Short- and Long-Term Effects of Cocaine on Enteric Neuronal Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Sampling
2.2. Primary Culture of Myenteric Neurons
2.3. Neuroimaging with Voltage Sensitive Dye
2.4. Neuroimaging: Acute and Chronic Effect of Cocaine on Isolated Enteric Neurons
2.5. Organ Bath Experiments: Effect of Cocaine on Gastric and Intestinal Motility In Vitro
2.6. Ussing Chamber Experiments: Effect of Cocaine on Intestinal Epithelial Secretion In Vitro
2.7. Statistical Analysis
3. Results
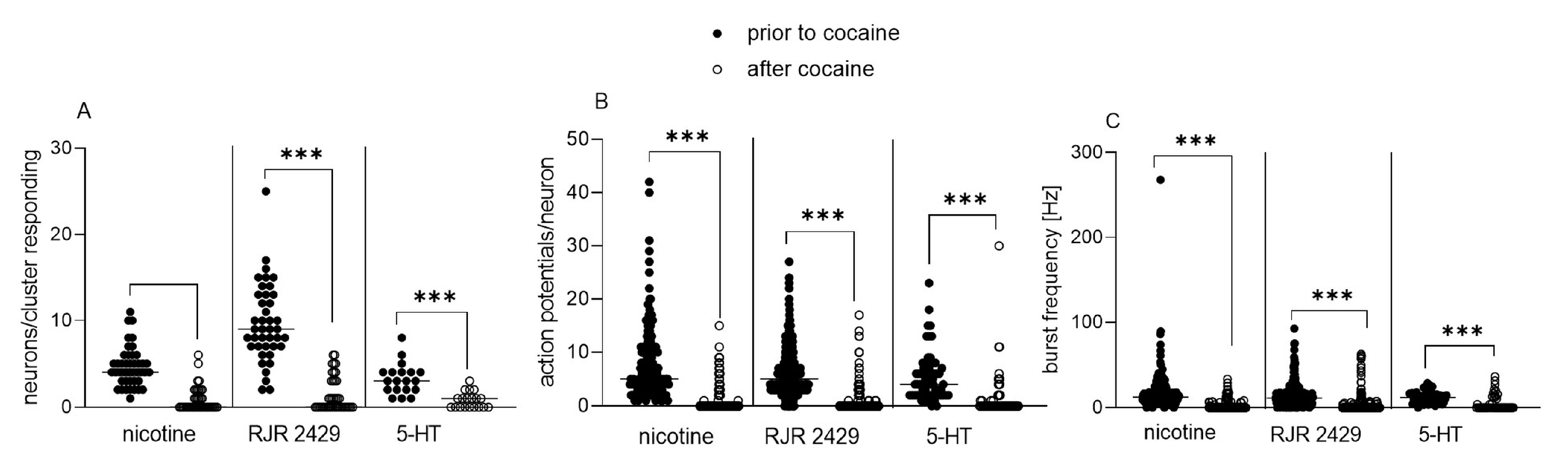
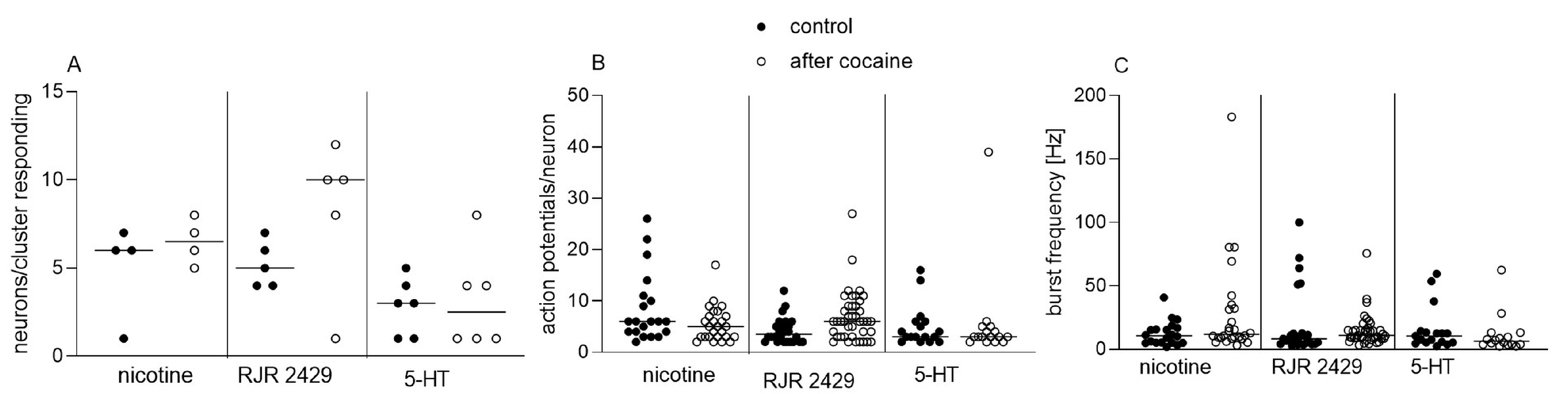
3.1. Acute and Chronic Effect of Cocaine on Isolated Enteric Neurons
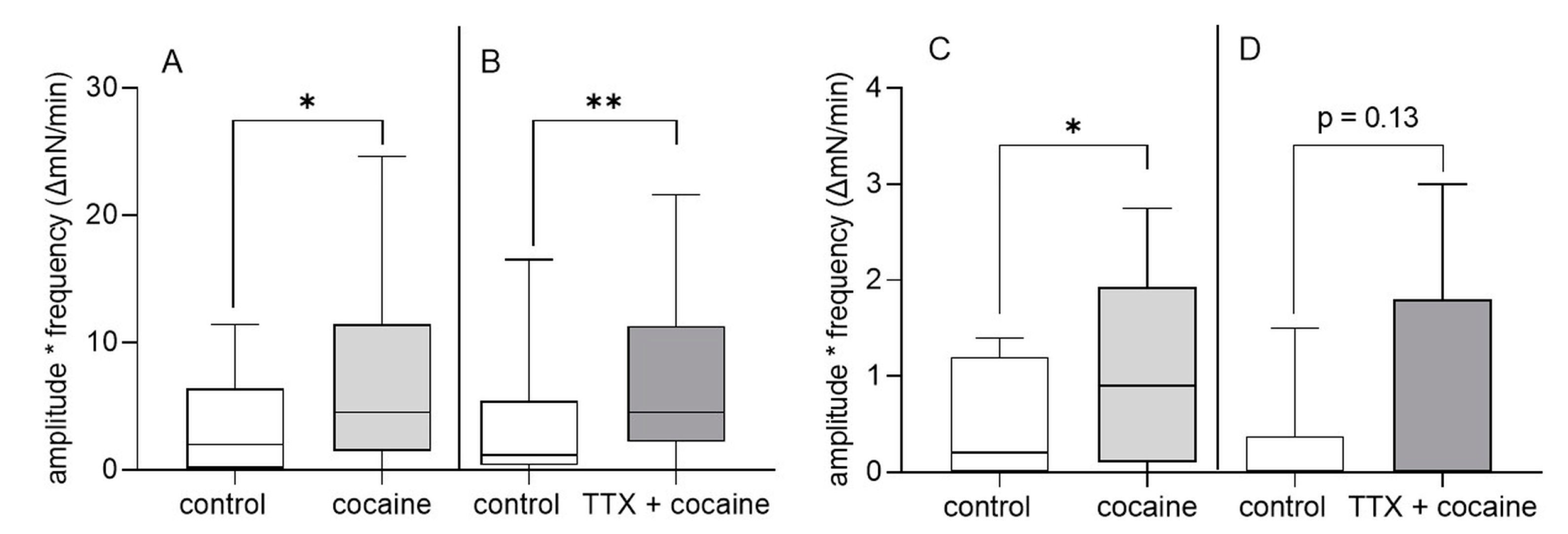
3.2. Organ Bath Experiments: Effect of Cocaine on Gastric and Intestinal Motility In Vitro
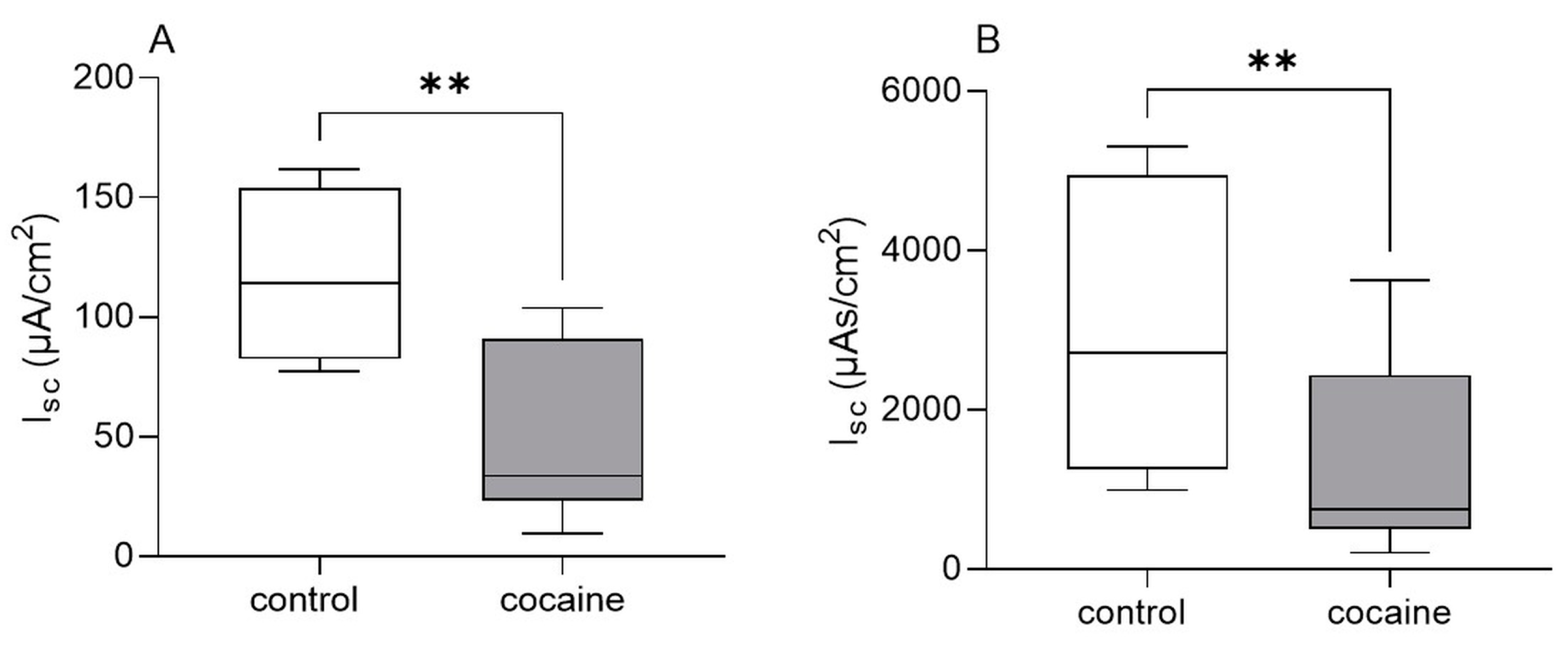
3.3. Ussing Chamber Experiments: Effect of Cocaine on Intestinal Epithelial Secretion In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drugs, E.M.C.F.; Addiction, D. European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Drugs, E.M.C.F.; Addiction, D. European Drug Report 2021: Trends and Developments; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Thomas, M.J.; Kalivas, P.W.; Shaham, Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008, 154, 327–342. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Orlansky, H.; Mytilineou, C.; Cohen, G. Amphetamine: Evaluation of d- and l-isomers as releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. J. Pharmacol. Exp. Ther. 1975, 194, 47–56. [Google Scholar]
- Reith, M.E.; Meisler, B.E.; Sershen, H.; Lajtha, A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem. Pharmacol. 1986, 35, 1123–1129. [Google Scholar] [CrossRef]
- Ritz, M.C.; Lamb, R.J.; Goldberg, S.R.; Kuhar, M.J. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 1987, 237, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Galloway, M.P. Neurochemical interactions of cocaine with dopaminergic systems. Trends Pharmacol. Sci. 1988, 9, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol. Sci. 2004, 25, 210–218. [Google Scholar] [CrossRef]
