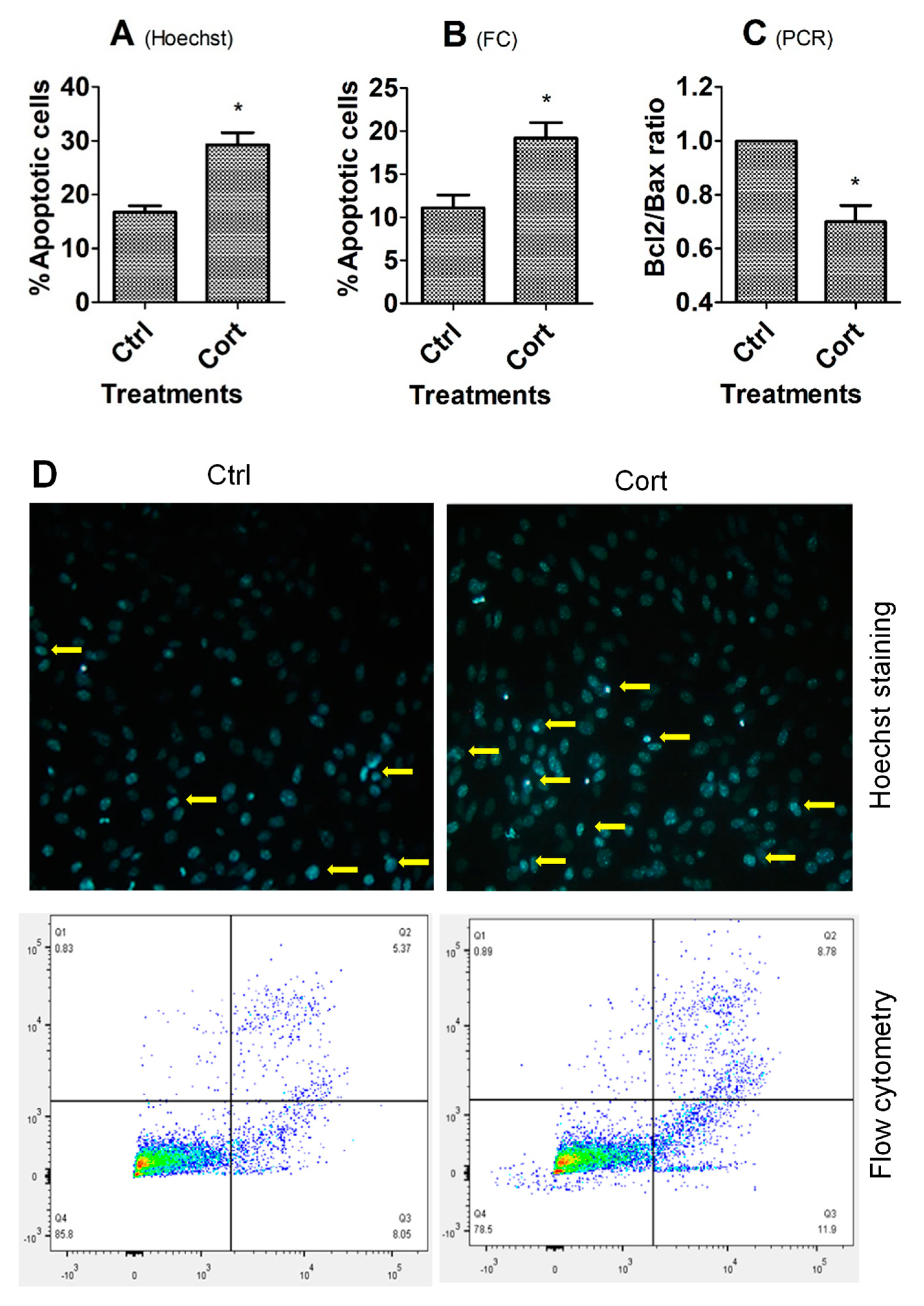
3.1. Culture with Corticosterone Increased Apoptosis of MGCs and OECs
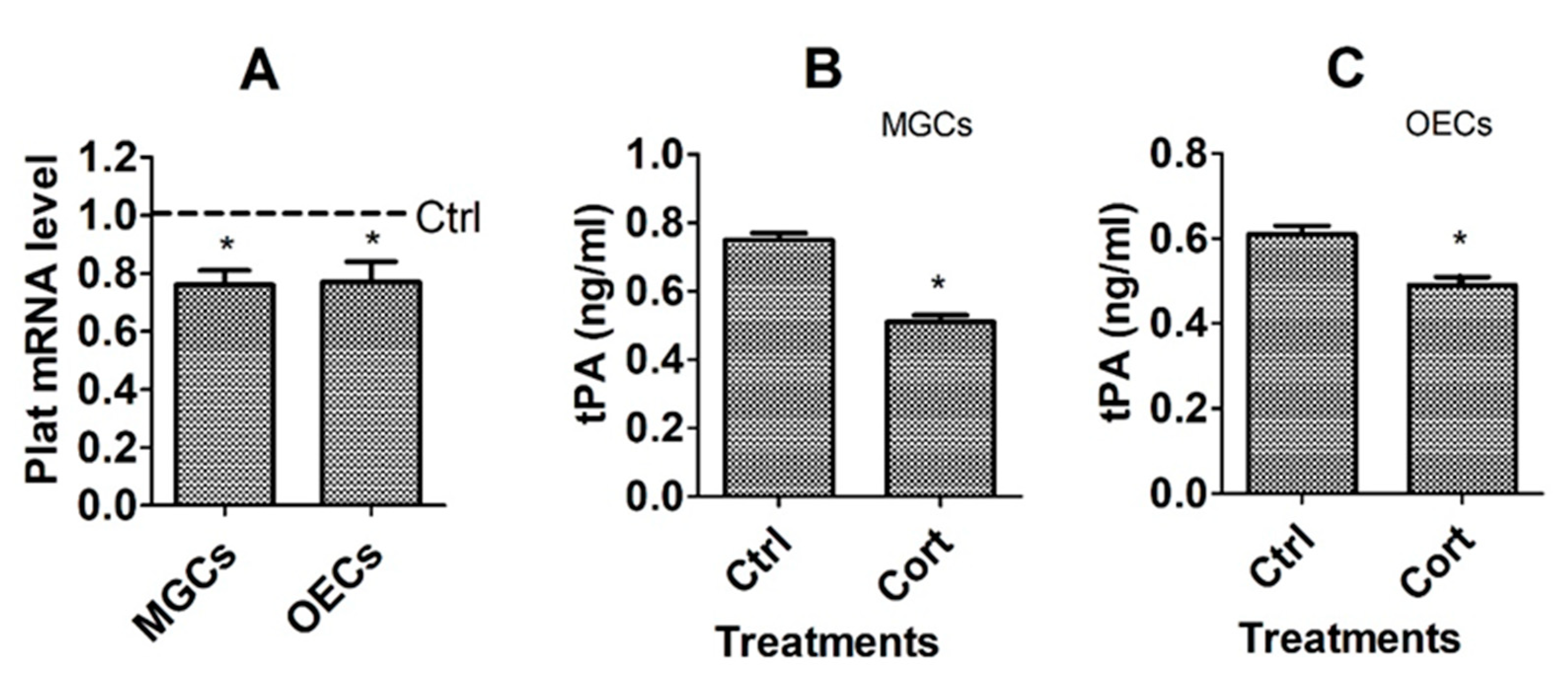
3.2. Culture with Corticosterone Decreased Expression of tPA Gene in MGCs and OECs
3.3. Culture with tPA Ameliorated Corticosterone-Induced Apoptosis of MGCs and OECs
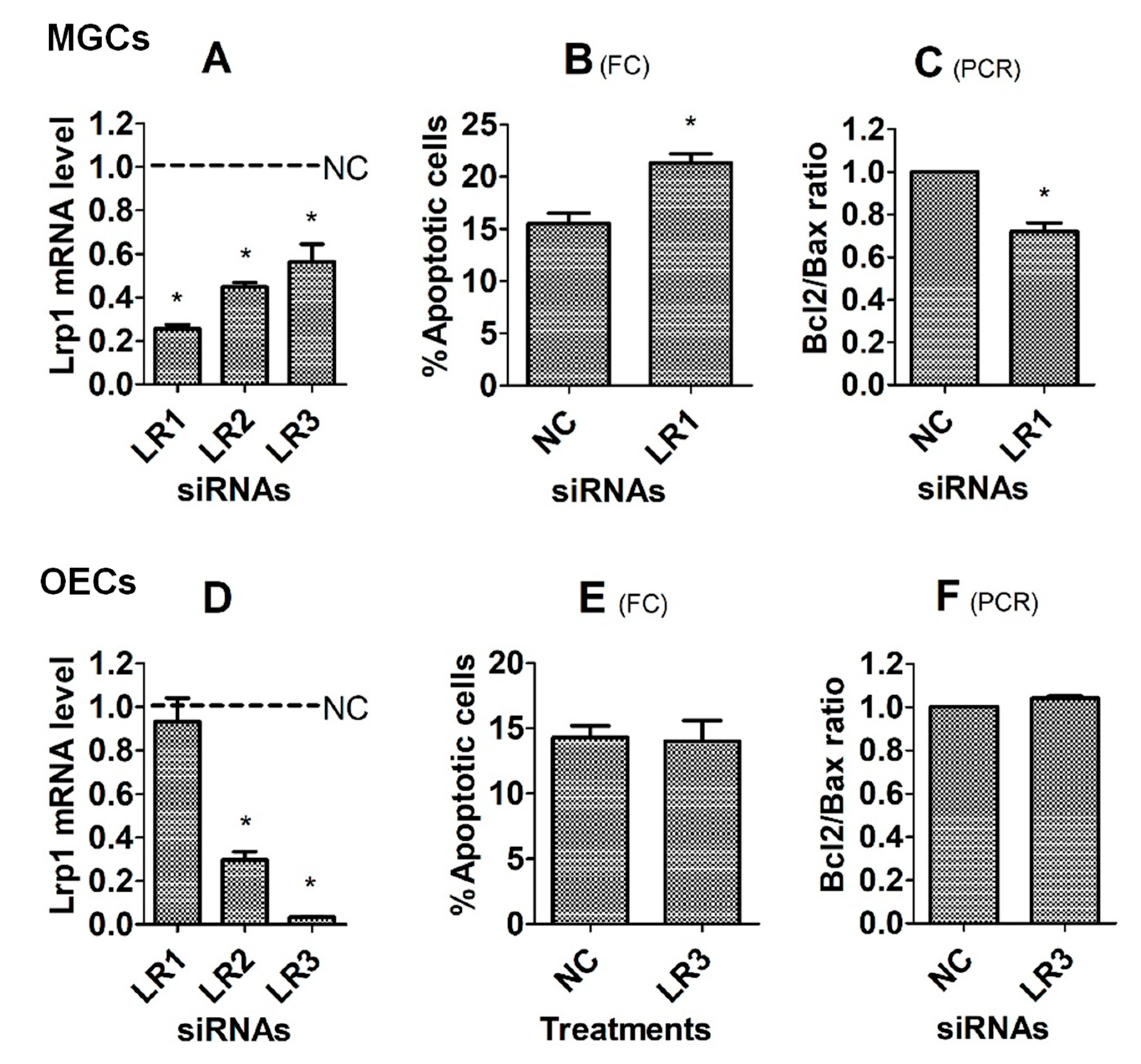
3.4. Effects of Knocking Down the Lrp1 Gene on Corticosterone-Induced Apoptosis of MGCs and OECs
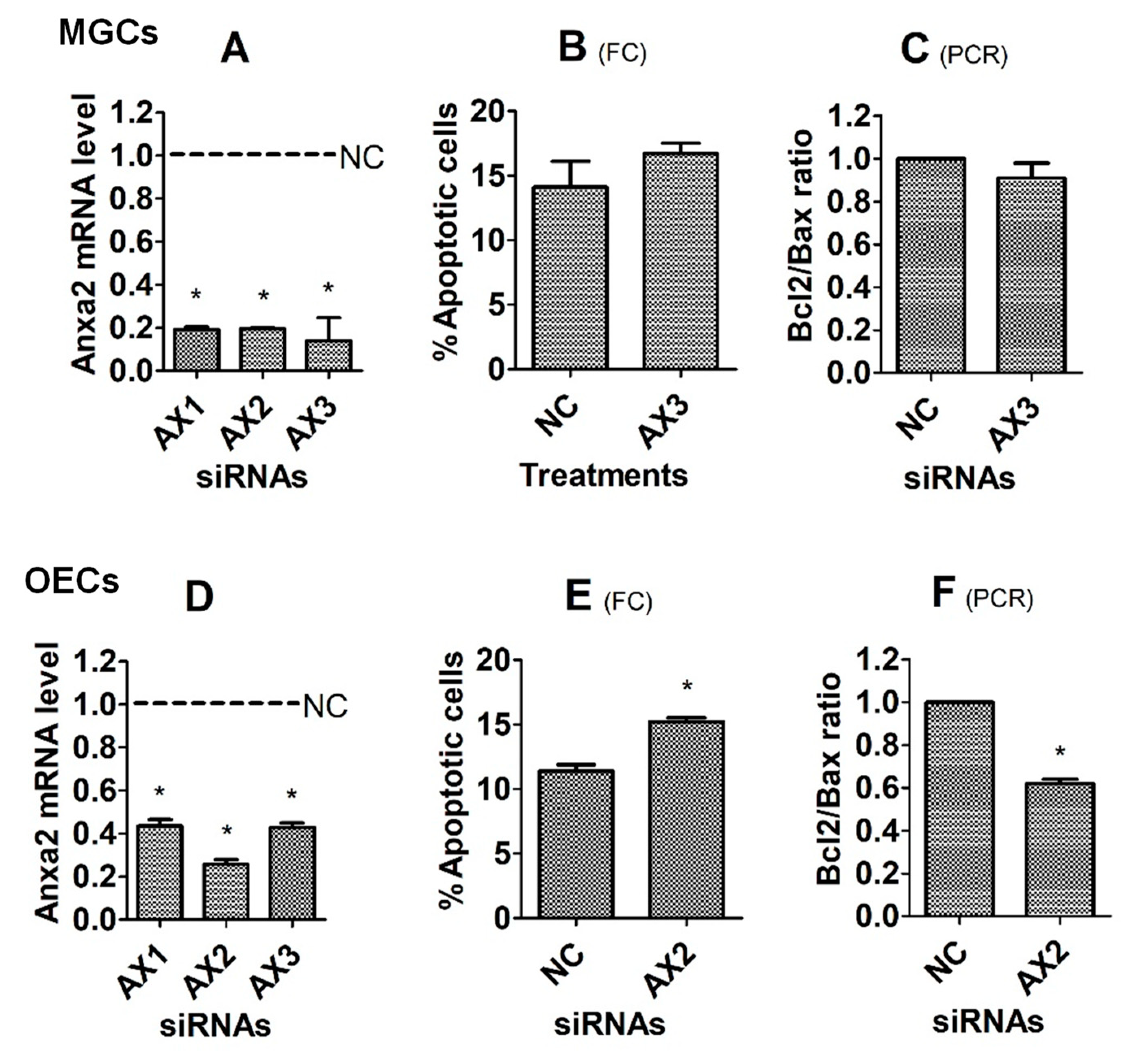
3.5. Effects of Knocking Down the Anxa2 Gene on Corticosterone-Induced Apoptosis of MGCs and OECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, S.Y.; Wang, J.Z.; Li, J.J.; Wei, D.L.; Sui, H.S.; Zhang, Z.H.; Zhou, P.; Tan, J.H. Maternal restraint stress diminishes the developmental potential of oocytes. Biol. Reprod. 2011, 84, 672–681. [Google Scholar] [CrossRef]
- Zheng, L.L.; Tan, X.W.; Cui, X.Z.; Yuan, H.J.; Li, H.; Jiao, G.Z.; Ji, C.L.; Tan, J.H. Preimplantation maternal stress impairs embryo development by inducing oviductal apoptosis with activation of the Fas system. Mol. Hum. Reprod. 2016, 22, 778–790. [Google Scholar] [CrossRef]
- Liang, B.; Wei, D.L.; Cheng, Y.N.; Yuan, H.J.; Lin, J.; Cui, X.Z.; Luo, M.J.; Tan, J.H. Restraint stress impairs oocyte developmental potential in mice: Role of CRH-induced apoptosis of ovarian cells. Biol. Reprod. 2013, 89, 64. [Google Scholar] [CrossRef]
- Yuan, H.J.; Han, X.; He, N.; Wang, G.L.; Gong, S.; Lin, J.; Gao, M.; Tan, J.H. Glucocorticoids impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the Fas system. Sci. Rep. 2016, 6, 24036. [Google Scholar] [CrossRef]
