Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives
Abstract
:1. Introduction
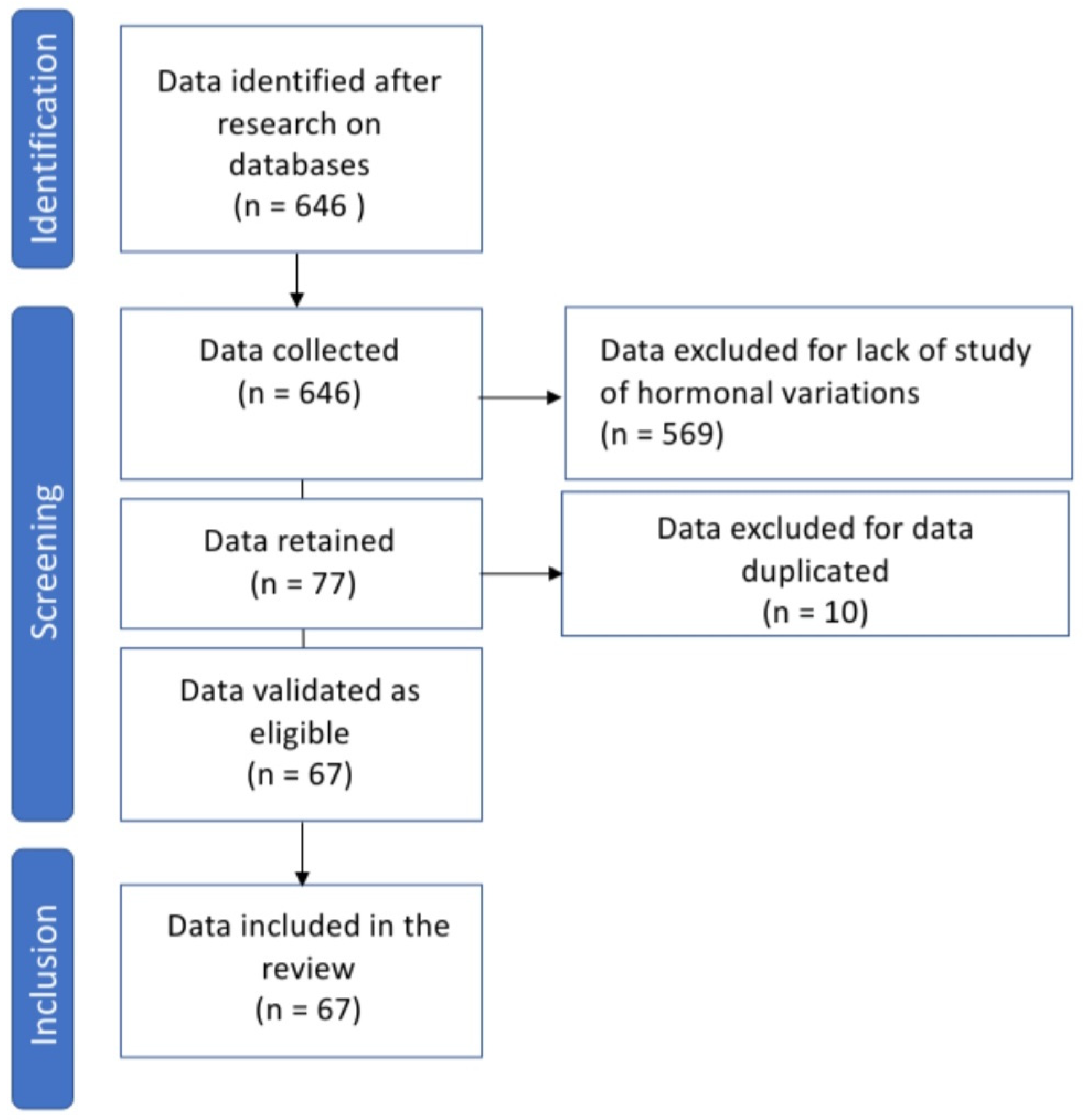
2. Methods
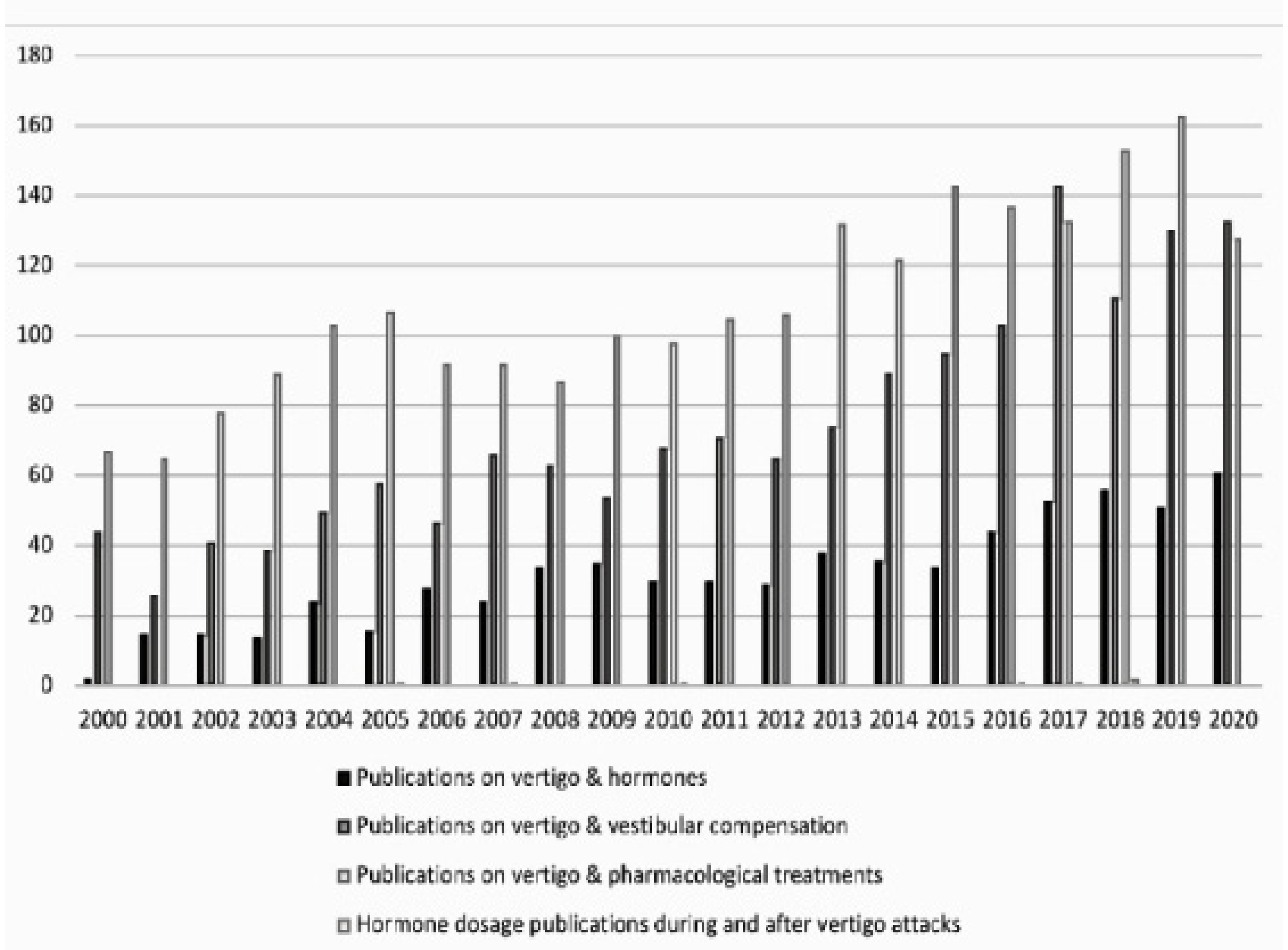
3. Results
4. Discussion
4.1. Why So Little Information?
4.2. Hormones and Vestibule: How to Move Forward?
4.3. Mapping the Expression of Hormone Receptors over the Whole Vestibular Sensory Network
4.4. Specify the Role of Hormones in Vestibular Physiology and Vertigo Pathophysiology
4.5. Follow the Hormonal Variations over the Different Phases of the Vestibular Syndrome
5. Conclusions
Funding
Conflicts of Interest
References
- Wangemann, P.; Liu, J.; Shimozono, M.; Scofield, M.A. Beta1-adrenergic receptors but not beta2-adrenergic or vasopressin receptors regulate K+ secretion in vestibular dark cells of the inner ear. J. Membr. Biol. 1999, 170, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; Koike, K.; Uno, A.; Uno, Y.; Kubo, T. Vestibular modulation of plasma vasopressin levels in rats. Brain Res. 2001, 914, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rastoldo, G.; Marouane, E.; El-Mahmoudi, N.; Péricat, D.; Watabe, I.; Lapotre, A.; Tonetto, A.; López-Juárez, A.; El-Ahmadi, A.; Caron, P.; et al. L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells 2022, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Kawao, N.; Takafuji, Y.; Ishida, M.; Okumoto, K.; Morita, H.; Muratani, M.; Kaji, H. Roles of the vestibular system in obesity and impaired glucose metabolism in high-fat diet-fed mice. PLoS ONE 2020, 15, e0228685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemungal, B.M.; Gresty, M.A.; Bronstein, A.M. The endocrine system, vertigo and balance. Curr. Opin. Neurol. 2001, 14, 27–34. [Google Scholar] [CrossRef]
- Patel, A.K.; Alexander, T.H.; Andalibi, A.; Ryan, A.F.; Doherty, J.K. Vestibular schwannoma quantitative polymerase chain reaction expression of estrogen and progesterone receptors. Laryngoscope 2008, 118, 1458–1463. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, A.E.; Wang, H.; Sahlin, L.; Hultcrantz, M. Mapping of estrogen receptors in the inner ear of mouse and rat. Hear. Res. 1999, 136, 29–34. [Google Scholar] [CrossRef]
- Motohashi, R.; Takumida, M.; Shimizu, A.; Konomi, U.; Fujita, K.; Hirakawa, K.; Suzuki, M.; Anniko, M. Effects of age and sex on the expression of estrogen receptor α and β in the mouse inner ear. Acta Otolaryngol. 2010, 130, 204–214. [Google Scholar] [CrossRef]
- Kumagami, H. Sex hormones in the kidney and endolymphatic sac. An immunohistological study. Acta Otolaryngol. 1994, 114, 48–51. [Google Scholar] [CrossRef]
- Grassi, S.; Frondaroli, A.; Di Mauro, M.; Pettorossi, V.E. Influence of testosterone on synaptic transmission in the rat medial vestibular nuclei: Estrogenic and androgenic effects. Neuroscience 2010, 171, 666–676. [Google Scholar] [CrossRef]
- Takeda, T.; Takeda, S.; Kitano, H.; Okada, T.; Kakigi, A. Endolymphatic hydrops induced by chronic administration of vasopressin. Hear. Res. 2000, 140, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; Kitahara, T.; Uno, A.; Kondoh, K.; Morihana, T.; Okumura, S.; Nakagawa, A.; Mitani, K.; Masumura, C.; Kubo, T. Vestibular function and vasopressin. Acta Otolaryngol. Suppl. 2004, 553, 50–53. [Google Scholar] [CrossRef]