- Schmidt, H.D.; Pierce, R.C. Cocaine-induced neuroadaptations in glutamate transmission: Potential therapeutic targets for craving and addiction. Ann. NY Acad. Sci. 2010, 1187, 35–75. [Google Scholar] [CrossRef]
- Kupchik, Y.M.; Scofield, M.D.; Rice, K.C.; Cheng, K.; Roques, B.P.; Kalivas, P.W. Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. J. Neurosci. 2014, 34, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Zhang, L.; Zhou, F.; Gong, S.; Gu, H.; De Biasi, M.; Zhou, F.M.; Dani, J.A. Cocaine inhibition of nicotinic acetylcholine receptors influences dopamine release. Front. Synaptic Neurosci. 2014, 6, 19. [Google Scholar] [CrossRef]
- Amenta, F.; Ricci, A.; Tayebati, S.K.; Zaccheo, D. The peripheral dopaminergic system: Morphological analysis, functional and clinical applications. Ital. J. Anat. Embryol. 2002, 107, 145–167. [Google Scholar] [PubMed]
- Magnaghi, V.; Ballabio, M.; Consoli, A.; Lambert, J.J.; Roglio, I.; Melcangi, R.C. GABA receptor-mediated effects in the peripheral nervous system: A cross-interaction with neuroactive steroids. J. Mol. Neurosci. 2006, 28, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Brookes, S.H. Architecture of enteric neural circuits involved in intestinal motility. Eur. Rev. Med. Pharmacol. Sci. 2008, 12 (Suppl. S1), 3–19. [Google Scholar] [PubMed]
- McConalogue, K.; Furness, J.B. Gastrointestinal neurotransmitters. Baillieres Clin. Endocrinol. Metab. 1994, 8, 51–76. [Google Scholar] [CrossRef]
- Obaid, A.L.; Koyano, T.; Lindstrom, J.; Sakai, T.; Salzberg, B.M. Spatiotemporal patterns of activity in an intact mammalian network with single-cell resolution: Optical studies of nicotinic activity in an enteric plexus. J. Neurosci. 1999, 19, 3073–3093. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J.; LePard, K.J.; Schneider, D.A.; Zhou, X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.G.; Jiang, N.; Huang, Y.B.; Ma, X.K.; Brek Eaton, J.; Gao, M.; Chang, Y.C.; Lukas, R.J.; Whiteaker, P.; Neisewander, J.; et al. Cocaine potently blocks neuronal α(3)β(4) nicotinic acetylcholine receptors in SH-SY5Y cells. Acta Pharmacol. Sin. 2020, 41, 163–172. [Google Scholar] [CrossRef]