- Yuan, H.J.; Li, Z.B.; Zhao, X.Y.; Sun, G.Y.; Wang, G.L.; Zhao, Y.Q.; Zhang, M.; Tan, J.H. Glucocorticoids impair oocyte competence and trigger apoptosis of ovarian cells via activating the TNF-alpha system. Reproduction 2020, 160, 129–140. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, Z.B.; Kong, Q.Q.; Han, X.; Xiao, B.; Li, X.; Chang, Z.L.; Tan, J.H. Restraint-induced corticotrophin-releasing hormone elevation triggers apoptosis of ovarian cells and impairs oocyte competence via activation of the Fas/FasL system. Biol. Reprod. 2018, 99, 828–837. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, Z.B.; Yuan, H.J.; Han, X.; Wu, J.S.; Feng, X.Y.; Zhang, M.; Tan, J.H. Restraint stress and elevation of corticotrophin-releasing hormone in female mice impair oocyte competence through activation of the tumour necrosis factor alpha (TNF-alpha) system. Reprod. Fertil. Dev. 2020, 32, 862–872. [Google Scholar] [CrossRef]
- Tan, X.W.; Ji, C.L.; Zheng, L.L.; Zhang, J.; Yuan, H.J.; Gong, S.; Zhu, J.; Tan, J.H. Corticotrophin-releasing hormone and corticosterone impair development of preimplantation embryos by inducing oviductal cell apoptosis via activating the Fas system: An in vitro study. Hum. Reprod. 2017, 32, 1583–1597. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Chen, R.R.; Kong, Q.Q.; An, J.S.; Zhao, X.Y.; Gong, S.; Yuan, H.J.; Tan, J.H. Corticosterone induced apoptosis of mouse oviduct epithelial cells independent of the TNF-alpha system. J. Reprod. Dev. 2021, 67, 43–51. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Gomez, C.F.; de Ridder, G.G.; Ray, R.; Pizzo, S.V. Binding of tissue-type plasminogen activator to the glucose-regulated protein 78 (GRP78) modulates plasminogen activation and promotes human neuroblastoma cell proliferation in vitro. J. Biol. Chem. 2014, 289, 25166–25176. [Google Scholar] [CrossRef]
- Kumada, M.; Niwa, M.; Hara, A.; Matsuno, H.; Mori, H.; Ueshima, S.; Matsuo, O.; Yamamoto, T.; Kozawa, O. Tissue type plasminogen activator facilitates NMDA-receptor-mediated retinal apoptosis through an independent fibrinolytic cascade. Investig. Opthalmol. Vis. Sci. 2005, 46, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, C.; Sun, K. Induction of Amnion Epithelial Apoptosis by Cortisol via tPA/Plasmin System. Endocrinology 2016, 157, 4487–4498. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Lin, L.; Tan, X.; Yang, J.; Bu, G.; Mars, W.M.; Liu, Y. tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J. Am. Soc. Nephrol. 2008, 19, 503–514. [Google Scholar] [CrossRef]
- Liang, C.; Ding, M.; Du, F.; Cang, J.; Xue, Z. Tissue plasminogen activator (tPA) attenuates propofol-induced apoptosis in developing hippocampal neurons. Springerplus 2016, 5, 475. [Google Scholar] [CrossRef]
- Chevilley, A.; Lesept, F.; Lenoir, S.; Ali, C.; Parcq, J.; Vivien, D. Impacts of tissue-type plasminogen activator (tPA) on neuronal survival. Front. Cell. Neurosci. 2015, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X. Plasminogen activator/plasminogen activator inhibitors in ovarian physiology. Front. Biosci. 2004, 9, 3356–3373. [Google Scholar] [CrossRef]
