FGF23 in Chronic Kidney Disease: Bridging the Heart and Anemia
Abstract
:1. FGF23 Signaling and Pathophysiology in CKD
2. FGF23 and Heart Involvement
3. FGF23 and Anemia in Patients with CKD
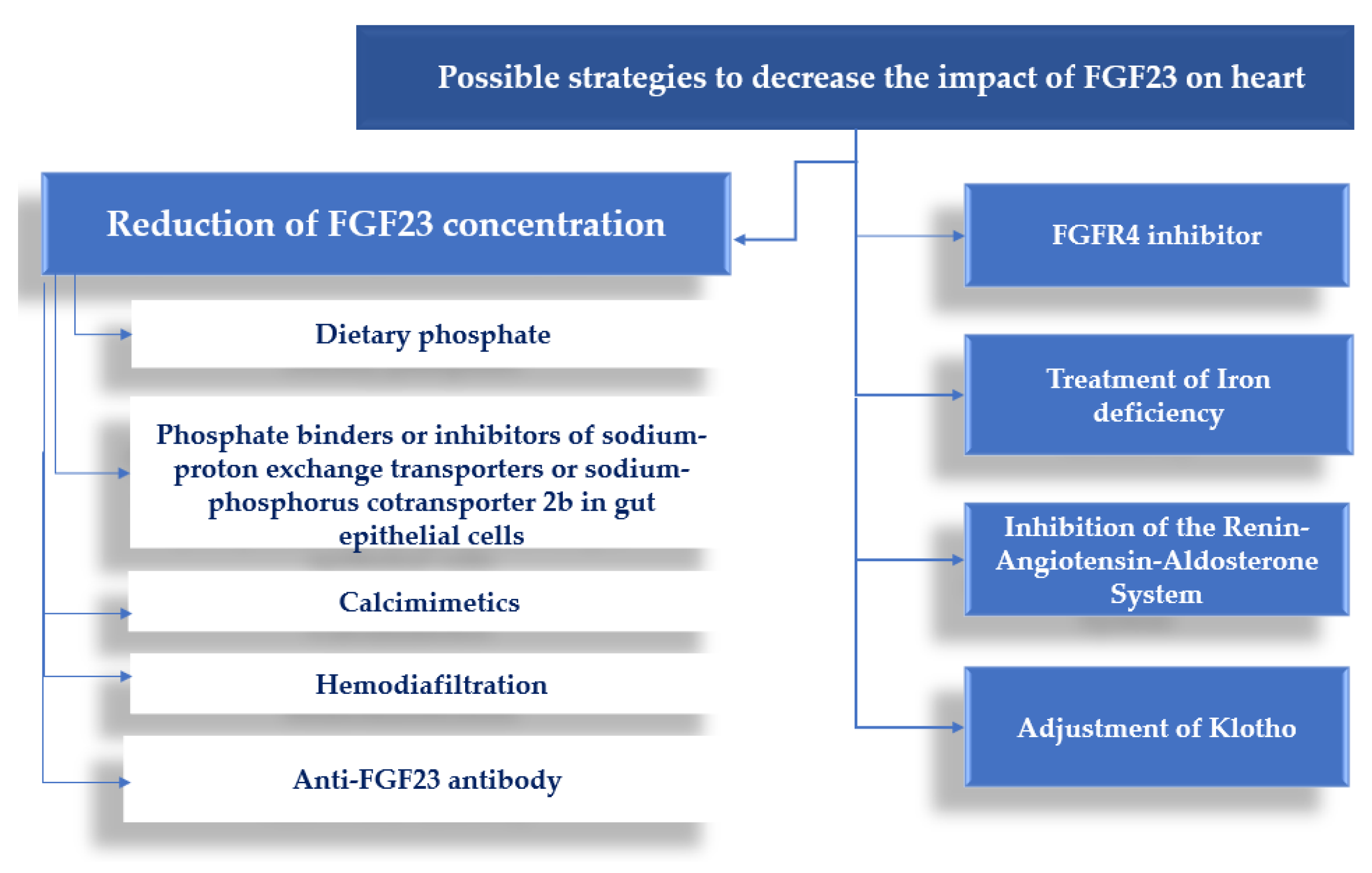
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.-C.; Moe, O.W.; et al. Regulation of Fibroblast Growth Factor-23 Signaling by Klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.; Beenken, A.; Ibrahimi, O.A.; Kalinina, J.; Olsen, S.K.; Eliseenkova, A.V.; Xu, C.; Neubert, T.A.; Zhang, F.; Linhardt, R.J.; et al. Molecular Insights into the Klotho-Dependent, Endocrine Mode of Action of Fibroblast Growth Factor 19 Subfamily Members. Mol. Cell. Biol. 2007, 27, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Goetz, R.; Nakada, Y.; Hu, M.C.; Kurosu, H.; Wang, L.; Nakatani, T.; Shi, M.; Eliseenkova, A.V.; Razzaque, M.S.; Moe, O.W.; et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl. Acad. Sci. USA 2009, 107, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues—With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef]
- Wang, Q.; Su, W.; Shen, Z.; Wang, R. Correlation between Soluble α-Klotho and Renal Function in Patients with Chronic Kidney Disease: A Review and Meta-Analysis. BioMed Res. Int. 2018, 2018, 9481475. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Bardet, C.; Prié, D. Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metabolism 2020, 103, 153865. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Quarles, L.D. FGF23 from Bench to Bedside Perspective. Am. J. Physiol. Ren. Physiol. 2016, 310, 1168–1174. [Google Scholar] [CrossRef]
- Introduction to Volume Two: Atlas of End-Stage Renal Disease in the United States. Am. J. Kidney Dis. 2014, 63, e151–e168. [CrossRef]
- Suassuna, P.G.D.A.; Sanders-Pinheiro, H.; de Paula, R.B. Uremic Cardiomyopathy: A New Piece in the Chronic Kidney Disease-Mineral and Bone Disorder Puzzle. Front. Med. 2018, 5, 206. [Google Scholar] [CrossRef]
- Remppis, A.; Ritz, E. NON-CORONARY HEART DISEASE IN DIALYSIS PATIENTS: Cardiac Problems in the Dialysis Patient: Beyond Coronary Disease. Semin. Dial. 2008, 21, 319–325. [Google Scholar] [CrossRef]
- Di Lullo, L.; Gorini, A.; Russo, D.; Santoboni, A.; Ronco, C. Left Ventricular Hypertrophy in Chronic Kidney Disease Patients: From Pathophysiology to Treatment. Cardiorenal Med. 2015, 5, 254–266. [Google Scholar] [CrossRef]
- Wang, X.; Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Kirkpantur, A.; Balci, M.; Gurbuz, O.A.; Afsar, B.; Canbakan, B.; Akdemir, R.; Ayli, M.D. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2010, 26, 1346–1354. [Google Scholar] [CrossRef]
- Grabner, A.; Amaral, A.P.; Schramm, K.; Singh, S.; Sloan, A.; Yanucil, C.; Li, J.; Shehadeh, L.A.; Hare, J.M.; David, V.; et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015, 22, 1020–1032. [Google Scholar] [CrossRef]
- Vainikka, S.; Joukov, V.; Wennström, S.; Bergman, M.; Pelicci, P.; Alitalo, K. Signal transduction by fibroblast growth factor receptor-4 (FGFR-4). Comparison with FGFR-1. J. Biol. Chem. 1994, 269, 18320–18326. [Google Scholar] [CrossRef]
