Diagnostic and Prognostic Comparison of Immune-Complex-Mediated Membranoproliferative Glomerulonephritis and C3 Glomerulopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Data Retrieval
2.2. Laboratory Analyses
2.3. Kidney Biopsies
2.4. Clinical Data
2.5. Complement Analyses
2.6. Statistical Analysis
3. Results
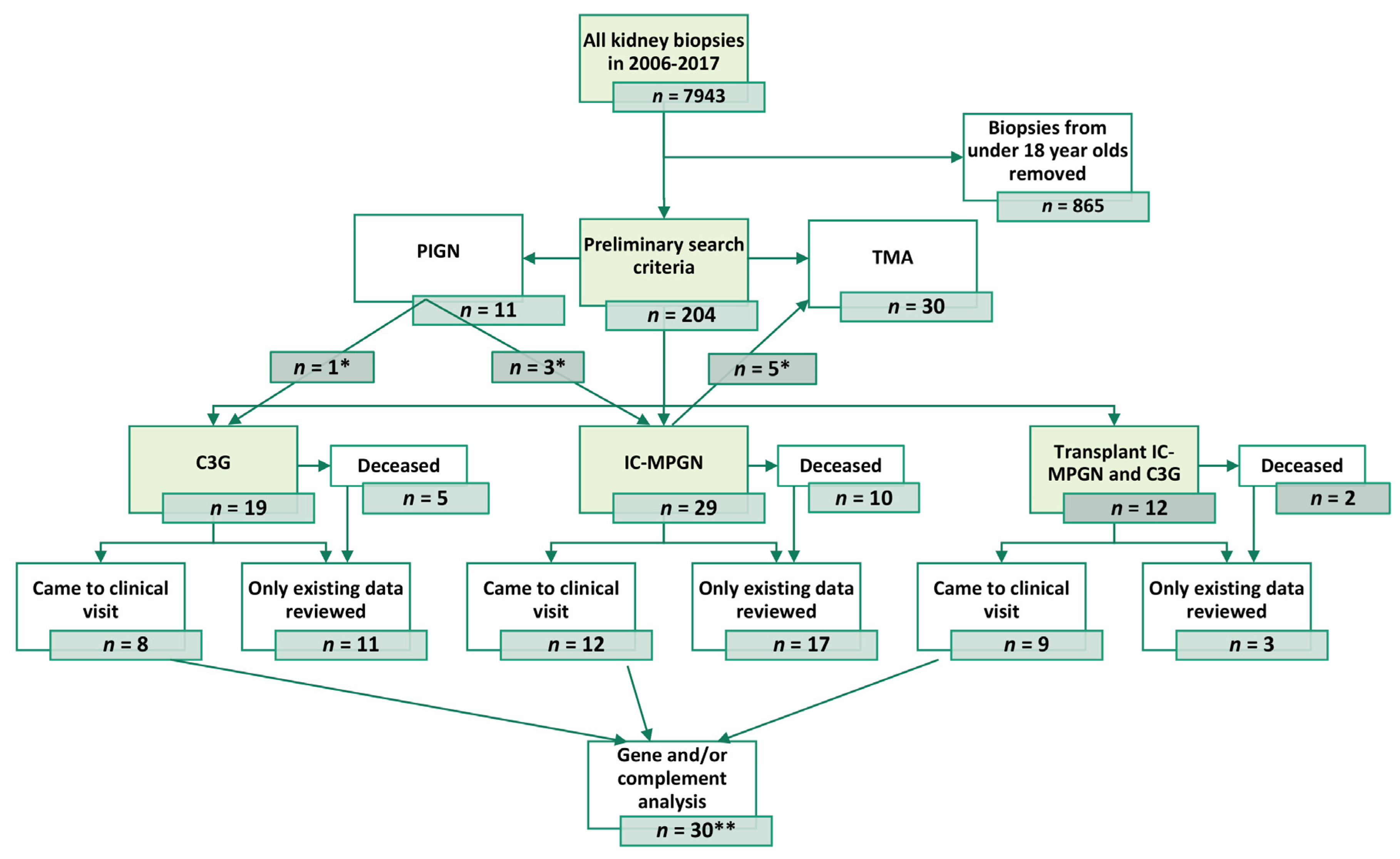
3.1. Patient Population
3.2. Clinical Characteristics
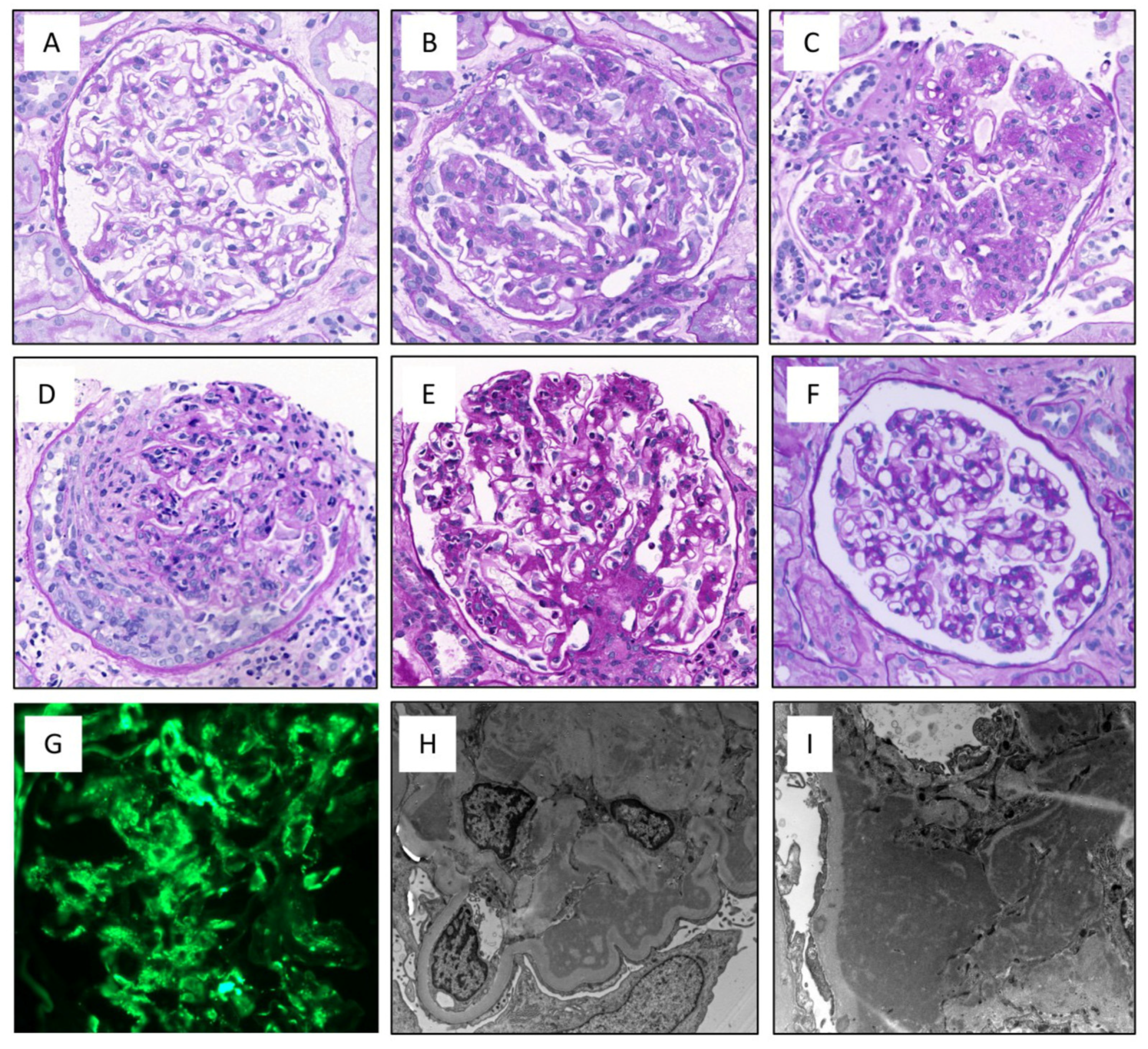
3.3. Histological Characteristics
3.4. Baseline Complement and Paraprotein Findings
3.5. Complement and Paraprotein Findings at Follow-Up
3.6. Treatment, Kidney, and Patient Survival
4. Discussion
4.1. Clinical Course, Survival Probability, and Complement Analyses
4.2. Histological and Clinical Features Predicting Prognosis
4.3. Strenghts and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masani, N.; Jhaveri, K.D.; Fishbane, S. Update on membranoproliferative GN. Clin. J. Am. Soc. Nephrol. 2014, 9, 600–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kher, K.; Schnaper, H.W.; Greenbaum, L.A. Clinical Pediatric Nephrology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Sethi, S.; Haas, M.; Markowitz, G.S.; D’Agati, V.D.; Rennke, H.G.; Jennette, J.C.; Bajema, I.M.; Alpers, C.E.; Chang, A.; Cornell, L.D.; et al. Mayo Clinic/Renal Pathology Society Consensus Report on Pathologic Classification, Diagnosis, and Reporting of GN. J. Am. Soc. Nephrol. 