- Katagiri, Y.; Takumida, M.; Hirakawa, K.; Anniko, M. Long-term administration of vasopressin can cause Ménière’s disease in mice. Acta Otolaryngol. 2014, 134, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, K.S. Vestibular Function Change in a Vasopressin-Induced Hydrops Model. Otol. Neurotol. 2017, 38, e495–e500. [Google Scholar] [CrossRef] [PubMed]
- Asmar, M.H.; Gaboury, L.; Saliba, I. Ménière’s Disease Pathophysiology: Endolymphatic Sac Immunohistochemical Study of Aquaporin-2, V2R Vasopressin Receptor, NKCC2, and TRPV4. Otolaryngol. Head Neck Surg. 2018, 158, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Z.L.; Wang, G.H.; Fan, J.W. Plasma vasopressin, an etiologic factor of motion sickness in rat and human? Neuroendocrinology 2005, 81, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.H.; Yang, Y.; Liu, H.X.; Xiao, S.F.; Qiu, W.X.; Wang, J.X.; Zhao, C.C.; Gui, Y.H.; Liu, G.Z.; Peng, B.; et al. Inner Ear Arginine Vasopressin-Vasopressin Receptor 2-Aquaporin 2 Signaling Pathway Is Involved in the Induction of Motion Sickness. J. Pharmacol. Exp. Ther. 2020, 373, 248–260. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Inokuchi, A.; Komiyama, S. Neuronal responses to vestibular stimulation in the guinea pig hypothalamic paraventricular nucleus. Eur. Arch. Otorhinolaryngol. 1997, 254, 95–100. [Google Scholar] [CrossRef]
- Markia, B.; Kovács Palkovits, M. Projections from the vestibular nuclei to the hypothalamic paraventricular nucleus: Morphological evidence for the existence of a vestibular stress pathway in the rat brain. Brain Struct. Funct. 2008, 213, 239–245. [Google Scholar] [CrossRef]
- Palkovits, M.; De Jong, W.; De Wied, D. Hypothalamic control of aldosterone production in sodium deficient rats. Neuroendocrinology 1974, 14, 297–309. [Google Scholar] [CrossRef]
- Sailesh, K.S.; Mukkadan, J.K. Vestibular modulation of endocrine secretions—A review. Int. J. Res. Health Sci. 2014, 2, 68–78. [Google Scholar]
- Tighilet, B.; Manrique, C.; Lacour, M. Stress axis plasticity during vestibular compensation in the adult cat. Neuroscience 2009, 160, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Van Sluijs, R.; Wilhelm, E.; Rondei, Q.; Omlin, X.; Crivelli, F.; Straumann, D.; Jäger, L.; Riener, R.; Achermann, P. Gentle rocking movements during sleep in the elderly. J. Sleep Res. 2020, 29, e12989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kompotis, K.; Hubbard, J.; Emmenegger, Y.; Perrault, A.; Mühlethaler, M.; Schwartz, S.; Bayer, L.; Franken, P. Rocking Promotes Sleep in Mice through Rhythmic Stimulation of the Vestibular System. Curr. Biol. 2019, 29, 392–401.e4. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.; Mauvieux, B.; Bulla, J.; Quarck, G.; Davenne, D.; Denise, P.; Philoxène, B.; Besnard, S. Vestibular loss disrupts daily rhythm in rats. J. Appl. Physiol. 2015, 118, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, P.M.; Jones, T.A.; Jones, S.M.; Fuller, C.A. Neurovestibular modulation of circadian and homeostatic regulation: Vestibulohypothalamic connection? Proc. Natl. Acad. Sci. USA 2002, 99, 15723–15728. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.; Dauvilliers, Y.; Koumar, O.-C.; Bouet, V.; Freret, T.; Besnard, S.; Dauphin, F.; Bessot, N. Dual orexin receptor antagonist induces changes in core body temperature in rats after exercise. Sci. Rep. 2019, 9, 18432. [Google Scholar] [CrossRef] [Green Version]