- Fozard, J.R.; Mobarok Ali, A.T.; Newgrosh, G. Blockade of serotonin receptors on autonomic neurones by (-)-cocaine and some related compounds. Eur. J. Pharmacol. 1979, 59, 195–210. [Google Scholar] [CrossRef]
- Nalbandian, H.; Sheth, N.; Dietrich, R.; Georgiou, J. Intestinal ischemia caused by cocaine ingestion: Report of two cases. Surgery 1985, 97, 374–376. [Google Scholar]
- Cregler, L.L.; Mark, H. Medical complications of cocaine abuse. N. Engl. J. Med. 1986, 315, 1495–1500. [Google Scholar] [CrossRef]
- Linder, J.D.; Mönkemüller, K.E.; Raijman, I.; Johnson, L.; Lazenby, A.J.; Wilcox, C.M. Cocaine-associated ischemic colitis. S. Med. J. 2000, 93, 909–913. [Google Scholar] [CrossRef]
- Ellis, C.N.; McAlexander, W.W. Enterocolitis associated with cocaine use. Dis. Colon. Rectum. 2005, 48, 2313–2316. [Google Scholar] [CrossRef]
- Gibbons, T.E.; Sayed, K.; Fuchs, G.J. Massive pan-gastrointestinal bleeding following cocaine use. World J. Pediatr. 2009, 5, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Scorza, C.; Piccini, C.; Martínez Busi, M.; Abin Carriquiry, J.A.; Zunino, P. Alterations in the Gut Microbiota of Rats Chronically Exposed to Volatilized Cocaine and Its Active Adulterants Caffeine and Phenacetin. Neurotox. Res. 2019, 35, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chivero, E.T.; Ahmad, R.; Thangaraj, A.; Periyasamy, P.; Kumar, B.; Kroeger, E.; Feng, D.; Guo, M.-L.; Roy, S.; Dhawan, P.; et al. Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization. Sci. Rep. 2019, 9, 12187. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Gong, X.; Xie, L.; Ma, B. Gut Microbiota Analysis in Rats with Methamphetamine-Induced Conditioned Place Preference. Front. Microbiol. 2017, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, D.D.; Walker, D.M.; Calipari, E.S.; Labonte, B.; Issler, O.; Pena, C.J.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep. 2016, 6, 35455. [Google Scholar] [CrossRef]
- Kugler, E.M.; Michel, K.; Zeller, F.; Demir, I.E.; Ceyhan, G.O.; Schemann, M.; Mazzuoli-Weber, G. Mechanical stress activates neurites and somata of myenteric neurons. Front. Cell. Neurosci. 2015, 9, 342. [Google Scholar] [CrossRef]