2016, 27, 1278–1287. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Fervenza, F.C. Membranoproliferative glomerulonephritis--a new look at an old entity. N. Engl. J. Med. 2012, 366, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Larsen, C.P.; Messias, N.C.; Walker, P.D.; Fidler, M.E.; Cornell, L.D.; Hernandez, L.H.; Alexander, M.P.; Sethi, S.; Nasr, S.H. Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney Int. 2015, 88, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, H.T.; Pickering, M.C. Clusters Not Classifications: Making Sense of Complement-Mediated Kidney Injury. J. Am. Soc. Nephrol. 2018, 29, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Goodship, T.H.; Cook, H.T.; Fakhouri, F.; Fervenza, F.C.; Fremeaux-Bacchi, V.; Kavanagh, D.; Nester, C.M.; Noris, M.; Pickering, M.C.; Rodriguez de Cordoba, S.; et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 539–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servais, A.; Noel, L.H.; Roumenina, L.T.; Le Quintrec, M.; Ngo, S.; Dragon-Durey, M.A.; Macher, M.A.; Zuber, J.; Karras, A.; Provot, F.; et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012, 82, 454–464. [Google Scholar] [CrossRef] [Green Version]
- Leung, N.; Bridoux, F.; Hutchison, C.A.; Nasr, S.H.; Cockwell, P.; Fermand, J.P.; Dispenzieri, A.; Song, K.W.; Kyle, R.A.; International, K.; et al. Monoclonal gammopathy of renal significance: When MGUS is no longer undetermined or insignificant. Blood 2012, 120, 4292–4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Markowitz, G.S.; Bomback, A.S.; Appel, G.B.; Herlitz, L.C.; Barry Stokes, M.; D’Agati, V.D. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int. 2014, 85, 450–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poppelaars, F.; Thurman, J.M. Complement-mediated kidney diseases. Mol. Immunol. 2020, 128, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.T. C3 glomerulopathy. F1000Res 2017, 6, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servais, A.; Noel, L.H.; Fremeaux-Bacchi, V.; Lesavre, P. C3 glomerulopathy. Contrib. Nephrol. 2013, 181, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Martín, B.; Smith, R.J.H. C3 Glomerulopathy. GeneReviews(®) [Internet] 2007 [updated 2018]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1425/. (accessed on 1 April 2020).