- Cavdar, S.; Onat, F.; Aker, R.; Sehirli, U.; San, T.; Yananli, H.R. The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. J. Anat. 2001, 198 Pt 4, 463–472. [Google Scholar] [CrossRef]
- Mickle, W.; Ades, H. Rostral Projection Pathway of the Vestibular System. Am. J. Physiol. Content 1954, 176, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8 (Suppl. S3), 34–42. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Yu, L.; He, Y.C.; Li, H.Z.; Zhu, J.N.; Wang, J.J. A role for orexin in central vestibular motor control. Neuron 2011, 69, 793–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Zhang, X.Y.; Chen, Z.P.; Zhuang, Q.X.; Zhu, J.N.; Wang, J.J. Orexin excites rat inferior vestibular nuclear neurons via co-activation of OX1 and OX2 receptors. J. Neural Transm. 2015, 122, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Takumida, M.; Anniko, M. Localization of melatonin and its receptors (melatonin 1a and 1b receptors) in the mouse inner ear. Acta Otolaryngol. 2019, 139, 948–952. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chen, J.; Guan, L.N.; Cao, X.L.; Li, Z.X.; Jiang, H.Q. Relationship between the level of estrogen, calcium and phosphorus concentration in serum with benign paroxysmal positional vertigo. J. Clin. Otorhinol. Head Neck Surg. 2019, 33, 497–500. [Google Scholar] [CrossRef]
- Feng, M.Y.; Zhuang, J.H.; Gu, H.H.; Tian, Q.; Zhang, Z.H. Changes of serum E2 and Otolin-1 levels in postmenopausal women with BPPV. J. Clin. Otorhinol. Head Neck Surg. 2019, 33, 1138–1141, 1147. [Google Scholar]
- Feng, M.Y.; Gu, H.H.; Tian, Q.; Yang, H.L.; Zhuang, J.H. Molecular Mediators of Estrogen Reduction-induced Otolith Shedding. Curr. Med. Sci. 2021, 41, 667–672. [Google Scholar] [CrossRef]
- Guerra, J.; Devesa, J. Causes and treatment of idiopathic benign paroxysmal positional vertigo based on endocrinological and other metabolic factors. J. Otol. 2020, 15, 155–160. [Google Scholar] [CrossRef]
- Jeong, S.H. Benign Paroxysmal Positional Vertigo Risk Factors Unique to Perimenopausal Women. Front. Neurol. 2020, 11, 589605. [Google Scholar] [CrossRef]
- Liu, D.H.; Kuo, C.H.; Wang, C.T.; Chiu, C.C.; Chen, T.J.; Hwang, D.K.; Kao, C.L. Age-Related Increases in Benign paroxysmal positional vertigo are reversed in women taking estrogen replacement therapy: A population-based study in Taiwan. Front. Aging Neurosci. 2017, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Ogun, O.A.; Büki, B.; Cohn, E.S.; Janky, K.L.; Lundberg, Y.W. Menopause and benign paroxysmal positional vertigo. Menopause 2014, 21, 886–889. [Google Scholar] [CrossRef] [Green Version]
