An Inverse Agonist of Estrogen-Related Receptor Gamma, GSK5182, Enhances Na+/I− Symporter Function in Radioiodine-Refractory Papillary Thyroid Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Chemicals, and Antibodies
2.2. Radioiodine Uptake Reporter Gene Assay
2.3. F18-FDG Uptake Assay
2.4. Clonogenic Assay
2.5. Western Blot
2.6. Quantitative RT-PCR
2.7. Statistical Analysis
3. Results
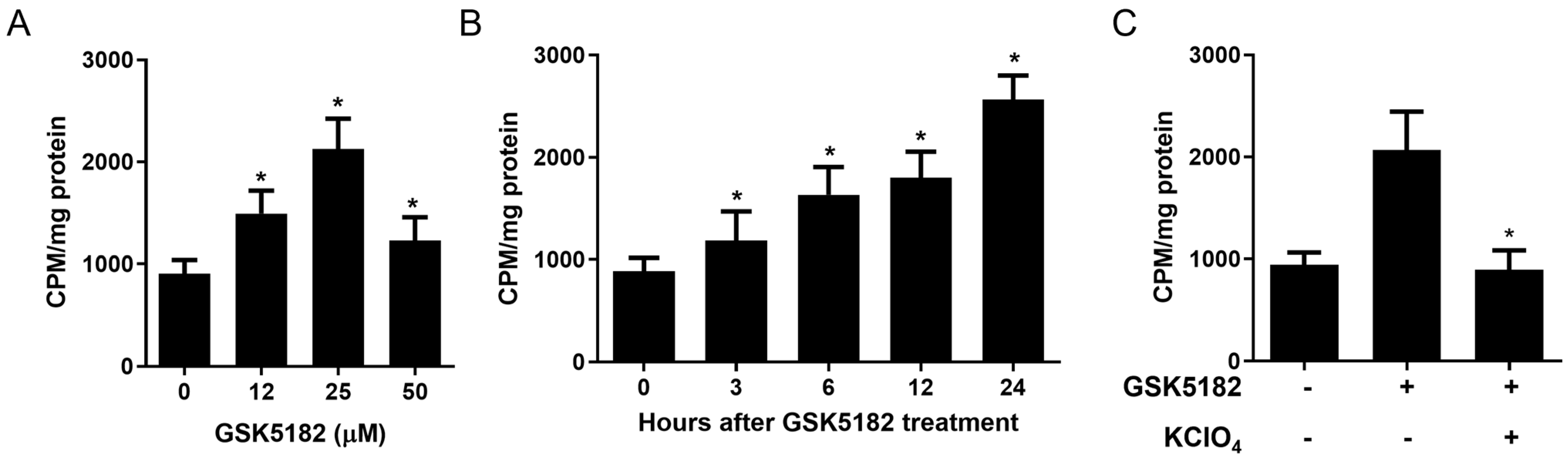
3.1. GSK5182 Enhanced Radioiodine Uptake in RAI-Refractory PTC Cells
3.2. Effects of GSK5182 on ERRγ and Iodide Handing Gene Expression in RAI-Refractory PTC Cells
3.3. Effects of Mitogen-Activated Protein (MAP) Kinase Signaling on GSK5182-Induced Radioiodine Uptake in RAI-Refractory PTC Cells
3.4. Effects of GSK5182 on Glucose Metabolism in RAI-Refractory PTC Cells
3.5. Restoration of Radioiodine Therapy Responsiveness in RAI-Refractory PTC Cells by GSK5182
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, J.-K. Sodium iodide symporter: Its role in nuclear medicine. J. Nucl. Med. 2002, 43, 1188–1200. [Google Scholar] [PubMed]
- Rosai, J. Poorly differentiated thyroid carcinoma: Introduction to the issue, its landmarks, and clinical impact. Endocr. Pathol. 2004, 15, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Gao, M.; Zhao, C.; Pan, Y.; Li, H.; Li, J.; Li, X. Low expression of sodium iodide symporter expression in aggressive variants of papillary thyroid carcinoma. Int. J. Clin. Oncol. 2014, 19, 800–804. [Google Scholar] [CrossRef]
- Galrao, A.L.; Camargo, R.Y.; Friguglietti, C.U.; Moraes, L.; Cerutti, J.M.; Serrano-Nascimento, C.; Suzuki, M.F.; Medeiros-Neto, G.; Rubio, I.G. Hypermethylation of a New Distal Sodium/Iodide Symporter (NIS) enhancer (NDE) is associated with reduced NIS expression in thyroid tumors. J. Clin. Endocrinol. Metab. 2014, 99, E944–E952. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.; Wojcicka, A.; Kotlarek, M.; Zhang, X.; Jazdzewski, K.; Jhiang, S.M. microRNA-339-5p modulates Na+/I− symporter-mediated radioiodide uptake. Endocr.-Relat. Cancer 2015, 22, 11–21. [Google Scholar] [CrossRef]
- Kogai, T.; Taki, K.; Brent, G. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr.-Relat. Cancer 2006, 13, 797–826. [Google Scholar] [CrossRef]
- Fenton, M.S.; Marion, K.M.; Salem, A.K.; Hogen, R.; Naeim, F.; Hershman, J.M. Sunitinib inhibits MEK/ERK and SAPK/JNK pathways and increases sodium/iodide symporter expression in papillary thyroid cancer. Thyroid 2010, 20, 965–974. [Google Scholar] [CrossRef]
- Kogai, T.; Sajid-Crockett, S.; Newmarch, L.S.; Liu, Y.-Y.; Brent, G.A. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J. Endocrinol. 2008, 199, 500. [Google Scholar] [CrossRef]