- Ito, N.; Ohashi, R.; Nagata, M. C3 glomerulopathy and current dilemmas. Clin. Exp. Nephrol. 2017, 21, 541–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zand, L.; Kattah, A.; Fervenza, F.C.; Smith, R.J.; Nasr, S.H.; Zhang, Y.; Vrana, J.A.; Leung, N.; Cornell, L.D.; Sethi, S. C3 glomerulonephritis associated with monoclonal gammopathy: A case series. Am. J. Kidney Dis. 2013, 62, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doshi, M.; Lahoti, A.; Danesh, F.R.; Batuman, V.; Sanders, P.W.; American Society of Nephrology Onco-Nephrology, F. Paraprotein-Related Kidney Disease: Kidney Injury from Paraproteins-What Determines the Site of Injury? Clin. J. Am. Soc. Nephrol. 2016, 11, 2288–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, S.; Sukov, W.R.; Zhang, Y.; Fervenza, F.C.; Lager, D.J.; Miller, D.V.; Cornell, L.D.; Krishnan, S.G.; Smith, R.J. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am. J. Kidney Dis. 2010, 56, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Alchi, B.; Jayne, D. Membranoproliferative glomerulonephritis. Pediatr. Nephrol. 2010, 25, 1409–1418. [Google Scholar] [CrossRef]
- Master Sankar Raj, V.; Gordillo, R.; Chand, D.H. Overview of C3 Glomerulopathy. Front. Pediatr. 2016, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Jozsi, M.; Reuter, S.; Nozal, P.; Lopez-Trascasa, M.; Sanchez-Corral, P.; Prohaszka, Z.; Uzonyi, B. Autoantibodies to complement components in C3 glomerulopathy and atypical hemolytic uremic syndrome. Immunol. Lett. 2014, 160, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Nasr, S.H.; Valeri, A.M.; Appel, G.B.; Sherwinter, J.; Stokes, M.B.; Said, S.M.; Markowitz, G.S.; D’Agati, V.D. Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Gurkan, S.; Fyfe, B.; Weiss, L.; Xiao, X.; Zhang, Y.; Smith, R.J. Eculizumab and recurrent C3 glomerulonephritis. Pediatr. Nephrol. 2013, 28, 1975–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaartinen, K.; Martola, L.; Raisanen-Sokolowski, A.; Meri, S. Recurrent allograft C3 glomerulonephritis and unsuccessful eculizumab treatment. Clin. Immunol. 2018, 187, 104–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivarelli, M.; Pasini, A.; Emma, F. Eculizumab for the treatment of dense-deposit disease. N. Engl. J. Med. 2012, 366, 1163–1165. [Google Scholar] [CrossRef]
- Bomback, A.S.; Smith, R.J.; Barile, G.R.; Zhang, Y.; Heher, E.C.; Herlitz, L.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D.; Canetta, P.A.; et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2012, 7, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daina, E.; Noris, M.; Remuzzi, G. Eculizumab in a patient with dense-deposit disease. N. Engl. J. Med. 2012, 366, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, J.A.; O’Rourke, D.M.; Courtney, A.E. Recurrent dense deposit disease after renal transplantation: An emerging role for complementary therapies. Am. J. Transplant. 2012, 12, 1046–1051. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Servais, A.; Fremeaux-Bacchi, V.; Lequintrec, M.; Salomon, R.; Blouin, J.; Knebelmann, B.; Grunfeld, J.P.; Lesavre, P.; Noel, L.H.; Fakhouri, F. Primary glomerulonephritis with isolated C3 deposits: A new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J. Med. Genet. 2007, 44, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Iatropoulos, P.; Noris, M.; Mele, C.; Piras, R.; Valoti, E.; Bresin, E.; Curreri, M.; Mondo, E.; Zito, A.; Gamba, S.; et al. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol. Immunol. 2016, 71, 131–142. [Google Scholar] [CrossRef]