- Vibert, D.; Kompis, M.; Häusler, R. Benign paroxysmal positional vertigo in older women may be related to osteoporosis and osteopenia. Ann. Otol. Rhino Laryngol. 2003, 112, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Zhang, L.; Li, G.H.; Zhang, W.W.; Wang, Y.P.; Geng, B. The change of female progesterone level and blood calcium concentration in perimenopausal women with benign paroxysmal positional vertigo. Chin. J. Otorhinol. Head Neck Surg. 2017, 52, 287–290. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Zhang, Y.; Vijayakumar, S.; Jones, S.M.; Lundberg, Y.W. Mechanism Underlying the Effects of Estrogen Deficiency on Otoconia. J. Assoc. Res. Otolaryngol. 2018, 19, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Yang, Z.D.; Li, W.X.; Shi, C.; Yu, Y.F. The relationship between the recurrence of benign paroxysmal positional vertigo and the level of bone mineral as well as estrogen in postmenopausal women. Chin. J. Otorhinol. Head Neck Surg. 2017, 52, 881–884. [Google Scholar] [CrossRef]
- Chiarella, G.; Russo, D.; Monzani, F.; Petrolo, C.; Fattori, B.; Pasqualetti, G.; Cassandro, E.; Costante, G. Hashimoto thyroiditis and vestibular dysfunction. Endocr. Pract. 2017, 23, 863–868. [Google Scholar] [CrossRef]
- Lin, W.L.; Chen, C.Y.; Hsu, T.Y.; Chen, W.K.; Lin, C.L.; Chen, H.C. Hypothyroidism is an independent risk factor for Ménière’s disease: A population-based cohort study. Medicine 2019, 98, e15166. [Google Scholar] [CrossRef]
- Choi, H.G.; Song, Y.S.; Wee, J.H.; Min, C.; Yoo, D.M.; Kim, S.Y. Analyses of the Relation between BPPV and Thyroid Diseases: A Nested Case-Control Study. Diagnostics 2021, 11, 329. [Google Scholar] [CrossRef]
- Miśkiewicz-Orczyk, K.A.; Lisowska, G.; Kajdaniuk, D.; Wojtulek, M. Can Hashimoto’s thyroiditis cause vertigo? Endokrynol. Pol. 2020, 71, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Doroszewska, G.; Kazmierczak, H. Hyperinsulinemia in vertigo, tinnitus and hearing loss. Otolaryngol. Pol. 2002, 56, 57–62. [Google Scholar]
- Gioacchini, F.M.; Albera, R.; Re, M.; Scarpa, A.; Cassandro, C.; Cassandro, E. Hyperglycemia and diabetes mellitus are related to vestibular organs dysfunction: Truth or suggestion? A literature review. Acta Diabetol. 2018, 55, 1201–1207. [Google Scholar] [CrossRef]
- Webster, G.; Sens, P.M.; Salmito, M.C.; Cavalcante, R.J.D.; Bonifácio dos Santos, P.R.; Muniz da Silva, A.L.; de Souza, C.F. Hyperinsulinemia and hyperglycemia: Risk factors for recurrence of benign paroxysmal positional vertigo. Braz. J. Otorhinolaryngol. 2015, 81, 347–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawron, W.; Pospiech, L.; Orendorz-Fraczkowska, K.; Noczynska, A. Are there any disturbances in vestibular organ of children and young adults with Type I diabetes? Diabetologia 2002, 45, 728–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittar, R.S.M.; Santos, M.D.A.; Mezzalira, R. Glucose metabolism disorders and vestibular manifestations: Evaluation through computerized dynamic posturography. Braz. J. Otorhinolaryngol. 2016, 82, 372–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Yu, X.; Jiang, W.; Zhang, C.; Ahan, T.; He, Y. Clinical significance of serum sex hormones in postmenopausal women with vestibular migraine: Potential role of estradiol. J. Int. Med. Res. 2021, 49, 3000605211016379. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Viirre, E. Vestibular migraine may be an important cause of dizziness/vertigo in perimenopausal period. Med. Hypotheses 2010, 75, 409–414. [Google Scholar] [CrossRef]