- Shang, H.; Zhao, J.; Yao, J.; Wang, H.; Dong, J.; Liao, L. Nevirapine increases sodium/iodide symporter-mediated radioiodide uptake by activation of TSHR/cAMP/CREB/PAX8 signaling pathway in dedifferentiated thyroid cancer. Front. Oncol. 2020, 10, 404. [Google Scholar] [CrossRef]
- Gao, X.; Wu, X.; Zhang, X.; Hua, W.; Zhang, Y.; Maimaiti, Y.; Gao, Z.; Zhang, Y. Inhibition of BRD4 suppresses tumor growth and enhances iodine uptake in thyroid cancer. Biochem. Biophys. Res. Commun. 2016, 469, 679–685. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Heinhuis, B.; Gerrits, D.; Netea, M.G.; Joosten, L.A.; Hermus, A.R.; Oyen, W.J.; Schweppe, R.E.; Haugen, B.R.; Boerman, O.C. mTOR Inhibition promotes TTF1-dependent redifferentiation and restores iodine uptake in thyroid carcinoma cell lines. J. Clin. Endocrinol. Metab. 2014, 99, E1368–E1375. [Google Scholar] [CrossRef] [PubMed]
- Giguére, V. To ERR in the estrogen pathway. Trends Endocrinol. Metab. 2002, 13, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Heard, D.J.; Norby, P.L.; Holloway, J.; Vissing, H. Human ERRγ, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: Tissue-specific isoforms are expressed during development and in the adult. Mol. Endocrinol. 2000, 14, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Choi, Y.-K.; Do, J.-Y.; Choi, Y.-K.; Ha, C.-M.; Lee, S.J.; Jeon, J.-H.; Lee, W.-K.; Choi, H.-S.; Park, K.-G. Estrogen-related receptor γ plays a key role in vascular calcification through the upregulation of BMP2 expression. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, D.-K.; Lee, J.-M.; Park, S.B.; Jeong, W.-I.; Kim, S.H.; Lee, I.-K.; Lee, C.-H.; Chiang, J.Y.; Choi, H.-S. Orphan nuclear receptor oestrogen-related receptor γ (ERRγ) plays a key role in hepatic cannabinoid receptor type 1-mediated induction of CYP7A1 gene expression. Biochem. J. 2015, 470, 181–193. [Google Scholar] [CrossRef]
- Kim, D.K.; Gang, G.T.; Ryu, D.; Koh, M.; Kim, Y.N.; Kim, S.S.; Park, J.; Kim, Y.H.; Sim, T.; Lee, I.K.; et al. Inverse agonist of nuclear receptor ERRgamma mediates antidiabetic effect through inhibition of hepatic gluconeogenesis. Diabetes 2013, 62, 3093–3102. [Google Scholar] [CrossRef]