- Ussing, H.H.; Zerahn, K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 1951, 23, 110–127. [Google Scholar] [CrossRef]
- Hall, F.S.; Sora, I.; Drgonova, J.; Li, X.F.; Goeb, M.; Uhl, G.R. Molecular mechanisms underlying the rewarding effects of cocaine. Ann. NY Acad. Sci. 2004, 1025, 47–56. [Google Scholar] [CrossRef]
- Nestler, E.J. The neurobiology of cocaine addiction. Sci. Pract. Perspect. 2005, 3, 4–10. [Google Scholar] [CrossRef]
- Tiwari, A.; Moghal, M.; Meleagros, L. Life threatening abdominal complications following cocaine abuse. J. R. Soc. Med. 2006, 99, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Khroyan, T.V.; Yasuda, D.; Toll, L.; Polgar, W.E.; Zaveri, N.T. High affinity α3β4 nicotinic acetylcholine receptor ligands AT-1001 and AT-1012 attenuate cocaine-induced conditioned place preference and behavioral sensitization in mice. Biochem. Pharmacol. 2015, 97, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, J.; Brown, E.; Schneider, D.; Caraballo-Lopez, Y.; Galligan, J.J. Pharmacological properties of nicotinic acetylcholine receptors expressed by guinea pig small intestinal myenteric neurons. J. Pharmacol. Exp. Ther. 2002, 302, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Howell, L.L.; Cunningham, K.A. Serotonin 5-HT2 receptor interactions with dopamine function: Implications for therapeutics in cocaine use disorder. Pharmacol. Rev. 2015, 67, 176–197. [Google Scholar] [CrossRef]
- Filip, M.; Bubar, M.J.; Cunningham, K.A. Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology 2006, 183, 482–489. [Google Scholar] [CrossRef]
- Fletcher, P.J.; Grottick, A.J.; Higgins, G.A. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 2002, 27, 576–586. [Google Scholar] [CrossRef]
- Huang, C.-C.; Liang, Y.-C.; Lee, C.-C.; Wu, M.-Y.; Hsu, K.-S. Repeated Cocaine Administration Decreases 5-HT2A Receptor-Mediated Serotonergic Enhancement of Synaptic Activity in Rat Medial Prefrontal Cortex. Neuropsychopharmacology 2009, 34, 1979–1992. [Google Scholar] [CrossRef]
