Adh Promotes Actinobacillus pleuropneumoniae Survival in Porcine Alveolar Macrophages by Inhibiting CHAC2-Mediated Respiratory Burst and Inflammatory Cytokine Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Strains, Cells and Culture Conditions
2.2. Regents, Antibodies and Plasmids
2.3. Adhesion and Intracellular Survival Assays
2.4. Immunofluorescence
2.5. RNA Extraction and qRT-PCR
2.6. Gene Chip Was Used to Detect the Expression Profile of Lung Cells
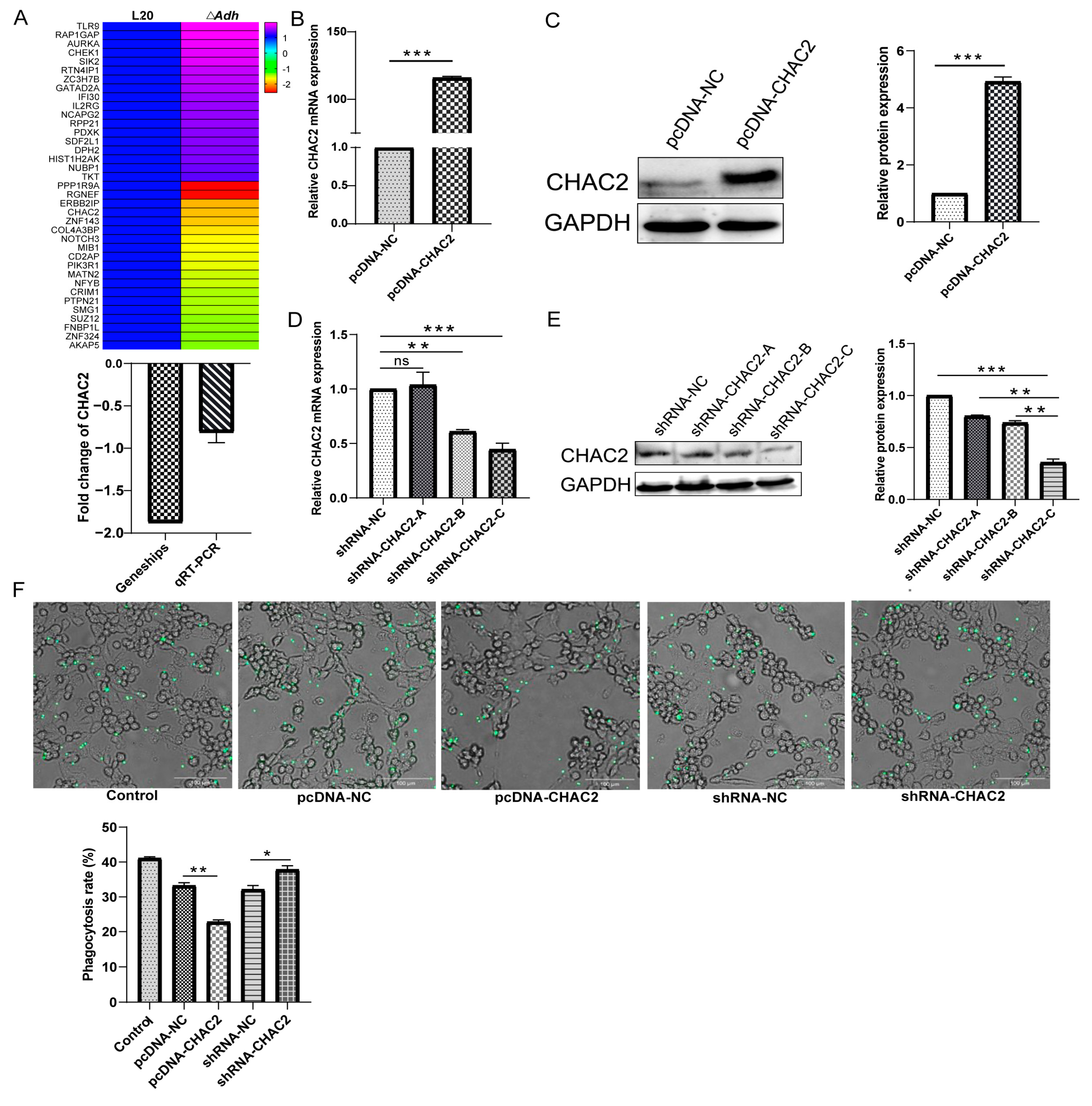
2.7. Construction and Verification of the Plasmid pcDNA-CHAC2 and shRNA-CHAC2
2.8. Western-Blot
2.9. Phagocytosis Experiments
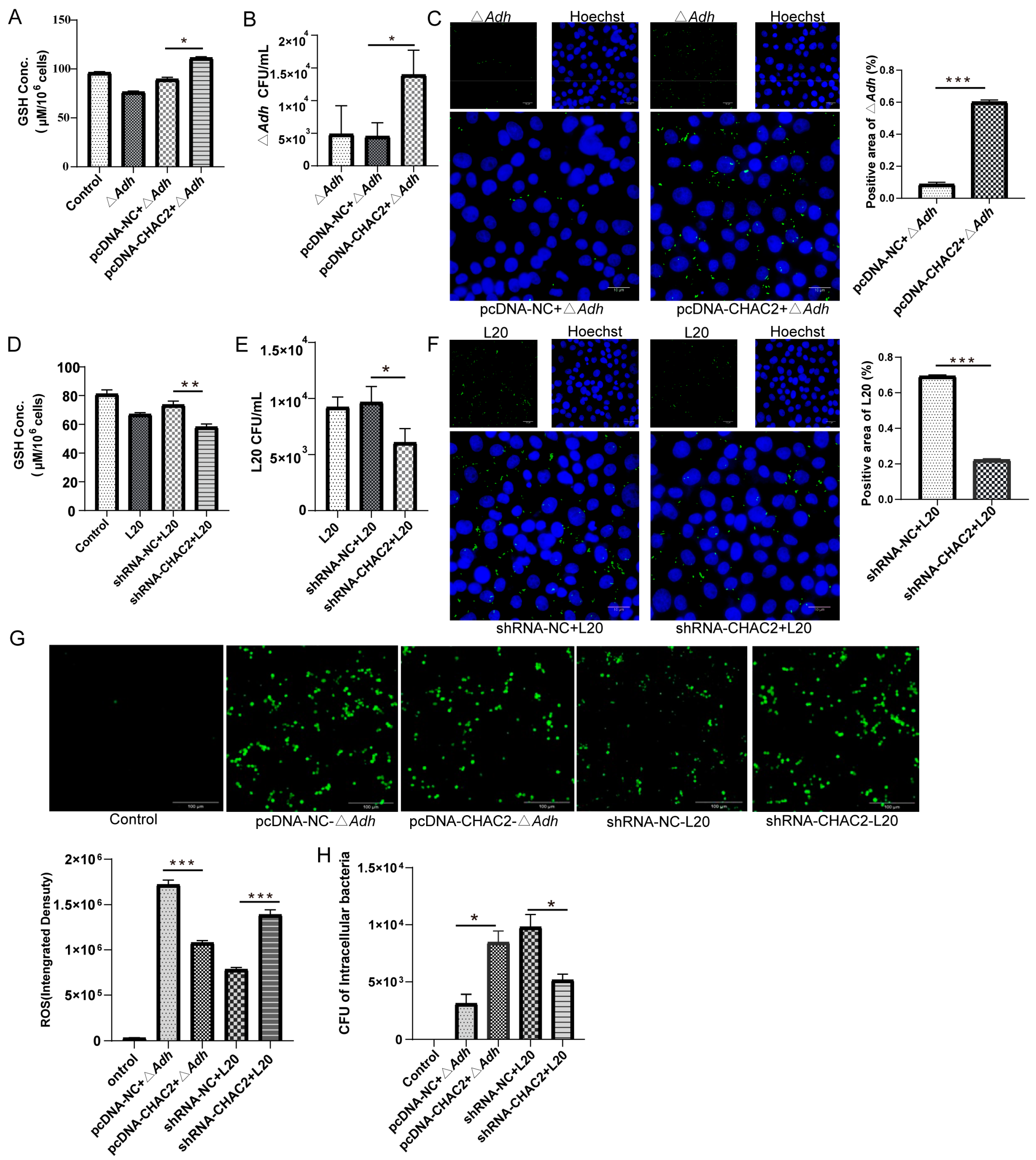
2.10. GSH Detection
2.11. ROS Level Detection
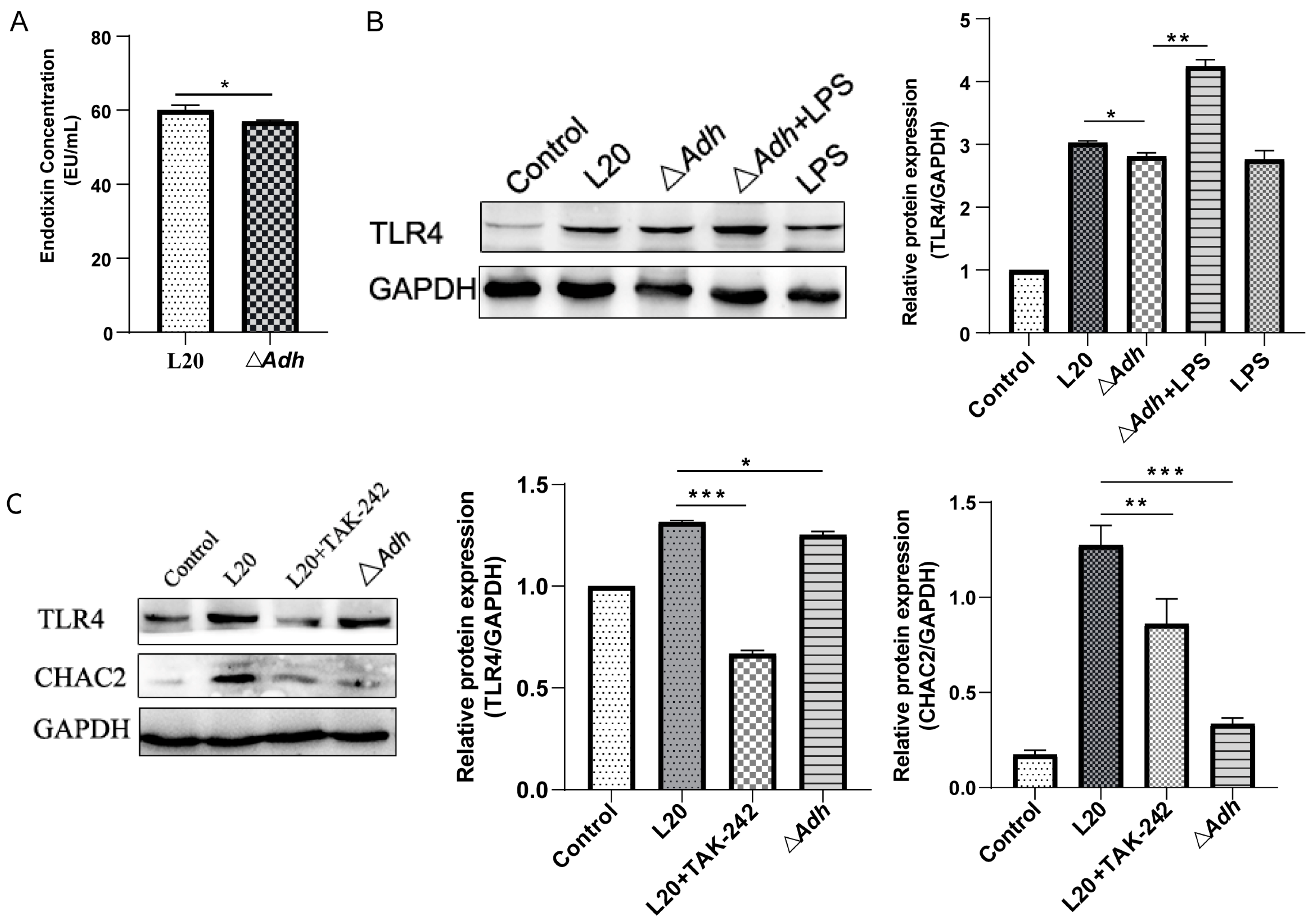
2.12. LPS Detection
2.13. Statistical Analysis
3. Results
3.1. Adh Deletion Decreases A. pleuropneumoniae Adhesion, Intracellular Survival in PAM and Cytokine Expressions
3.2. CHAC2 Expression Inhibits the Phagocytic Capacity of PAM
3.3. CHAC2 Inhibits ROS Generation in PAM to Improve A. pleuropneumoniae Survival
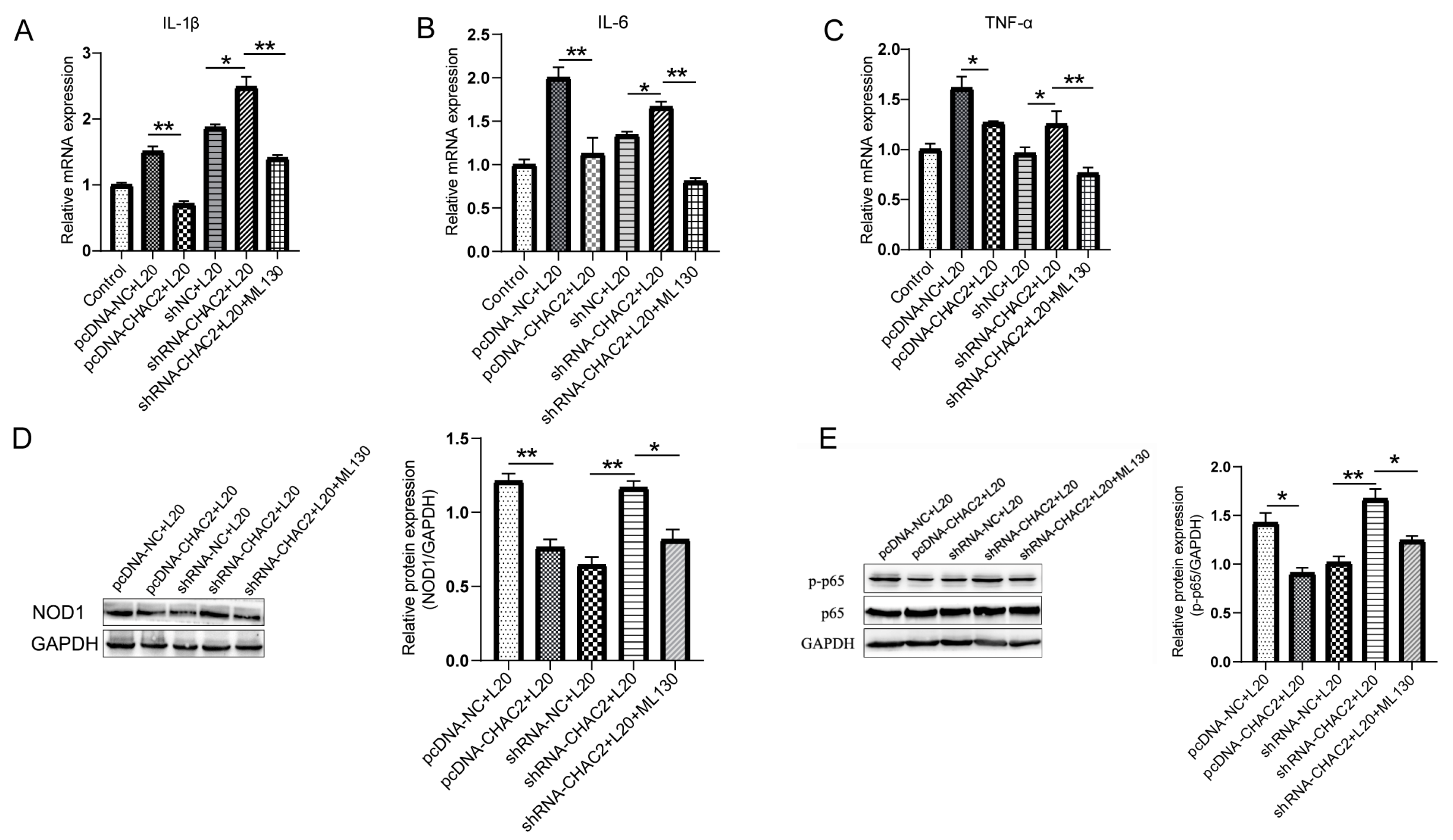
3.4. CHAC2 Inhibits Inflammatory Cytokine Expression by NOD1/NF-κB Signaling Path Way during A. pleuropneumoniae Infection
3.5. CHAC2 Is Regulated by Adh via the LPS-TLR4 Pathway
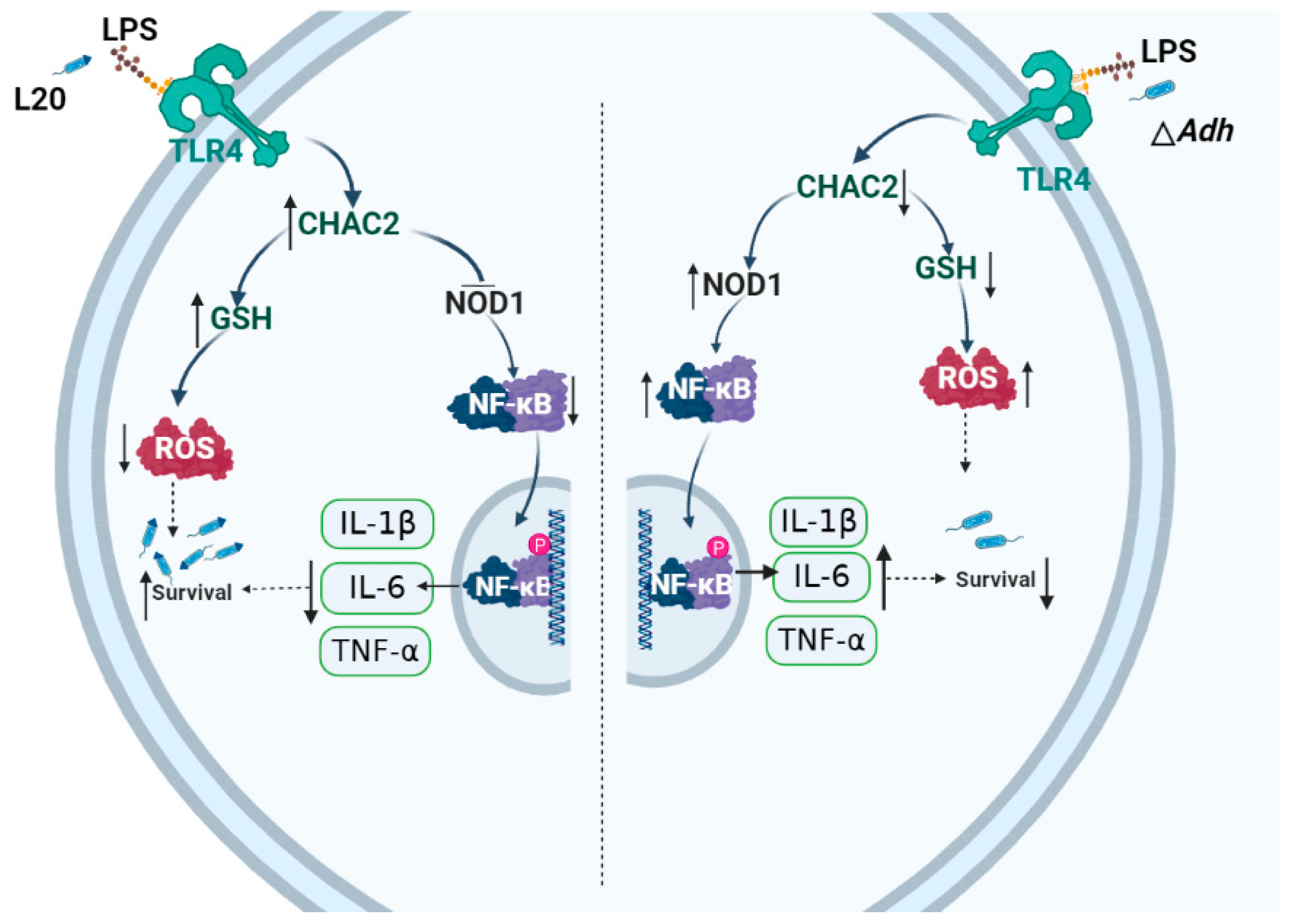