- Ray, C.A.; Monahan, K.D. Vestibulo sympathetic reflex in humans neural interactions between cardio vascular reflexes. Clin. Exp. Pharmacol. Physiol. 2002, 29, 98–102. [Google Scholar] [CrossRef]
- Aoki, M.; Asai, M.; Nishihori, T.; Mizuta, K.; Ito, Y.; Ando, K.J. The Relevance of an Elevation in the Plasma Vasopressin Levels to the Pathogenesis of Ménière’s Attack. J. Neuroendocrinol. 2007, 11, 901–906. [Google Scholar] [CrossRef]
- Rybak, L.P. Metabolic disorders of the vestibular system. Otolaryngol. Head Neck Surg. 1995, 112, 128–132. [Google Scholar] [CrossRef]
- Degerman, E.; Rauch, U.; Lindberg, S.; Caye-Thomasen, P.; Hultgårdh, A.; Magnusson, M. Expression of insulin signaling components in the sensory epithelium of the human saccule. Cell Tissue Res. 2013, 352, 469–478. [Google Scholar] [CrossRef]
- Aoki, M.; Hayashi, H.; Kuze, B.; Mizuta, K.; Ito, Y. The association of the plasma vasopressin level during attacks with a prognosis of Ménière’s disease. Int. J. Audiol. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Kitahara, T.; Okamoto, H.; Fukushima, M.; Sakagami, M.; Ito, T.; Yamashita, A.; Ota, I.; Yamanaka, T. A Two-Year Randomized Trial of Interventions to Decrease Stress Hormone Vasopressin Production in Patients with Ménière’s Disease—A Pilot Study. PLoS ONE 2016, 11, e0158309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.S.; Lange, M.E.; Megerian, C.A. Serum antidiuretic hormone levels in patients with unilateral Ménière’s disease. Laryngoscope 2003, 113, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Ando, K.; Kuze, B.; Mizuta, K.; Hayashi, T.; Ito, Y. The association of antidiuretic hormone levels with an attack of Ménière’s disease. Clin. Otolaryngol. 2005, 30, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Hornibrook, J.; George, P.; Gourley, J. Vasopressin in definite Ménière’s disease with positive electrocochleographic findings. Acta Otolaryngol. 2011, 131, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Okayasu, T.; Ito, T.; Fujita, H.; Ueda, K. Endolymphatic Sac Drainage Surgery and Plasma Stress Hormone Vasopressin Levels in Ménière’s Disease. Front. Neurol. 2021, 12, 722217. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Maekawa, C.; Kizawa, K.; Horii, A.; Doi, K. Plasma vasopressin and V2 receptor in the endolymphatic sac in patients with delayed endolymphatic hydrops. Otol. Neurotol. 2009, 30, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Doi, K.; Maekawa, C.; Kizawa, K.; Horii, A.; Kubo, T.; Kiyama, H. Ménière’s attacks occur in the inner ear with excessive vasopressin type-2 receptors. J. Neuroendocrinol. 2008, 12, 1295–1300. [Google Scholar] [CrossRef]
- Takeda, T.; Takeda, S.; Kakigi, A.; Okada, T.; Nishioka, R.; Taguchi, D.; Nishimura, M.; Nakatani, H. Hormonal aspects of Ménière’s disease on the basis of clinical and experimental studies. ORL J. Otorhinolaryngol. Relat. Spec. 2010, 71 (Suppl. S1), 1–9. [Google Scholar] [CrossRef]
- Falkenius-Schmidt, K.; Rydmarker, S.; Horner, K.C. Hyperprolactinemia in some Ménière patients even in the absence of incapacitating vertigo. Hear. Res. 2005, 203, 154–158. [Google Scholar] [CrossRef]