- Wilkins, B.J.; Dai, Y.-S.; Bueno, O.F.; Parsons, S.A.; Xu, J.; Plank, D.M.; Jones, F.; Kimball, T.R.; Molkentin, J.D. Calcineurin/NFAT Coupling Participates in Pathological, but not Physiological, Cardiac Hypertrophy. Circ. Res. 2004, 94, 110–118. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujita, S.-I.; Kizawa, S.; Morita, H.; Ishizaka, N. Association between FGF23, α-Klotho, and Cardiac Abnormalities among Patients with Various Chronic Kidney Disease Stages. PLoS ONE 2016, 11, e0156860. [Google Scholar] [CrossRef]
- Grabner, A.; Schramm, K.; Silswal, N.; Hendrix, M.; Yanucil, C.; Czaya, B.; Singh, S.; Wolf, M.; Hermann, S.; Stypmann, J.; et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci. Rep. 2017, 7, 1993. [Google Scholar] [CrossRef]
- Leifheit-Nestler, M.; Grabner, A.; Hermann, L.; Richter, B.; Schmitz, K.; Fischer, D.-C.; Yanucil, C.; Faul, C.; Haffner, D. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol. Dial. Transplant. 2017, 32, 1493–1503. [Google Scholar] [CrossRef]
- Touchberry, C.; Green, T.M.; Tchikrizov, V.; Mannix, J.E.; Mao, T.F.; Carney, B.W.; Girgis, M.; Vincent, R.J.; Wetmore, L.A.; Dawn, B.; et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am. J. Physiol. Metab. 2013, 304, E863–E873. [Google Scholar] [CrossRef] [PubMed]
- Figurek, A.; Rroji, M.; Spasovski, G. The Complexity of FGF23 Effects on Cardiomyocytes in Normal and Uremic Milieu. Cells 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Eitner, F.; Richter, B.; Schwänen, S.; Szaroszyk, M.; Vogt, I.; Grund, A.; Thum, T.; Heineke, J.; Haffner, D.; Leifheit-Nestler, M. Comprehensive Expression Analysis of Cardiac Fibroblast Growth Factor 23 in Health and Pressure-induced Cardiac Hypertrophy. Front. Cell Dev. Biol. 2022, 9, 791479. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Haffner, D. Paracrine Effects of FGF23 on the Heart. Front. Endocrinol. 2018, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Kirchhoff, F.; Nespor, J.; Richter, B.; Soetje, B.; Klintschar, M.; Heineke, J.; Haffner, D. Fibroblast growth factor 23 is induced by an activated renin–angiotensin–aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol. Dial. Transplant. 2018, 33, 1722–1734. [Google Scholar] [CrossRef]
- Smith, E.R.; Tan, S.-J.; Holt, S.G.; Hewitson, T.D. FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci. Rep. 2017, 7, 3345. [Google Scholar] [CrossRef]
- Smith, E.R.; Holt, S.G.; Hewitson, T.D. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-β autoinduction. Int. J. Biochem. Cell Biol. 2017, 92, 63–78. [Google Scholar] [CrossRef]
- Hao, H.; Li, X.; Li, Q.; Lin, H.; Chen, Z.; Xie, J.; Xuan, W.; Liao, W.; Bin, J.; Huang, X.; et al. FGF23 promotes myocardial fibrosis in mice through activation of β-catenin. Oncotarget 2016, 7, 64649–64664. [Google Scholar] [CrossRef]
- Andrukhova, O.; Slavic, S.; I Odörfer, K.; Erben, R.G. Experimental Myocardial Infarction Upregulates Circulating Fibroblast Growth Factor-23. J. Bone Miner. Res. 2015, 30, 1831–1839. [Google Scholar] [CrossRef]
- Lam, A.P.; Gottardi, C.J. β-catenin signaling. Curr. Opin. Rheumatol. 2011, 23, 562–567. [Google Scholar] [CrossRef]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef]