- Carroll, P.M.; Richards, W.G.; Darrow, A.L.; Wells, J.M.; Strickland, S. Preimplantation mouse embryos express a cell surface receptor for tissue-plasminogen activator. Development 1993, 119, 191–198. [Google Scholar] [CrossRef]
- Roldan-Olarte, M.; Garcia, D.C.; Jimenez-Diaz, M.; Valdecantos, P.A.; Miceli, D.C. In vivo and in vitro expression of the plasminogen activators and urokinase type plasminogen activator receptor (u-PAR) in the pig oviduct. Anim. Reprod. Sci. 2012, 136, 90–99. [Google Scholar] [CrossRef]
- Redlitz, A.; Plow, E.F. Receptors for plasminogen and t-PA: An update. Baillieres. Clin. Haematol. 1995, 8, 313–327. [Google Scholar] [CrossRef]
- Zhuo, M.; Holtzman, D.M.; Li, Y.; Osaka, H.; DeMaro, J.; Jacquin, M.; Bu, G. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J. Neurosci. 2000, 20, 542–549. [Google Scholar] [CrossRef]
- Siao, C.J.; Tsirka, S.E. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J. Neurosci. 2002, 22, 3352–3358. [Google Scholar] [CrossRef] [PubMed]
- Gveric, D.; Herrera, B.M.; Cuzner, M.L. tPA receptors and the fibrinolytic response in multiple sclerosis lesions. Am. J. Pathol. 2005, 166, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.D.; Carletti, M.Z.; Hong, X.; Christenson, L.K. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol. Reprod. 2008, 79, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Carletti, M.Z.; Christenson, L.K. Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction 2009, 137, 843–855. [Google Scholar] [CrossRef]