- Horner, K.C.; Guieu, R.; Magnan, J.; Chays, A.; Cazals, Y. Prolactinoma in some Ménière’s patients—Is stress involved? Neuropsychopharmacology 2002, 26, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Aoki, M.; Yokota, Y.; Hayashi, T.; Kuze, B.; Murai, M.; Mizuta, K.; Ito, Y. Disorder of the saliva melatonin circadian rhythm in patients with Ménière’s disease. Acta Neurol. Scand. 2006, 113, 256–261. [Google Scholar] [CrossRef]
- Mateijsen, D.J.; Kingma, C.M.; De Jong, P.E.; Wit, H.P.; Albers, F.W. Aldosterone assessment in patients with Menière’s disease. ORL J. Otorhinolaryngol. Relat. Spec. 2001, 63, 280–286. [Google Scholar] [CrossRef]
- Jian, H.; Yu, G.; Chen, G.; Lin, N.; Wang, H. Correlation between auditory-vestibular functions and estrogen levels in postmenopausal patients with Ménière’s disease. J. Clin. Lab. Anal. 2019, 33, e22626. [Google Scholar] [CrossRef] [Green Version]
- Grillo, C.M.; Maiolino, L.; Rapisarda, A.M.C.; Caruso, G.; Palermo, G.; Caruso, S. Effects of hormone therapy containing 2 mg drospirenone and 1 mg 17β-estradiol on postmenopausal exacerbation of Ménière’s disease: Preliminary study. Exp. Ther. Med. 2021, 22, 1103. [Google Scholar] [CrossRef] [PubMed]
- Owada, S.; Yamamoto, M.; Suzuki, M.; Yoshida, T.; Nomura, T. Clinical evaluation of vertigo in menopausal women. Nihon Jibiinkoka Gakkai Kaiho 2012, 115, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.C.; Honrubia, V. Premenstrual exacerbation of Ménière’s disease revisited. Otolaryngol. Clin. N. Am 2010, 43, 1029–1040. [Google Scholar] [CrossRef]
- Horner, K.C.; Cazals, Y. Stress hormones in Ménière’s disease and acoustic neuroma. Brain Res. Bull. 2005, 66, 1–8. [Google Scholar] [CrossRef]
- Horner, K.C.; Cazals, Y. Stress in hearing and balance in Ménière’s disease. Noise Health 2003, 5, 29–34. [Google Scholar] [PubMed]
- Juhn, S.K.; Li, W.; Kim, J.Y.; Javel, E.; Levine, S.; Odland, R.M. Effect of stress-related hormones on inner ear fluid homeostasis and function. Am. J. Otol. 1999, 20, 800–806. [Google Scholar] [PubMed]
- Van Cruijsen, N.; Dullaart, R.P.; Wit, H.P.; Albers, F.W. Analysis of cortisol and other stress-related hormones in patients with Ménière’s disease. Otol. Neurotol. 2005, 26, 1214–1219. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.L.; Wang, G.H. The relationship of the content of AVP and the expression of V1b receptors in some brain areas with the sexual difference in the susceptibility of motion sickness in rats. Acta Otolaryngol. Suppl. 2011, 27, 46–50. [Google Scholar]
- Abdel-Salam, M.; Awad, O.; El-Badry, M.; Ibrahim, A.; Ibrahiem, M.H. The possible effect of human menopausal gonadotropin on the audio-vestibular system. Auris Nasus Larynx 2018, 45, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Agrawal, Y.; Darlington, C.L. Sexual dimorphism in vestibular function and dysfunction. J. Neurophysiol. 2019, 121, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Rzewnicki, I.; Knapp, P.; Kluz-Kowal, A.B.; Kuryliszyn-Moskal, A.; Terlikowski, R. Activity of vestibular organs in menopausal women non-users hormone replacement therapy (HRT). Otolaryngol. Pol. 2010, 64, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Bustamante, M.; Del Cid Chua, C.; Vázquez, M.; Bello Dotel, L.; Baez Recalde, M. Estrogen and neurotological diders in women sexual hormones and neurotological disorders in women. Rev. Fac. Cien. Med. Univ. Nac Cordoba 2020, 77, 351–355. [Google Scholar] [CrossRef]
