CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD2
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Depilation-Induced Hair Cycle Progression
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. Immunohistochemistry (IHC)
2.5. Immunoblotting
2.6. Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
2.7. Cell Culture
2.8. Immunocytochemistry
2.9. In Vivo Hair Regrowth Test
2.10. Wound-Induced Hair Follicle Neogenesis Assay
2.11. Ex Vivo Vibrissa Follicle Culture
2.12. Alkaline Phosphatase Staining
2.13. Statistical Analysis
3. Results
3.1. The Expression Patterns of Ptgds Correlate and Inversely Correlate with Those of Cxxc5 and Β-Catenin, Respectively
3.2. PGD2 Suppresses Hair Growth via CXXC5
3.3. Blockade of CXXC5 Function Recovers Hair Growth Suppressed by PGD2
3.4. Inhibition of CXXC5 Function Restores WIHN Reduced by PGD2
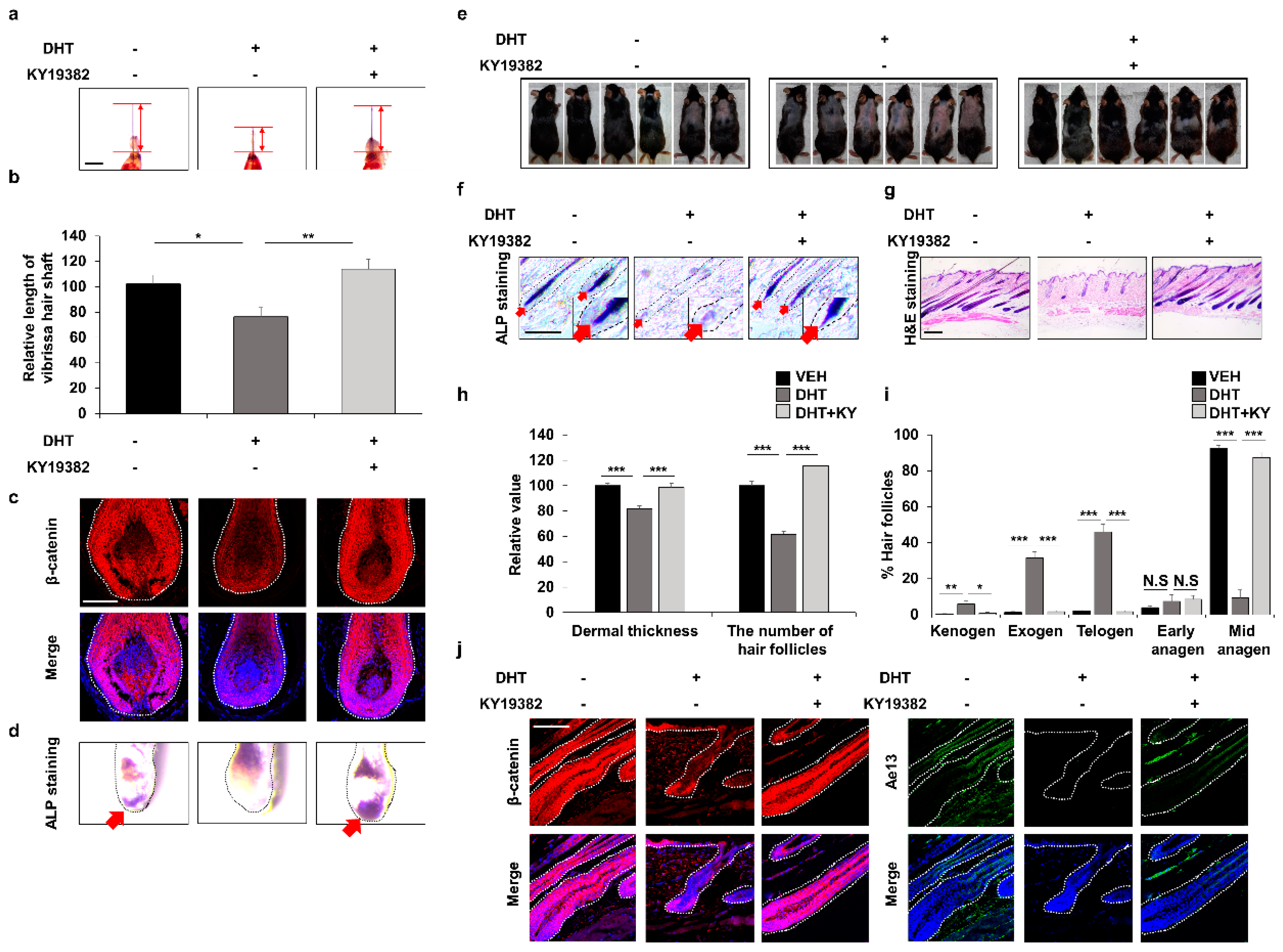
3.5. Efficient Improvement of the Androgenetic Alopecia by Inhibition of Functions of Both GSK-3β and CXXC5
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.C.; Rose, C.; Yoon, G.S.; Lee, S.J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Premanand, A.; Reena Rajkumari, B. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch. Dermatol. Res. 2018, 310, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Vasserot, A.P.; Geyfman, M.; Poloso, N.J. Androgenetic alopecia: Combing the hair follicle signaling pathways for new therapeutic targets and more effective treatment options. Expert Opin. Ther. Targets 2019, 23, 755–771. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012, 4, 126ra134. [Google Scholar] [CrossRef]
- Nelson, A.M.; Loy, D.E.; Lawson, J.A.; Katseff, A.S.; Fitzgerald, G.A.; Garza, L.A. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J. Investig. Dermatol. 2013, 133, 881–889. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Sennett, R.; Rezza, A.; Clavel, C.; Grisanti, L.; Zemla, R.; Najam, S.; Rendl, M. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev. Biol. 2014, 385, 179–188. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Seo, S.H.; Lee, D.H.; Pi, L.Q.; Lee, W.S.; Choi, K.Y. Targeting of CXXC5 by a Competing Peptide Stimulates Hair Regrowth and Wound-Induced Hair Neogenesis. J. Investig. Dermatol. 2017, 137, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yang, D.H.; Shin, S.W.; Kim, M.Y.; Yoon, J.H.; Kim, S.; Park, H.C.; Kang, D.W.; Min, D.; Hur, M.W.; et al. CXXC5 is a transcriptional activator of Flk-1 and mediates bone morphogenic protein-induced endothelial cell differentiation and vessel formation. FASEB J. 2014, 28, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yoon, J.Y.; Yun, J.H.; Cho, K.W.; Lee, S.H.; Rhee, Y.M.; Jung, H.S.; Lim, H.J.; Lee, H.; Choi, J.; et al. CXXC5 is a negative-feedback regulator of the Wnt/β-catenin pathway involved in osteoblast differentiation. Cell Death Differ. 2015, 22, 912–920. [Google Scholar] [CrossRef]
- Leirós, G.J.; Attorresi, A.I.; Balañá, M.E. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br. J. Dermatol. 2012, 166, 1035–1042. [Google Scholar] [CrossRef]