- Eberhardt, W.; Engels, C.; Muller, R.; Pfeilschifter, J. Mechanisms of dexamethasone-mediated inhibition of cAMP-induced tPA expression in rat mesangial cells. Kidney Int. 2002, 62, 809–821. [Google Scholar] [CrossRef]
- Kwon, K.J.; Cho, K.S.; Lee, S.H.; Kim, J.N.; Joo, S.H.; Ryu, J.H.; Ignarro, L.J.; Han, S.H.; Shin, C.Y. Regulation of tissue plasminogen activator/plasminogen activator inhibitor-1 by hydrocortisone in rat primary astrocytes. J. Neurosci. Res. 2011, 89, 1059–1069. [Google Scholar] [CrossRef]
- Kathju, S.; Heaton, J.H.; Bruzdzinski, C.J.; Gelehrter, T.D. Synergistic induction of tissue-type plasminogen activator gene expression by glucocorticoids and cyclic nucleotides in rat HTC hepatoma cells. Endocrinology 1994, 135, 1195–1204. [Google Scholar] [CrossRef]
- Liot, G.; Roussel, B.D.; Lebeurrier, N.; Benchenane, K.; Lopez-Atalaya, J.P.; Vivien, D.; Ali, C. Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J. Neurochem. 2006, 98, 1458–1464. [Google Scholar] [CrossRef]
- Yoeruek, E.; Spitzer, M.S.; Tatar, O.; Biedermann, T.; Grisanti, S.; Luke, M.; Bartz-Schmidt, K.U.; Szurman, P. Toxic effects of recombinant tissue plasminogen activator on cultured human corneal endothelial cells. Investig. Opthalmol. Vis. Sci. 2008, 49, 1392–1397. [Google Scholar] [CrossRef]
- Bertrand, T.; Lesept, F.; Chevilley, A.; Lenoir, S.; Aimable, M.; Briens, A.; Hommet, Y.; Bardou, I.; Parcq, J.; Vivien, D. Conformations of tissue plasminogen activator (tPA) orchestrate neuronal survival by a crosstalk between EGFR and NMDAR. Cell Death Dis. 2015, 6, e1924. [Google Scholar] [CrossRef]