- Ishii, C.; Nishino, L.K.; Alberto, C.; Campos, H. De Vestibular characterization in the menstrual cycle. Braz. J. Otorhinolaryngol. 2009, 75, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Corazzi, V.; Ciorba, A.; Skarzynski, P.H.; Skarżyńska, M.B.; Bianchini, C.; Stomeo, F.; Bellini, T.; Pelucchi, S.; Hatzopoulos, S. Gender differences in audio-vestibular disorders. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420929174. [Google Scholar] [CrossRef]
- Korsunska, L.L.; Meshcheryakova, A.V. The epidemiology of vestibular dysfunction in women with perimenopausal period. Crime. J. Exp. Clin. Med. 2013, 3, 14–17. [Google Scholar]
- Gliddon, C.M.; Darlington, C.L.; Smith, P.F. Activation of the hypothalamic-pituitary-adrenal axis following vestibular deafferentation in pigmented guinea pig. Brain Res. 2003, 964, 306–310. [Google Scholar] [CrossRef]
- Pan, L.; Qi, R.; Wang, J.; Zhou, W.; Liu, J.; Cai, Y. Evidence for a Role of Orexin/Hypocretin System in Vestibular Lesion-Induced Locomotor Abnormalities in Rats. Front. Neurosci. 2016, 10, 355. [Google Scholar] [CrossRef] [Green Version]
- Gliddon, C.M.; Smith, P.F.; Darlington, C.L. Interaction between the hypothalamic-pituitary-adrenal axis and behavioural compensation following unilateral vestibular deafferentation. Acta Otolaryngol. 2003, 123, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D. Neural substrates linking balance control and anxiety. Physiol. Behav. 2002, 77, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.N.; Kirkeby, S.; Vikeså, J.; Nielsen, F.C.; Cayé-Thomasen, P. The human endolymphatic sac expresses natriuretic peptides. Laryngoscope 2017, 127, E201–E208. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, T.; Bicer, Y.O.; Serin, E.; Seyhan, S.; Koybasi Sanal, S. Salivary α-amylase levels in vertigo: Can it be an autonomic dysfunction? Ear Nose Throat J. 2018, 97, 278–282. [Google Scholar] [CrossRef]
- Cozma, S.; Ghiciuc, C.M.; Damian, L.; Pasquali, V.; Saponaro, A.; Lupusoru, E.C.; Patacchioli, F.R.; Dima-Cozma, L.C. Distinct activation of the sympathetic adreno-medullar system and hypothalamus pituitary adrenal axis following the caloric vestibular test in healthy subjects. PLoS ONE 2018, 13, e0193963. [Google Scholar] [CrossRef] [Green Version]
- Dagilas, A.; Kimiskidis, V.; Aggelopoulou, M.; Kapaki, E.; Fitili, C.; Libitaki, G.; Papagiannopoulos, S.; Kazis, D.; Kazis, A.; Aidonis, A. Changes in blood neurotransmitter and steroid levels during evoked vertigo. Otol. Neurotol. 2005, 26, 476–480. [Google Scholar] [CrossRef]
- Kahraman, S.S.; Ozcan, O.; Arli, C.; Ustun, I.; Erduran, R.; Akoglu, E.; Gokce, C. Calcium Homeostasis During Attack and Remission in Patients With Idiopathic Benign Paroxysmal Positional Vertigo. Otol. Neurotol. 2016, 37, 1388–1392. [Google Scholar] [CrossRef]
- Saman, Y.; Arshad, Q.; Dutia, M.; Rea, P. Stress and the vestibular system. Int. Rev. Neurobiol. 2020, 152, 221–236. [Google Scholar] [CrossRef]