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Auger, E.; Deslandes, V.; Ramjeet, M.; Contreras, I.; Nash, J.H.; Harel, J.; Gottschalk, M.; Olivier, M.; Jacques, M. Host-pathogen interactions of Actinobacillus pleuropneumoniae with porcine lung and tracheal epithelial cells. Infect. Immun. 2009, 77, 1426–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiers, K.; De Waele, T.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F. Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet. Res. 2010, 41, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Qin, W.; Yang, S.; Zhai, R.; Zhou, L.; Sun, C.; Pan, F.; Ji, Q.; Wang, Y.; Gu, J.; et al. The Adh adhesin domain is required for trimeric autotransporter Apa1-mediated Actinobacillus pleuropneumoniae adhesion, autoaggregation, biofilm formation and pathogenicity. Vet. Microbiol. 2015, 177, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, A.R.; Malik, A.; Goldman, A. Recent advances in the understanding of trimeric autotransporter adhesins. Med. Microbiol. Immunol. 2020, 209, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Qin, W.; Zhang, J.; Bao, C.; Zhang, H.; Che, Y.; Sun, C.; Gu, J.; Feng, X.; Du, C.; et al. Adh enhances Actinobacillus pleuropneumoniae pathogenicity by binding to OR5M11 and activating p38 which induces apoptosis of PAMs and IL-8 