- Craig, D.A.; Clarke, D.E. Pharmacological characterization of a neuronal receptor for 5-hydroxytryptamine in guinea pig ileum with properties similar to the 5-hydroxytryptamine receptor. J. Pharmacol. Exp. Ther. 1990, 252, 1378–1386. [Google Scholar]
- Kilbinger, H.; Wolf, D. Effects of 5-HT4 receptor stimulation on basal and electrically evoked release of acetylcholine from guinea-pig myenteric plexus. Naunyn Schmiedebergs Arch. Pharmacol. 1992, 345, 270–275. [Google Scholar] [CrossRef]
- Gershon, M.D.; Takaki, M.; Tamir, H.; Branchek, T. The enteric neural receptor for 5-hydroxytryptamine. Experientia 1985, 41, 863–868. [Google Scholar] [CrossRef]
- Ruetsch, Y.A.; Böni, T.; Borgeat, A. From cocaine to ropivacaine: The history of local anesthetic drugs. Curr. Top. Med. Chem. 2001, 1, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Vignali, S.; Peter, N.; Ceyhan, G.; Demir, I.E.; Zeller, F.; Senseman, D.; Michel, K.; Schemann, M. Recordings from human myenteric neurons using voltage-sensitive dyes. J. Neurosci. Methods 2010, 192, 240–248. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, Y.; Srinivasan, S.; Mittal, R.K. Morphological, immunocytochemical, and functional characterization of esophageal enteric neurons in primary culture. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G129–G138. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Unterweger, P.; Parry, L.J.; Bornstein, J.C.; Foong, J.P. VPAC1 receptors regulate intestinal secretion and muscle contractility by activating cholinergic neurons in guinea pig jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G748–G758. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Neunlist, M.; Schemann, M.; Frieling, T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J. Physiol. 2001, 536, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Cooke, H.J.; Shonnard, K.; Highison, G.; Wood, J.D. Effects of neurotransmitter release on mucosal transport in guinea pig ileum. Am. J. Physiol. 1983, 245, G745–G750. [Google Scholar] [CrossRef]
- Cooke, H.J. Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am. J. Physiol. 1984, 246, G263–G267. [Google Scholar] [CrossRef]