- Kim, D.K.; Ryu, D.; Koh, M.; Lee, M.W.; Lim, D.; Kim, M.J.; Kim, Y.H.; Cho, W.J.; Lee, C.H.; Park, S.B.; et al. Orphan nuclear receptor estrogen-related receptor gamma (ERRgamma) is key regulator of hepatic gluconeogenesis. J. Biol. Chem. 2012, 287, 21628–21639. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.R.; Koh, M.; Kim, Y.D.; Lee, J.M.; Chanda, D.; Park, S.B.; Min, J.J.; Lee, C.H.; Park, T.S.; et al. Estrogen-related receptor gamma (ERRgamma) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J. Biol. Chem. 2011, 286, 38035–38042. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeong, J.H.; Lee, J.M.; Kim, K.S.; Park, S.H.; Kim, Y.D.; Koh, M.; Shin, M.; Jung, Y.S.; Kim, H.S.; et al. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat. Med. 2014, 20, 419–424. [Google Scholar] [CrossRef]
- Singh, T.D.; Jeong, S.Y.; Lee, S.-W.; Ha, J.-H.; Lee, I.-K.; Kim, S.H.; Kim, J.; Cho, S.J.; Ahn, B.-C.; Lee, J. Inverse agonist of estrogen-related receptor γ enhances sodium iodide symporter function through mitogen-activated protein kinase signaling in anaplastic thyroid cancer cells. J. Nucl. Med. 2015, 56, 1690–1696. [Google Scholar] [CrossRef]
- Chen, K.; Wallis, J.W.; McLellan, M.D.; Larson, D.E.; Kalicki, J.M.; Pohl, C.S.; McGrath, S.D.; Wendl, M.C.; Zhang, Q.; Locke, D.P. BreakDancer: An algorithm for high-resolution mapping of genomic structural variation. Nat. Methods 2009, 6, 677–681. [Google Scholar] [CrossRef]
- Schmutzler, C.; Koehrle, J. Innovative strategies for the treatment of thyroid cancer. Eur. J. Endocrinol 2000, 143, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Vivaldi, A.; Miasaki, F.; Ciampi, R.; Agate, L.; Collecchi, P.; Capodanno, A.; Pinchera, A.; Elisei, R. Re-differentiation of thyroid carcinoma cell lines treated with 5-Aza-2′-deoxycytidine and retinoic acid. Mol. Cell. Endocrinol. 2009, 307, 142–148. [Google Scholar] [CrossRef]
- Hay, I.D.; Thompson, G.B.; Grant, C.S.; Bergstralh, E.J.; Dvorak, C.E.; Gorman, C.A.; Maurer, M.S.; McIver, B.; Mullan, B.P.; Oberg, A.L. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): Temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J.Surg. 2002, 26, 879–885. [Google Scholar] [CrossRef]
- Aashiq, M.; Silverman, D.A.; Na’ara, S.; Takahashi, H.; Amit, M. Radioiodine-refractory thyroid cancer: Molecular basis of redifferentiation therapies, management, and novel therapies. Cancers 2019, 11, 1382. [Google Scholar] [CrossRef]
- Oh, J.M.; Ahn, B.-C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics 2021, 11, 6251. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Blakely, R.D. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science 1999, 285, 763–766. [Google Scholar] [CrossRef]
- Buffet, C.; Wassermann, J.; Hecht, F.; Leenhardt, L.; Dupuy, C.; Groussin, L.; Lussey-Lepoutre, C. Redifferentiation of radioiodine-refractory thyroid cancers. Endocr. Relat. Cancer 2020, 27, R113–R132. [Google Scholar] [CrossRef]






| Primary Antibodies | Secondary Antibodies | ||||||