- Bao, H.; Jiang, M.; Zhu, M.; Sheng, F.; Ruan, J.; Ruan, C. Overexpression of Annexin II affects the proliferation, apoptosis, invasion and production of proangiogenic factors in multiple myeloma. Int. J. Hematol. 2009, 90, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liang, L.; Qin, T.; Xiao, S.; Liang, R. Encephalomyocarditis Virus 2A Protein Inhibited Apoptosis by Interaction with Annexin A2 through JNK/c-Jun Pathway. Viruses 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Campenot, R.B.; Vance, D.E.; Vance, J.E. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 2007, 27, 1933–1941. [Google Scholar] [CrossRef]
- Yan, C.; Yu, H.; Liu, Y.; Wu, P.; Wang, C.; Zhao, H.; Yang, K.; Shao, Q.; Zhong, Y.; Zhao, W.; et al. c-Abl Tyrosine Kinase-Mediated Neuronal Apoptosis in Subarachnoid Hemorrhage by Modulating the LRP-1-Dependent Akt/GSK3beta Survival Pathway. J. Mol. Neurosci. 2021, 71, 2514–2525. [Google Scholar] [CrossRef]
- Sharma, M.; Blackman, M.R.; Sharma, M.C. Antibody-directed neutralization of annexin II (ANX II) inhibits neoangiogenesis and human breast tumor growth in a xenograft model. Exp. Mol. Pathol. 2012, 92, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Wu, S.C.; Tian, X.C.; Barber, M.; Hoagland, T.; Riesen, J.; Lee, K.H.; Tu, C.F.; Cheng, W.T.; Yang, X. Production of cloned pigs by whole-cell intracytoplasmic microinjection. Biol. Reprod. 2003, 69, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Samiec, M.; Skrzyszowska, M. Molecular conditions of the cell nucleus remodelling/reprogramming process and nuclear transferred embryo development in the intraooplasmic karyoplast injection technique: A review. Czech J. Anim. Sci. 2005, 50, 185–195. [Google Scholar] [CrossRef]