- Pickering, M.C.; D’Agati, V.D.; Nester, C.M.; Smith, R.J.; Haas, M.; Appel, G.B.; Alpers, C.E.; Bajema, I.M.; Bedrosian, C.; Braun, M.; et al. C3 glomerulopathy: Consensus report. Kidney Int. 2013, 84, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noris, M.; Remuzzi, G. Glomerular Diseases Dependent on Complement Activation, Including Atypical Hemolytic Uremic Syndrome, Membranoproliferative Glomerulonephritis, and C3 Glomerulopathy: Core Curriculum 2015. Am. J. Kidney Dis. 2015, 66, 359–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustad, P.; Felding, P.; Franzson, L.; Kairisto, V.; Lahti, A.; Mårtensson, A.; Hyltoft Petersen, P.; Simonsson, P.; Steensland, H.; Uldall, A. The Nordic Reference Interval Project 2000: Recommended reference intervals for 25 common biochemical properties. Scand. J. Clin. Lab. Invest. 2004, 64, 271–284. [Google Scholar] [CrossRef]
- Sethi, S.; Fervenza, F.C. Membranoproliferative glomerulonephritis: Pathogenetic heterogeneity and proposal for a new classification. Semin. Nephrol. 2011, 31, 341–348. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasinska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Seshan, S.V.; Barisoni, L.; Amann, K.; Bajema, I.M.; Becker, J.U.; Joh, K.; Ljubanovic, D.; Roberts, I.S.D.; Roelofs, J.J.; et al. Consensus definitions for glomerular lesions by light and electron microscopy: Recommendations from a working group of the Renal Pathology Society. Kidney Int. 2020, 98, 1120–1134. [Google Scholar] [CrossRef]
- Watson, R.; Lindner, S.; Bordereau, P.; Hunze, E.-M.; Tak, F.; Ngo, S.; Zipfel, P.F.; Skerka, C.; Dragon-Durey, M.-A.; Marchbank, K.J. Standardisation of the factor H autoantibody assay. Immunobiology 2014, 219, 9–16. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Woo, S.A.; Ju, H.Y.; Kwon, S.H.; Lee, J.-H.; Choi, S.J.; Han, D.C.; Hwang, S.D.; Hong, S.-Y.; Jin, S.-Y.; Gil, H.-W. Reanalysis of membranoproliferative glomerulonephritis patients according to the new classification: A multicenter study. Kidney Res. Clin. Pract. 2014, 33, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Noris, M.; Daina, E.; Remuzzi, G. Membranoproliferative glomerulonephritis: No longer the same disease and may need very different treatment. Nephrol. Dial. Transplant. 2021, 38, 283–290. [Google Scholar] [CrossRef]
- Sethi, S.; Zand, L.; Leung, N.; Smith, R.J.H.; Jevremonic, D.; Herrmann, S.S.; Fervenza, F.C. Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Bhutani, G.; Nasr, S.H.; Said, S.M.; Sethi, S.; Fervenza, F.C.; Morice, W.G.; Kurtin, P.J.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; et al. Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin. Proc. 2015, 90, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Jokiranta, T.S.; Solomon, A.; Pangburn, M.K.; Zipfel, P.F.; Meri, S. Nephritogenic lambda light chain dimer: A unique human miniautoantibody against complement factor H. J. Immunol. 1999, 163, 4590–4596. [Google Scholar] [CrossRef]