- Czaya, B.; Faul, C. FGF23 and inflammation—a vicious coalition in CKD. Kidney Int. 2019, 96, 813–815. [Google Scholar] [CrossRef]
- Singh, S.; Grabner, A.; Yanucil, C.; Schramm, K.; Czaya, B.; Krick, S.; Czaja, M.J.; Bartz, R.; Abraham, R.; Di Marco, G.S.; et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016, 90, 985–996. [Google Scholar] [CrossRef]
- Fitzpatrick, E.A.; Han, X.; Xiao, Z.; Quarles, L.D. Role of Fibroblast Growth Factor-23 in Innate Immune Responses. Front. Endocrinol. 2018, 9, 320. [Google Scholar] [CrossRef]
- McKnight, Q.; Jenkins, S.; Li, X.; Nelson, T.; Marlier, A.; Cantley, L.G.; Finberg, K.E.; Fretz, J.A. IL-1β Drives Production of FGF-23 at the Onset of Chronic Kidney Disease in Mice. J. Bone Miner. Res. 2020, 35, 1352–1362. [Google Scholar] [CrossRef]
- Bacchetta, J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Wesseling-Perry, K.; Gales, B.; Adams, J.S.; Salusky, I.B.; Hewison, M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J. Bone Miner. Res. 2012, 28, 46–55. [Google Scholar] [CrossRef]
- Francis, C.; David, V. Inflammation regulates fibroblast growth factor 23 production. Curr. Opin. Nephrol. Hypertens. 2016, 25, 325–332. [Google Scholar] [CrossRef]
- Frieler, R.A.; Mortensen, R.M. Immune Cell and Other Noncardiomyocyte Regulation of Cardiac Hypertrophy and Remodeling. Circulation 2015, 131, 1019–1030. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Fang, J.; Ren, J.; Li, H.; Xiao, Z.; Quarles, L.D. Novel Regulators of Fgf23 Expression and Mineralization in Hyp Bone. Mol. Endocrinol. 2009, 23, 1505–1518. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Tang, W.; Jiang, X.; Rowe, D.W.; Quarles, L.D. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Metab. 2006, 291, E38–E49. [Google Scholar] [CrossRef]
- Pi, M.; Ye, R.; Han, X.; Armstrong, B.; Liu, X.; Chen, Y.; Sun, Y.; Quarles, L.D. Cardiovascular Interactions between Fibroblast Growth Factor-23 and Angiotensin II. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, P.; Melenovsky, V.; Kotrc, M.; Benes, J.; Jabor, A.; Franekova, J.; Lemaire, S.; Kautzner, J.; Jarolim, P. Association of Fibroblast Growth Factor-23 Levels and Angiotensin-Converting Enzyme Inhibition in Chronic Systolic Heart Failure. JACC: Hear. Fail. 2015, 3, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; David, V.; Martin, A.; Huang, J.; Li, H.; Jiao, Y.; Gu, W.; Quarles, L.D. A Comparative Transcriptome Analysis Identifying FGF23 Regulated Genes in the Kidney of a Mouse CKD Model. PLoS ONE 2012, 7, e44161. [Google Scholar] [CrossRef] [PubMed]
- Kuga, K.; Kusakari, Y.; Uesugi, K.; Semba, K.; Urashima, T.; Akaike, T.; Minamisawa, S. Fibrosis growth factor 23 is a promoting factor for cardiac fibrosis in the presence of transforming growth factor-β1. PLoS ONE 2020, 15, e0231905. [Google Scholar] [CrossRef]
- Fajol, A.; Chen, H.; Umbach, A.T.; Quarles, L.D.; Lang, H.; Föller, M. Enhanced FGF23 production in mice expressing PI3K-insensitive GSK3 is normalized by β-blocker treatment. FASEB J. 2015, 30, 994–1001. [Google Scholar] [CrossRef]