release. Sci. Rep. 2016, 6, 24058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Zhou, L.; Sun, C.; Feng, X.; Du, C.; Gao, Y.; Ji, Q.; Yang, S.; Wang, Y.; Han, W.; et al. Apa is a trimeric autotransporter adhesin of Actinobacillus pleuropneumoniae responsible for autoagglutination and host cell adherence. J. Basic Microbiol. 2012, 52, 598–607. [Google Scholar] [CrossRef]
- Sarantis, H.; Grinstein, S. Subversion of Phagocytosis for Pathogen Survival. Cell Host Microbe 2012, 12, 419–431. [Google Scholar] [CrossRef] [Green Version]
- Flannagan, R.S.; Cosio, G.; Grinstein, S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 2009, 7, 355–366. [Google Scholar] [CrossRef]
- Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies Used by Bacteria to Grow in Macrophages. Microbiol. Spectr. 2016, 4, 701–725. [Google Scholar] [CrossRef] [Green Version]
- Cano, V.; March, C.; Insua, J.L.; Aguilo, N.; Llobet, E.; Moranta, D.; Regueiro, V.; Brennan, G.P.; Isabel Millan-Lou, M.; Martin, C.; et al. Klebsiella pneumoniae survives within macrophages by avoiding delivery to lysosomes. Cell. Microbiol. 2015, 17, 1537–1560. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Fei, W.; Shi, Q.; Li, Q.; Kuang, Y.; Wang, C.; He, C.; Hu, X. CHAC2, downregulated in gastric and colorectal cancers, acted as a tumor suppressor inducing apoptosis and autophagy through unfolded protein response. Cell Death Dis. 2017, 8, e3009. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.K.; Park, J.S.; Jang, J.Y.; Kim, K.R.; Vo, T.T.L.; Kim, K.W.; Han, B.W. Structural and Functional Analyses of Human ChaC2 in Glutathione Metabolism. Biomolecules 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, W.X.; Zhu, R.V.; Bao, C.T.; Xiao, J.M.; Liu, B.J.; Sun, M.; Feng, X.; Gu, J.M.; Li, Y.; Lei, L.C. Porcine circovirus type 2 promotes Actinobacillus pleuropneumoniae survival during coinfection of porcine alveolar macrophages by inhibiting ROS production. Vet. Microbiol. 2019, 233, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, H.; Jiang, X.; Liao, X.; Yue, M.; Li, X.; Fang, W. PrsA contributes to Streptococcus suis serotype 2 pathogenicity by modulating secretion of selected virulence factors. Vet. Microbiol. 2019, 236, 108375. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Joffe, A.M.; Bakalar, M.H.; Fletcher, D.A. Macrophage phagocytosis assay with reconstituted target particles. Nat. Protoc. 2020, 15, 2230–2246. [Google Scholar] [CrossRef]
- Kaur, A.; Gautam, R.; Srivastava, R.; Chandel, A.; Kumar, A.; Karthikeyan, S.; Bachhawat, A.K. ChaC2, an Enzyme for Slow Turnover of Cytosolic Glutathione. J. Biol. Chem. 2017, 292, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.R.