- Chauvet, S.; Roumenina, L.T.; Aucouturier, P.; Marinozzi, M.C.; Dragon-Durey, M.A.; Karras, A.; Delmas, Y.; Le Quintrec, M.; Guerrot, D.; Jourde-Chiche, N.; et al. Both Monoclonal and Polyclonal Immunoglobulin Contingents Mediate Complement Activation in Monoclonal Gammopathy Associated-C3 Glomerulopathy. Front. Immunol. 2018, 9, 2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iatropoulos, P.; Daina, E.; Curreri, M.; Piras, R.; Valoti, E.; Mele, C.; Bresin, E.; Gamba, S.; Alberti, M.; Breno, M.; et al. Cluster Analysis Identifies Distinct Pathogenetic Patterns in C3 Glomerulopathies/Immune Complex-Mediated Membranoproliferative GN. J. Am. Soc. Nephrol. 2018, 29, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Chauvet, S.; Hauer, J.J.; Petitprez, F.; Rabant, M.; Martins, P.V.; Baudouin, V.; Delmas, Y.; Jourde-Chiche, N.; Cez, A.; Ribes, D.; et al. Results from a national-wide retrospective cohort measure the impact of C3 and soluble C5b-9 levels on kidney outcomes in C3 glomerulopathy. Kidney Int. 2022, 102, 904–916. [Google Scholar] [CrossRef]
- Fakhouri, F.; Le Quintrec, M.; Frémeaux-Bacchi, V. Practical management of C3 glomerulopathy and Ig-mediated MPGN: Facts and uncertainties. Kidney Int. 2020, 98, 1135–1148. [Google Scholar] [CrossRef]
- Chauvet, S.; Berthaud, R.; Devriese, M.; Mignotet, M.; Vieira Martins, P.; Robe-Rybkine, T.; Miteva, M.A.; Gyulkhandanyan, A.; Ryckewaert, A.; Louillet, F.; et al. Anti-Factor B Antibodies and Acute Postinfectious GN in Children. J. Am. Soc. Nephrol. 2020, 31, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Marinozzi, M.C.; Roumenina, L.T.; Chauvet, S.; Hertig, A.; Bertrand, D.; Olagne, J.; Frimat, M.; Ulinski, T.; Deschênes, G.; Burtey, S.; et al. Anti-Factor B and Anti-C3b Autoantibodies in C3 Glomerulopathy and Ig-Associated Membranoproliferative GN. J. Am. Soc. Nephrol. 2017, 28, 1603–1613. [Google Scholar] [CrossRef] [Green Version]
- Caravaca-Fontán, F.; Trujillo, H.; Alonso, M.; Díaz-Encarnación, M.; Cabello, V.; Ariceta, G.; Quintana, L.F.; Marco, H.; Barros, X.; Ramos, N.; et al. Validation of a Histologic Scoring Index for C3 Glomerulopathy. Am. J. Kidney Dis. 2021, 77, 684–695. [Google Scholar] [CrossRef]




| Baseline Variable | IC-MPGN, n = 37 | C3G, n = 23 |
|---|---|---|
| Age, years (range) | 52 (5–78) | 54 (16–79) |
| Male sex, n (%) | 23 (62) | 13 (57) |
| BMI (kg/m2) (SD) | 28.8 (6.3), n = 34 | 26 (4.3), n = 22 |
| Smoking | ||
| Current, n (%) | 9 (27), n = 34 | 7 (32), n = 22 |
| Former, n (%) | 14 (41), n = 34 | 11 (50), n = 22 |
| Diabetes, n (%) | 2 (6), n = 34 | 4 (17) |
| Hypertension, n (%) * | 36 (97) | 23 (100) |
| Rheumatic disease, n (%) | 2 (6), n = 34 | 4 (17) |
| Chronic infection, n (%) | 7 (21), n = 34 | 3 (13) |
| Malignancy, n (%) | 2 (6), n = 34 | 4 (17) |
| Plasma cell dyscrasia, n (%) ** | 8 (24), n = 34 | 6 (26) |
| Cardiovascular disease, n (%) | 10 (29), n = 34 | 7 (30) |
| Duration of renal findings before diagnosis, years, (range) | 1.3 (0–9), n = 27 | 1.4 (0–7) |
| Macroscopic hematuria, n (%) | 4 (13), n = 31 | 6 (27), n = 22 |
| Diagnostic biopsy | ||
| Transplant, n (%) | 1 (3) | 2 (9) |
| Native kidney, n (%) | 36 (97) | 21 (91) |
| Diagnostic biopsy taken before index biopsy, n (%) | 7 (19) | 1 (4) |