- Xie, J.; Yoon, J.; An, S.-W.; Kuro, M.; Kuro-O, M.; Huang, C.-L. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J. Am. Soc. Nephrol. 2015, 26, 1150–1160. [Google Scholar] [CrossRef]
- Epstein, M.; Freundlich, M. The intersection of mineralocorticoid receptor activation and the FGF23–Klotho cascade: A duopoly that promotes renal and cardiovascular injury. Nephrol. Dial. Transplant. 2021, 37, 211–221. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Sonmez, A.; Saglam, M.; Yaman, H.; Kilic, S.; Demirkaya, E.; Eyileten, T.; Caglar, K.; Oguz, Y.; Vural, A.; et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010, 78, 679–685. [Google Scholar] [CrossRef]
- van Venrooij, N.A.; Pereira, R.C.; Tintut, Y.; Fishbein, M.C.; Tumber, N.; Demer, L.L.; Salusky, I.B.; Wesseling-Perry, K. FGF23 protein expression in coronary arteries is associated with impaired kidney function. Nephrol. Dial. Transplant. 2014, 29, 1525–1532. [Google Scholar] [CrossRef]
- Richter, B.; Haller, J.; Haffner, D.; Leifheit-Nestler, M. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflüg. Archiv-Eur. J. Physiol. 2016, 468, 1621–1635. [Google Scholar] [CrossRef]
- Dörr, K.; Kainz, A.; Oberbauer, R. Lessons from effect of etelcalcetide on left ventricular hypertrophy in patients with end-stage kidney disease. Curr. Opin. Nephrol. Hypertens. 2022, 31, 339–343. [Google Scholar] [CrossRef]
- Savica, V.; Calò, L.A.; Monardo, P.; Santoro, D.; Mallamace, A.; Muraca, U.; Bellinghieri, G. Salivary Phosphorus and Phosphate Content of Beverages: Implications for the Treatment of Uremic Hyperphosphatemia. J. Ren. Nutr. 2009, 19, 69–72. [Google Scholar] [CrossRef]
- Monardo, P.; Lacquaniti, A.; Campo, S.; Bucca, M.; di Tocco, T.C.; Rovito, S.; Ragusa, A.; Santoro, A. Updates on hemodialysis techniques with a common denominator: The personalization of the dialytic therapy. Semin. Dial. 2021, 34, 183–195. [Google Scholar] [CrossRef]
- Zhao, S.-J.; Wang, Z.-X.; Chen, L.; Wang, F.-X.; Kong, L.-D. Effect of different phosphate binders on fibroblast growth factor 23 levels in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2021. [Google Scholar] [CrossRef]
- Babitt, J.L.; Sitara, D. Crosstalk between fibroblast growth factor 23, iron, erythropoietin, and inflammation in kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 304–310. [Google Scholar] [CrossRef]
- Imel, E.A.; Peacock, M.; Gray, A.K.; Padgett, L.R.; Hui, S.L.; Econs, M.J. Iron Modifies Plasma FGF23 Differently in Autosomal Dominant Hypophosphatemic Rickets and Healthy Humans. J. Clin. Endocrinol. Metab. 2011, 96, 3541–3549. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef]
- Flamme, I.; Ellinghaus, P.; Urrego, D.; Krüger, T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS ONE 2017, 12, e0186979. [Google Scholar] [CrossRef]
- Hanudel, M.; Eisenga, M.; Rappaport, M.; Chua, K.J.; Qiao, B.; Jung, C.-L.; Gabayan, V.; Gales, B.; Ramos, G.; Jong, M.; et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2018, 34, 2057–2065. [Google Scholar] [CrossRef]