- Kitagawa, T.; Matsuda, K.; Inui, S.; Takenaka, H.; Katoh, N.; Itami, S.; Kishimoto, S.; Kawata, M. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J. Clin. Endocrinol. Metab. 2009, 94, 1288–1294. [Google Scholar] [CrossRef]
- Thompson, V.C.; Day, T.K.; Bianco-Miotto, T.; Selth, L.A.; Han, G.; Thomas, M.; Buchanan, G.; Scher, H.I.; Nelson, C.C.; Greenberg, N.M.; et al. A gene signature identified using a mouse model of androgen receptor-dependent prostate cancer predicts biochemical relapse in human disease. Int. J. Cancer 2012, 131, 662–672. [Google Scholar] [CrossRef]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Lee, D.H.; Shim, J.; Park, J.; Kim, Y.R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.H.; Choi, K.Y. KY19382, a novel activator of Wnt/β-catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.Y.; Kim, H.Y.; Lee, Y.M.; Kim, H.; Nam, K.A.; Roh, M.R.; Min do, S.; Chung, K.Y.; Choi, K.Y. The Dishevelled-binding protein CXXC5 negatively regulates cutaneous wound healing. J. Exp. Med. 2015, 212, 1061–1080. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Södersten, E.; Duckworth, J.K.; Cascante, A.; Fritz, N.; Sacchetti, P.; Cervenka, I.; Bryja, V.; Hermanson, O. CXXC5 is a novel BMP4-regulated modulator of Wnt signaling in neural stem cells. J. Biol. Chem. 2009, 284, 3672–3681. [Google Scholar] [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.Q.; Ai, W.B.; Hu, Q.T.; Zhang, Q.J.; Wan, L.Y.; Wang, X.L.; Liu, C.B.; Wu, J.F. Hes1, an important gene for activation of hepatic stellate cells, is regulated by Notch1 and TGF-β/BMP signaling. World J. Gastroenterol. 2015, 21, 878–887. [Google Scholar] [CrossRef]
- Morikawa, M.; Koinuma, D.; Tsutsumi, S.; Vasilaki, E.; Kanki, Y.; Heldin, C.H.; Aburatani, H.; Miyazono, K. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011, 39, 8712–8727. [Google Scholar] [CrossRef]
- Davidson, P.; Hardy, M.H. The development of mouse vibrissae in vivo and in vitro. J. Anat. 1952, 86, 342–356. [Google Scholar]
- Fan, C.; Luedtke, M.A.; Prouty, S.M.; Burrows, M.; Kollias, N.; Cotsarelis, G. Characterization and quantification of wound-induced hair follicle neogenesis using in vivo confocal scanning laser microscopy. Ski. Res. Technol. 2011, 17, 387–397. [Google Scholar] [CrossRef]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, H.; Ni, H.; Ma, X.H.; Mills, N.; Yang, Z.M. Expression and regulation of lipocalin-type prostaglandin d synthase in rat testis and epididymis. Biol. Reprod. 2004, 70, 1088–1095. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.S.; Min do, S.; Kim, H.Y.; Choi, K.Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Androgenetic alopecia in women. J. Investig. Dermatol. Symp. Proc. 2003, 8, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Ustuner, E.T. Cause of androgenic alopecia: Crux of the matter. Plast. Reconstr. Surg. Glob. Open 2013, 1, e64. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Garza, L.A. The Negative Regulator CXXC5: Making WNT Look a Little Less Dishevelled. J. Investig. Dermatol. 2017, 137, 2248–2250. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef]