- Miyashita, N.; Kubo, Y.; Yonai, M.; Kaneyama, K.; Saito, N.; Sawai, K.; Minamihashi, A.; Suzuki, T.; Kojima, T.; Nagai, T. Cloned cows with short telomeres deliver healthy offspring with normal-length telomeres. J. Reprod. Dev. 2011, 57, 636–642. [Google Scholar] [CrossRef]
- Skrzyszowska, M.; Samiec, M. Generating Cloned Goats by Somatic Cell Nuclear Transfer-Molecular Determinants and Application to Transgenics and Biomedicine. Int. J. Mol. Sci. 2021, 22, 7490. [Google Scholar] [CrossRef]
- Shahzad, Q.; Xu, H.Y.; Pu, L.; Waqas, M.; Wadood, A.A.; Xie, L.; Lu, K.H.; Liang, X.; Lu, Y. Developmental potential of buffalo embryos cultured in serum free culture system. Theriogenology 2020, 149, 38–45. [Google Scholar] [CrossRef]
- Nicacio, A.C.; Simões, R.; de Paula-Lopes, F.F.; de Barros, F.R.; Peres, M.A.; Assumpção, M.E.; Visintin, J.A. Effects of different cryopreservation methods on post-thaw culture conditions of in vitro produced bovine embryos. Zygote 2012, 20, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, N.; Kunj, N.; Tiwari, S.; Saraiya, M.; Majumdar, S.S. Optimization of embryo culture conditions for increasing efficiency of cloning in buffalo (Bubalus bubalis) and generation of transgenic embryos via cloning. Cloning Stem Cells 2009, 11, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fabjan, J.M.G.; Batista, R.I.T.P.; Freitas, V.J.F.; Mermillod, P. In Vitro Culture of Embryos from LOPU-Derived Goat Oocytes. Methods Mol. Biol. 2019, 2006, 141–153. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences (5′—3′) | Amplified Product Size (bp) |