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R. Glutathione: Role in Oxidative/Nitrosative Stress, Antioxidant Defense, and Treatments. Chemistryselect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Li, B.; Fang, J.; Zuo, Z.C.; Yin, S.R.; He, T.T.; Yang, M.X.; Deng, J.L.; Shen, L.H.; Ma, X.P.; Yu, S.M.; et al. Activation of Porcine Alveolar Macrophages by Actinobacillus pleuropneumoniae Lipopolysaccharide via the Toll-Like Receptor 4/NF-kappa B-Mediated Pathway. Infect. Immun. 2018, 86, e00642-17. [Google Scholar] [CrossRef] [Green Version]
- Tobias, T.J.; Klinkenberg, D.; Bouma, A.; van den Broek, J.; Daemen, A.J.; Wagenaar, J.A.; Stegeman, J.A. A cohort study on Actinobacillus pleuropneumoniae colonisation in suckling piglets. Prev. Vet. Med. 2014, 114, 223–230. [Google Scholar] [CrossRef]
- Bosse, J.T.; Janson, H.; Sheehan, B.J.; Beddek, A.J.; Rycroft, A.N.; Kroll, J.S.; Langford, P.R. Actinobacillus pleuropneumoniae: Pathobiology and pathogenesis of infection. Microbes Infect. 2002, 4, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, Q.; Han, W.; Wang, Z.; Pang, S.; Zhu, H.; Tan, K.; Liu, X.; Langford, P.R.; Huang, Q.; et al. Identification of FtpA, a Dps-Like Protein Involved in Anti-Oxidative Stress and Virulence in Actinobacillus pleuropneumoniae. J. Bacteriol. 2022, 204, e00326-21. [Google Scholar] [CrossRef] [PubMed]