- Tosti, A.; Zaiac, M.N.; Canazza, A.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Alis, R.; Lucia, A.; Emanuele, E. Topical application of the Wnt/β-catenin activator methyl vanillate increases hair count and hair mass index in women with androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15, 469–474. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Morroniside regulates hair growth and cycle transition via activation of the Wnt/β-catenin signaling pathway. Sci. Rep. 2018, 8, 13785. [Google Scholar] [CrossRef]
- Jo, S.J.; Choi, S.J.; Yoon, S.Y.; Lee, J.Y.; Park, W.S.; Park, P.J.; Kim, K.H.; Eun, H.C.; Kwon, O. Valproic acid promotes human hair growth in in vitro culture model. J. Dermatol. Sci. 2013, 72, 16–24. [Google Scholar] [CrossRef]
- Jo, S.J.; Shin, H.; Park, Y.W.; Paik, S.H.; Park, W.S.; Jeong, Y.S.; Shin, H.J.; Kwon, O. Topical valproic acid increases the hair count in male patients with androgenetic alopecia: A randomized, comparative, clinical feasibility study using phototrichogram analysis. J. Dermatol. 2014, 41, 285–291. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol. Cell. Endocrinol. 2017, 439, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.S.; Kilbourne, E.J.; Peano, B.J.; Chippari, S.; Kenney, T.; McNally, C.; Wang, W.; Harris, H.A.; Winneker, R.C.; Nagpal, S.; et al. A mouse model of androgenetic alopecia. Endocrinology 2010, 151, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. = Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.M. Mouse models for human hair loss disorders. J. Anat. 2003, 202, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Schoeb, T.R.; Bajpai, P.; Slominski, A.; Singh, K.K. Reversing wrinkled skin and hair loss in mice by restoring mitochondrial function. Cell Death Dis. 2018, 9, 735. [Google Scholar] [CrossRef]
- Orasan, M.S.; Roman, I.I.; Coneac, A.; Muresan, A.; Orasan, R.I. Hair loss and regeneration performed on animal models. Med. Pharm. Rep. 2016, 89, 327–334. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, Y.C.; Park, J.; Kim, Y.-R.; Choi, S.; Kim, G.-U.; Kim, E.; Hwang, Y.; Kim, H.; Han, G.; Lee, S.-H.; et al. CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD2. Cells 2023, 12, 555. https://doi.org/10.3390/cells12040555
Ryu YC, Park J, Kim Y-R, Choi S, Kim G-U, Kim E, Hwang Y, Kim H, Han G, Lee S-H, et al. CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD2. Cells. 2023; 12(4):555. https://doi.org/10.3390/cells12040555
Chicago/Turabian StyleRyu, Yeong Chan, Jiyeon Park, You-Rin Kim, Sehee Choi, Geon-Uk Kim, Eunhwan Kim, Yumi Hwang, Heejene Kim, Gyoonhee Han, Soung-Hoon Lee, and et al. 2023. "CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD2" Cells 12, no. 4: 555. https://doi.org/10.3390/cells12040555
APA StyleRyu, Y. C., Park, J., Kim, Y.-R., Choi, S., Kim, G.-U., Kim, E., Hwang, Y., Kim, H., Han, G., Lee, S.-H., & Choi, K.-Y. (2023). CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD2. Cells, 12(4), 555. https://doi.org/10.3390/cells12040555