- Carey, H.V.; Tien, X.Y.; Wallace, L.J.; Cooke, H.J. Muscarinic receptor subtypes mediating the mucosal response to neural stimulation of guinea pig ileum. Am. J. Physiol. 1987, 253, G323–G329. [Google Scholar] [CrossRef]
- Kuwahara, A.; Bowen, S.; Wang, J.; Condon, C.; Cooke, H.J. Epithelial responses evoked by stimulation of submucosal neurons in guinea pig distal colon. Am. J. Physiol. 1987, 252, G667–G674. [Google Scholar] [CrossRef]
- Diener, M.; Knobloch, S.F.; Bridges, R.J.; Keilmann, T.; Rummel, W. Cholinergic-mediated secretion in the rat colon: Neuronal and epithelial muscarinic responses. Eur. J. Pharmacol. 1989, 168, 219–229. [Google Scholar] [CrossRef]
- Traynor, T.R.; Brown, D.R.; O’Grady, S.M. Regulation of ion transport in porcine distal colon: Effects of putative neurotransmitters. Gastroenterology 1991, 100, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. Incentive-sensitization and addiction. Addiction 2001, 96, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hope, B.T.; Widnell, K.L. Drug addiction: A model for the molecular basis of neural plasticity. Neuron 1993, 11, 995–1006. [Google Scholar] [CrossRef]
- Nestler, E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001, 2, 119–128. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kolb, B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci. 1999, 11, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Egashira, K.; Morgan, K.G.; Morgan, J.P. Effects of cocaine on excitation-contraction coupling of aortic smooth muscle from the ferret. J. Clin. Investig. 1991, 87, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.; Yamamoto, H.; Kobayashi, S.; Kanaide, H. Extracellular Ca2+-dependent potentiation by cocaine of serotonin- and norepinephrine-induced contractions in rat vascular smooth muscle. Circ. Res. 1993, 72, 1191–1201. [Google Scholar] [CrossRef]
- Du, C.; Park, K.; Allen, C.P.; Hu, X.-T.; Volkow, N.D.; Pan, Y. Ca2+ channel blockade reduces cocaine’s vasoconstriction and neurotoxicity in the prefrontal cortex. Transl. Psychiatry 2021, 11, 459. [Google Scholar] [CrossRef]