- Haesebrouck, F.; Chiers, K.; Van Overbeke, I.; Ducatelle, R. Actinobacillus pleuropneumoniae infections in pigs: The role of virulence factors in pathogenesis and protection. Vet. Microbiol. 1997, 58, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Master, S.S.; Rampini, S.K.; Davis, A.S.; Keller, C.; Ehlers, S.; Springer, B.; Timmins, G.S.; Sander, P.; Deretic, V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 2008, 3, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fremond, C.M.; Togbe, D.; Doz, E.; Rose, S.; Vasseur, V.; Maillet, I.; Jacobs, M.; Ryffel, B.; Quesniaux, V.F.J. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 2007, 179, 1178–1189. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Jiang, H.; Zhu, R.; Liu, B.; Xiao, J.; Li, Z.; Chen, P.; Langford, P.R.; Zhang, F.; Lei, L. Differences in pig respiratory tract and peripheral blood immune responses to Actinobacillus pleuropneumoniae. Vet. Microbiol. 2020, 247, 108755. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Wang, L.; Qin, W.; Ruidong, Z.; Liu, S.; Zhang, H.; Sun, C.; Feng, X.; Gu, J.; Du, C.; Han, W.; et al. Differential gene expression profiling of Actinobacillus pleuropneumoniae during induction of primary alveolar macrophage apoptosis in piglets. Microb. Pathog. 2015, 78, 74–86. [Google Scholar] [CrossRef]