- Saman, Y.; Bamiou, D.E.; Gleeson, M.; Dutia, M.B. Interactions between Stress and Vestibular Compensation—A Review. Front. Neurol. 2012, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Yetiser, S. Review of the pathology underlying benign paroxysmal positional vertigo. J. Int. Med. Res. 2020, 48, 0300060519892370. [Google Scholar] [CrossRef] [Green Version]
- Serra, A.P.; Lopes, K.C.; Dorigueto, R.S.; Ganança, F.F. Blood glucose and insulin levels in patients with peripheral vestibular disease. Braz. J. Otorhinolaryngol. 2009, 75, 701–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pathology and Method of the Research | Hormonal Variation | References |
|---|---|---|
| VPPB | ||
| - BPPV and estrogen, BPPV and peri-menopause, BPPV and postmenopausal, | Estradiol (women in pre and post menopause) | [34,35,36,37,38,39,40,41,42,43,44] |
| - BPPV and thyroid | Thyroid pathologies | [47,48] |
| - BPPV and cortisol | ||
| - BPPV and stress hormones | ||
| - BPPV and vasopressin | ||
| - BPPV and insulin | Insulin | [51] |
| Ménière disease | ||
| - Ménière disease and vasopressin | Vasopressin | [2,56,59,60,61,62,63,64,65,66,67,68] |
| - Ménière disease and prolactin | Prolactin | [69,70] |
| - Ménière disease and melatonin | Melatonin | [71] |
| - Ménière disease and aldosterone | Aldosterone | [72] |
| - Ménière disease and menopause | Sexual hormones | [73,74,75,76] |
| - Ménière and stress hormones | Stress hormones | [77,78,79,80] |
| Vestibular migraine | ||
| - vestibular migraine and hormones | Estradiol | [54,55] |
| - vestibular migraine and menopause | ||
| - vestibular migraine and estrogen/vestibular migraine and menstrual cycle | ||
| - vestibular migraine and thyroid | ||
| - vestibular migraine and insulin | ||
| - vestibular migraine and cortisol | ||
| Vestibular dysfunction | ||
| - vestibular dysfunction and vasopressin | Vasopressin | [2,12,13,15,81] |
| - vestibular dysfunction and insulin | Insulin | [52,53] |
| - vestibular dysfunction and estrogen | Estradiol | [82,83,84,85,86,87,88] |
| - vestibular dysfunction and menopause | [89] | |
| - vestibular dysfunction and cortisol | Cortisol | |
| Vestibular system | ||
| Vestibular system and hypothalamus | Hypothalamus | [2,22,89,90,91,92,93] |
| Acute vertigo | ||
| - acute vertigo and hormones | Different hormones | [57,60,94,95,96,97] |
| - acute vertigo and cortisol | ||
| - acute and thyroid vertigo | ||
| - acute vertigo and insulin | ||
| - acute vertigo and progesterone | ||
| - acute vertigo and estrogen | ||
| - acute vertigo and vasopressin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khiati, R.; Tighilet, B.; Besnard, S.; Chabbert, C. Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives. Cells 2023, 12, 656. https://doi.org/10.3390/cells12040656
El Khiati R, Tighilet B, Besnard S, Chabbert C. Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives. Cells. 2023; 12(4):656. https://doi.org/10.3390/cells12040656
Chicago/Turabian StyleEl Khiati, Rhizlane, Brahim Tighilet, Stéphane Besnard, and Christian Chabbert. 2023. "Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives" Cells 12, no. 4: 656. https://doi.org/10.3390/cells12040656
APA StyleEl Khiati, R., Tighilet, B., Besnard, S., & Chabbert, C. (2023). Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives. Cells, 12(4), 656. https://doi.org/10.3390/cells12040656