- Wegener, J.W.; Schulla, V.; Koller, A.; Klugbauer, N.; Feil, R.; Hofmann, F. Control of intestinal motility by the Ca(v)1.2 L-type calcium channel in mice. FASEB J. 2006, 20, 1260–1262. [Google Scholar] [CrossRef]
- Parekh, A.B.; Penner, R. Store depletion and calcium influx. Physiol. Rev. 1997, 77, 901–930. [Google Scholar] [CrossRef]
- Bolton, T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979, 59, 606–718. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Rayner, C.K.; Wu, K.L.; Chuah, S.K.; Tai, W.C.; Chou, Y.P.; Chiu, Y.C.; Chiu, K.W.; Hu, T.H. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J. Gastroenterol. 2011, 17, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Schemann, M.; Michel, K.; Zeller, F.; Hohenester, B.; Rühl, A. Region-specific effects of STW 5 (Iberogast) and its components in gastric fundus, corpus and antrum. Phytomedicine 2006, 13 (Suppl. S5), 90–99. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, B.; Rühl, A.; Kelber, O.; Schemann, M. The herbal preparation STW5 (lberogast) has potent and region-specific effects on gastric motility. Neurogastroenterol. Motil. 2004, 16, 765–773. [Google Scholar] [CrossRef]
- Annaházi, A.; Schröder, A.; Schemann, M. Region-specific effects of the cysteine protease papain on gastric motility. Neurogastroenterol. Motil. 2021, 33, e14105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfers, K.; Menne, L.; Colnaghi, L.; Hoppe, S.; Mazzuoli-Weber, G. Short- and Long-Term Effects of Cocaine on Enteric Neuronal Functions. Cells 2023, 12, 577. https://doi.org/10.3390/cells12040577
Elfers K, Menne L, Colnaghi L, Hoppe S, Mazzuoli-Weber G. Short- and Long-Term Effects of Cocaine on Enteric Neuronal Functions. Cells. 2023; 12(4):577. https://doi.org/10.3390/cells12040577
Chicago/Turabian StyleElfers, Kristin, Laura Menne, Luca Colnaghi, Susanne Hoppe, and Gemma Mazzuoli-Weber. 2023. "Short- and Long-Term Effects of Cocaine on Enteric Neuronal Functions" Cells 12, no. 4: 577. https://doi.org/10.3390/cells12040577
APA StyleElfers, K., Menne, L., Colnaghi, L., Hoppe, S., & Mazzuoli-Weber, G. (2023). Short- and Long-Term Effects of Cocaine on Enteric Neuronal Functions. Cells, 12(4), 577. https://doi.org/10.3390/cells12040577