- Seregina, T.A.; Petrushanko, I.Y.; Shakulov, R.S.; Zaripov, P.I.; Makarov, A.A.; Mitkevich, V.A.; Mironov, A.S. The Inactivation of LPS Biosynthesis Genes in E. coli Cells Leads to Oxidative Stress. Cells 2022, 11, 2667. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhu, R.; Jiang, H.; Li, Z.; Jiang, X.; Li, F.; Zhang, F.; Feng, X.; Gu, J.; Li, N.; et al. Adh Promotes Actinobacillus pleuropneumoniae Survival in Porcine Alveolar Macrophages by Inhibiting CHAC2-Mediated Respiratory Burst and Inflammatory Cytokine Expression. Cells 2023, 12, 696. https://doi.org/10.3390/cells12050696
Zhu J, Zhu R, Jiang H, Li Z, Jiang X, Li F, Zhang F, Feng X, Gu J, Li N, et al. Adh Promotes Actinobacillus pleuropneumoniae Survival in Porcine Alveolar Macrophages by Inhibiting CHAC2-Mediated Respiratory Burst and Inflammatory Cytokine Expression. Cells. 2023; 12(5):696. https://doi.org/10.3390/cells12050696
Chicago/Turabian StyleZhu, Junhui, Rining Zhu, Hexiang Jiang, Ziheng Li, Xuan Jiang, Fengyang Li, Fuxian Zhang, Xin Feng, Jingmin Gu, Na Li, and et al. 2023. "Adh Promotes Actinobacillus pleuropneumoniae Survival in Porcine Alveolar Macrophages by Inhibiting CHAC2-Mediated Respiratory Burst and Inflammatory Cytokine Expression" Cells 12, no. 5: 696. https://doi.org/10.3390/cells12050696