- Czaya, B.; Faul, C. The Role of Fibroblast Growth Factor 23 in Inflammation and Anemia. Int. J. Mol. Sci. 2019, 20, 4195. [Google Scholar] [CrossRef]
- Coe, L.M.; Madathil, S.V.; Casu, C.; Lanske, B.; Rivella, S.; Sitara, D. FGF-23 Is a Negative Regulator of Prenatal and Postnatal Erythropoiesis. J. Biol. Chem. 2014, 289, 9795–9810. [Google Scholar] [CrossRef] [PubMed]
- Agoro, R.; Montagna, A.; Goetz, R.; Aligbe, O.; Singh, G.; Coe, L.M.; Mohammadi, M.; Rivella, S.; Sitara, D. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 2018, 32, 3752–3764. [Google Scholar] [CrossRef] [PubMed]
- Biruete, A.; E Metzger, C.; Chen, N.X.; A Swallow, E.; Vrabec, C.; Clinkenbeard, E.L.; Stacy, A.J.; Srinivasan, S.; O’Neill, K.; Avin, K.G.; et al. Effects of ferric citrate and intravenous iron sucrose on markers of mineral, bone, and iron homeostasis in a rat model of CKD-MBD. Nephrol. Dial. Transplant. 2022, 37, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.; Courbon, G.; Gerber, C.; Neuburg, S.; Wang, X.; Dussold, C.; Capella, M.; Qi, L.; Isakova, T.; Mehta, R.; et al. Ferric citrate reduces fibroblast growth factor 23 levels and improves renal and cardiac function in a mouse model of chronic kidney disease. Kidney Int. 2019, 96, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, H.; An, S.Y.; Lee, M.; Cha, M.-U.; Park, J.T.; Yoo, T.-H.; Lee, K.-B.; Kim, Y.-H.; Sung, S.-A.; et al. Circulating Fibroblast Growth Factor-23 Levels are Associated with an Increased Risk of Anemia Development in Patients with Nondialysis Chronic Kidney Disease. Sci. Rep. 2018, 8, 7294. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Cai, X.; Hodakowski, A.; Lee, J.; Leonard, M.; Ricardo, A.; Chen, J.; Hamm, L.; Sondheimer, J.; Dobre, M.; et al. Fibroblast Growth Factor 23 and Anemia in the Chronic Renal Insufficiency Cohort Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 1795–1803. [Google Scholar] [CrossRef]
- Wolf, M.; Chertow, G.M.; MacDougall, I.C.; Kaper, R.; Krop, J.; Strauss, W. Randomized trial of intravenous iron-induced hypophosphatemia. J. Clin. Investig. 2018, 3, e124486. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Pasqualetti, P.; Di Tocco, T.C.; Campo, S.; Rovito, S.; Bucca, M.; Ragusa, A.; Monardo, P. Ferric carboxymaltose versus ferric gluconate in hemodialysis patients: Reduction of erythropoietin dose in 4 years of follow-up. Kidney Res. Clin. Pr. 2020, 39, 334–343. [Google Scholar] [CrossRef]
- Hempel, J.C.; Poppelaars, F.; da Costa, M.G.; Franssen, C.F.; de Vlaam, T.P.; Daha, M.R.; Berger, S.P.; Seelen, M.A.; Gaillard, C.A. Distinct in vitro Complement Activation by Various Intravenous Iron Preparations. Am. J. Nephrol. 2016, 45, 49–59. [Google Scholar] [CrossRef]
- Fishbane, S.; Block, G.A.; Loram, L.; Neylan, J.; Pergola, P.E.; Uhlig, K.; Chertow, G.M. Effects of Ferric Citrate in Patients with Nondialysis-Dependent CKD and Iron Deficiency Anemia. J. Am. Soc. Nephrol. 2017, 28, 1851–1858. [Google Scholar] [CrossRef]
- A Block, G.; E Pergola, P.; Fishbane, S.; Martins, J.G.; LeWinter, R.D.; Uhlig, K.; Neylan, J.F.; Chertow, G.M. Effect of ferric citrate on serum phosphate and fibroblast growth factor 23 among patients with nondialysis-dependent chronic kidney disease: Path analyses. Nephrol. Dial. Transplant. 2018, 34, 1115–1124. [Google Scholar] [CrossRef]