|---|---|---|
| Plat | F: ACCGAAAGCTGACGTGGGAA | 128 |
| R: CTGCCAAGGGTGTGAGGTGA | ||
| Bcl-2 | F: TTCGGGATGGAGTAAACTGG | 157 |
| R: TGGATCCAAGGCTCTAGGTG | ||
| Bax | F: TGCAGAGGATGATTGCTGAC | 173 |
| R: GATCAGCTCGGGCACTTTAG | ||
| Lrp1 | F: GACGAGTGTTCCGTGTATGG | 384 |
| R: CAGGCTGAGGGAGATGTTG | ||
| Anxa2 | F: CACGAAATCCTGTGCAAGCTC | 293 |
| R: AGGCCCAAAATCACCGTCTC | ||
| Gapdh | F: AAACCRGCCAAGTATGATGA | 244 |
| R: GTGGTCCAGGGTTTCTTACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Q.; Cheng, H.; Yang, Y.-Q.; An, J.-S.; Zhang, M.; Gong, S.; Luo, M.-J.; Tan, J.-H. Role of tPA in Corticosterone-Induced Apoptosis of Mouse Mural Granulosa and Oviductal Epithelial Cells. Cells 2023, 12, 455. https://doi.org/10.3390/cells12030455
Hua Q, Cheng H, Yang Y-Q, An J-S, Zhang M, Gong S, Luo M-J, Tan J-H. Role of tPA in Corticosterone-Induced Apoptosis of Mouse Mural Granulosa and Oviductal Epithelial Cells. Cells. 2023; 12(3):455. https://doi.org/10.3390/cells12030455
Chicago/Turabian StyleHua, Qi, Hao Cheng, Yong-Qing Yang, Jin-Song An, Min Zhang, Shuai Gong, Ming-Jiu Luo, and Jing-He Tan. 2023. "Role of tPA in Corticosterone-Induced Apoptosis of Mouse Mural Granulosa and Oviductal Epithelial Cells" Cells 12, no. 3: 455. https://doi.org/10.3390/cells12030455
APA StyleHua, Q., Cheng, H., Yang, Y.-Q., An, J.-S., Zhang, M., Gong, S., Luo, M.-J., & Tan, J.-H. (2023). Role of tPA in Corticosterone-Induced Apoptosis of Mouse Mural Granulosa and Oviductal Epithelial Cells. Cells, 12(3), 455. https://doi.org/10.3390/cells12030455