|---|---|---|---|---|---|---|---|
| Name | Dilution Factor | Catalog Number | Manufacturer | Name | Dilution Factor | Catalog Number | Manufacturer |
| NIS | 1:4000 | MS-1653 | Thermo Fisher | Anti-Mouse IgG, HRP-linked Antibody | 1:8000 | 402B | PROMEGA |
| TTF-1 | 1:1000 | SC-53136 | Santa Cruz | Anti-Mouse IgG, HRP-linked Antibody | 1:2000 | 402B | PROMEGA |
| PAX-8 | 1:1000 | SC-81353 | Santa Cruz | Anti-Mouse IgG, HRP-linked Antibody | 1:2000 | 402B | PROMEGA |
| Thyroperoxidase (TPO) | 1:1000 | SC-58432 | Santa Cruz | Anti-Mouse IgG, HRP-linked Antibody | 1:2000 | 402B | PROMEGA |
| TSH-receptor (TSHR) | 1:1000 | SC-58432 | Santa Cruz | Anti-Mouse IgG, HRP-linked Antibody | 1:2000 | 402B | PROMEGA |
| ERRγ | 1:1000 | PP-H6812-00 | R&D system | Anti-mouse IgG Isotype, HRP-linked Antibody | 1:2000 | 402B | PROMEGA |
| Phospho-p44/42 MAPK (Erk1/2) | 1:1000 | 4377 | Cell Signaling | Anti-Rabbit IgG, HRP-linked Antibody | 1:10,000 | 401B | PROMEGA |
| GLUT-1 | 1:1000 | sc-377228 | Santa Cruz | Anti-Mouse IgG, HRP-linked Antibody | 1:10,000 | 401B | PROMEGA |
| GLUT-4 | 1:1000 | ab33780 | Abcam | Anti-Rabbit IgG, HRP-linked Antibody | 1:10,000 | 401B | PROMEGA |
| β-actin | 1:5000 | ab8227 | Abcam | Anti-Rabbit HRP-linked Antibody | 1:000 | 401B | PROMEGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, T.D.; Lee, J.E.; Son, K.H.; Lee, B.R.; Kim, S.K.; Gulwani, D.; Sarangthem, V.; Jeon, Y.H. An Inverse Agonist of Estrogen-Related Receptor Gamma, GSK5182, Enhances Na+/I− Symporter Function in Radioiodine-Refractory Papillary Thyroid Cancer Cells. Cells 2023, 12, 470. https://doi.org/10.3390/cells12030470
Singh TD, Lee JE, Son KH, Lee BR, Kim SK, Gulwani D, Sarangthem V, Jeon YH. An Inverse Agonist of Estrogen-Related Receptor Gamma, GSK5182, Enhances Na+/I− Symporter Function in Radioiodine-Refractory Papillary Thyroid Cancer Cells. Cells. 2023; 12(3):470. https://doi.org/10.3390/cells12030470
Chicago/Turabian StyleSingh, Thoudam Debraj, Jae Eon Lee, Kwang Hee Son, Bo Ra Lee, Sang Kyoon Kim, Deepak Gulwani, Vijaya Sarangthem, and Yong Hyun Jeon. 2023. "An Inverse Agonist of Estrogen-Related Receptor Gamma, GSK5182, Enhances Na+/I− Symporter Function in Radioiodine-Refractory Papillary Thyroid Cancer Cells" Cells 12, no. 3: 470. https://doi.org/10.3390/cells12030470
APA StyleSingh, T. D., Lee, J. E., Son, K. H., Lee, B. R., Kim, S. K., Gulwani, D., Sarangthem, V., & Jeon, Y. H. (2023). An Inverse Agonist of Estrogen-Related Receptor Gamma, GSK5182, Enhances Na+/I− Symporter Function in Radioiodine-Refractory Papillary Thyroid Cancer Cells. Cells, 12(3), 470. https://doi.org/10.3390/cells12030470