- Maruyama, N.; Otsuki, T.; Yoshida, Y.; Nagura, C.; Kitai, M.; Shibahara, N.; Tomita, H.; Maruyama, T.; Abe, M. Ferric Citrate Decreases Fibroblast Growth Factor 23 and Improves Erythropoietin Responsiveness in Hemodialysis Patients. Am. J. Nephrol. 2018, 47, 406–414. [Google Scholar] [CrossRef]
- Moe, S.M.; Chertow, G.M.; Parfrey, P.S.; Kubo, Y.; Block, G.A.; Correa-Rotter, R.; Drueke, T.B.; Herzog, C.A.; London, G.M.; Mahaffey, K.W.; et al. Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis. Circulation 2015, 132, 27–39. [Google Scholar] [CrossRef]
- Wolf, M.; Koch, T.A.; Bregman, D.B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013, 28, 1793–1803. [Google Scholar] [CrossRef]
- Bielesz, B.; Reiter, T.; Hammerle, F.P.; Winnicki, W.; Bojic, M.; Gleiss, A.; Kieweg, H.; Ratzinger, F.; Sunder-Plassmann, G.; Marculescu, R. The Role of Iron and Erythropoietin in the Association of Fibroblast Growth Factor 23 with Anemia in Chronic Kidney Disease in Humans. J. Clin. Med. 2020, 9, 2640. [Google Scholar] [CrossRef]
- Gupta, N.; Wish, J.B. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am. J. Kidney Dis. 2017, 69, 815–826. [Google Scholar] [CrossRef]
- Noonan, M.L.; Clinkenbeard, E.L.; Ni, P.; Swallow, E.A.; Tippen, S.P.; Agoro, R.; Allen, M.R.; White, K.E. Erythropoietin and a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHDi) lowers FGF23 in a model of chronic kidney disease (CKD). Physiol. Rep. 2020, 8, e14434. [Google Scholar] [CrossRef]
- Hanudel, M.R.; Wong, S.; Jung, G.; Qiao, B.; Gabayan, V.; Zuk, A.; Ganz, T. Amelioration of chronic kidney disease-associated anemia by vadadustat in mice is not dependent on erythroferrone. Kidney Int. 2021, 100, 79–89. [Google Scholar] [CrossRef]
- Hasegawa, H.; Nagano, N.; Urakawa, I.; Yamazaki, Y.; Iijima, K.; Fujita, T.; Yamashita, T.; Fukumoto, S.; Shimada, T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010, 78, 975–980. [Google Scholar] [CrossRef]
- Shalhoub, V.; Shatzen, E.M.; Ward, S.C.; Davis, J.; Stevens, J.; Bi, V.; Renshaw, L.; Hawkins, N.; Wang, W.; Chen, C.; et al. FGF23 neutralization improves chronic kidney disease–associated hyperparathyroidism yet increases mortality. J. Clin. Investig. 2012, 122, 2543–2553. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figurek, A.; Rroji, M.; Spasovski, G. FGF23 in Chronic Kidney Disease: Bridging the Heart and Anemia. Cells 2023, 12, 609. https://doi.org/10.3390/cells12040609
Figurek A, Rroji M, Spasovski G. FGF23 in Chronic Kidney Disease: Bridging the Heart and Anemia. Cells. 2023; 12(4):609. https://doi.org/10.3390/cells12040609
Chicago/Turabian StyleFigurek, Andreja, Merita Rroji, and Goce Spasovski. 2023. "FGF23 in Chronic Kidney Disease: Bridging the Heart and Anemia" Cells 12, no. 4: 609. https://doi.org/10.3390/cells12040609
APA StyleFigurek, A., Rroji, M., & Spasovski, G. (2023). FGF23 in Chronic Kidney Disease: Bridging the Heart and Anemia. Cells, 12(4), 609. https://doi.org/10.3390/cells12040609