| Baseline Variable | IC-MPGN, n = 35 | C3G, n = 23 |
|---|---|---|
| eGFR (SD) * | 56 (32) | 49 (27), n = 22 |
| eGFR decreased, n (%) | 22 (63) | 16 (73), n = 22 |
| S-creat, µmol/L (SD) | 155 (99) | 166 (121) |
| Urine dipstick proteinuria positive, n (%) | 33 (97), n = 34 | 21 (100), n = 21 |
| Urine alb/creat, mg/mmol (SD) | 192.6 (160.6), n = 15 | 158.8 (171.5), n = 9 |
| Urine protein excretion, g/24 h (SD) | 5.3 (4.2), n = 34 | 3.6 (3.3), n = 21 |
| Nephrotic proteinuria (<3 g/24 h), n (%) | 23 (68), n = 34 | 9 (43), n = 21 |
| Microscopic hematuria, n (%) | 28 (90), n = 31 | 20 (100), n = 20 |
| Alb, g/L (SD) | 27 (6) | 25 (6) |
| Hb, g/L (SD) | 116 (18) | 116 (19) |
| CRP, mg/L (SD) | 13 (20) | 37 (81) |
| LDL, mmol/L (SD) | 3.5 (1.5) | 3.3. (1.7) |
| ENA-Ab positivity, n (%) | 0 (0) | 1 (14) |
| Variable | IC-MPGN, n = 37 | C3G, n = 21 * | p-Value |
|---|---|---|---|
| Glomerular changes | |||
| % of sclerotic glomeruli | 17 (47), n = 36 | 11 (52) | 1.000 |
| Biopsies containing crescents, n (%) | 5 (13.5) | 4 (19) | 0.710 |
| Mesangial matrix expansion | |||
| none, n (%) | 10 (27) | 6 (29) | 1.000 |
| mild, n (%) | 8 (22) | 6 (29) | 0.761 |
| moderate, n (%) | 6 (16) | 2 (10) | 0.703 |
| strong, n (%) | 13 (35) | 7 (33) | 1.000 |
| Lobulated pattern of glomeruli, n (%) | 20 (54) | 9 (43) | 0.811 |
| Doubled GBM | |||
| None, n (%) Mild, n (%) Moderate, n (%) Strong, n (%) | 3 (8) 7 (19) 3 (8) 24 (65) | 4 (19) 5 (24) 2 (10) 10 (48) | 0.415 0.751 1.000 0.648 |
| Tubulointerstitial changes | |||
| Total interstitial inflammation | |||
| None, n (%) Mild, n (%) Moderate, n (%) Strong, n (%) | 27 (73) 4 (11) 4 (11) 2 (5) | 10 (48) 8 (38) 2 (10) 1 (5) | 0.379 0.062 1.000 1.000 |
| Interstitial fibrosis | |||
| None, n (%) Mild, n (%) Moderate, n (%) Strong, n (%) | 29 (78) 4 (11) 2 (5) 2 (5) | 10 (48) 9 (43) 2 (10) 0 (0) | 0.375 0.059 0.623 0.537 |
| Tubular atrophy | |||
| None, n (%) Mild, n (%) Moderate, n (%) Strong, n (%) | 24 (65) 11 (30) 0 (0) 2 (5) | 10 (48) 10 (48) 0 (0) 1 (5) | 0.648 0.437 1.000 1.000 |
| Arteriolar sclerosis | |||
| None, n (%) Mild, n (%) Moderate, n (%) Strong, n (%) | 17 (49), n = 35 12 (34), n = 35 6 (17), n = 35 0 (0), n = 35 | 11 (52) 8 (38) 2 (10) 0 (0) | 1.000 1.000 0.7 1.000 |
| Variable | IC-MPGN, n = 23 | C3G, n = 17 |
|---|---|---|
| Complement proteins | ||
| S-C3, g/L (SD) Decreased (lower limit of normal 0.5 g/L), n (%) | 1.0 (0.2), n = 21 1 (5) | 0.9 (0.3) 1 (7) |
| S-C4, g/L (SD) Decreased C4 (lower limit of normal >0.12), n (%) | 0.2 (0.1), n = 20 2 (10) | 0.2 (0.1) 3 (21) |
| FHR1-3 heterozygous deletion, n (%) | 5 (29), n = 17 | 4 (31), n = 13 |
| Functional complement analysis | ||
| S-CH100AI, % (SD) Decreased, n (%) | 93 (31), n = 18 1 (6) | 75 (33), n = 14 2 (14) |
| S-CH100CI, % (SD) Decreased, n (%) | 95 (25), n = 18 2 (11) | 77 (34), n = 14 4 (29) |
| S-CH100L, % (SD) Decreased, n (%) | 50 (53), n = 18 8 (44) | 58 (42), n = 14 3 (21) |
| Complement autoantibodies | ||
| C3Nef positivity, n (%) | 0 (0), n = 22 | 2 (18), n = 11 |
| Factor H-antibody positivity, n (%) * | 1 (6), n = 18 | 1 (8), n = 13 |
| Factor B antibody positivity, n (%) | 1 (6), n = 17 | 2 (15), n = 13 |
| C3b-antibody positivity, n (%) | 1 (6), n = 17 | 2 (15), n = 13 |
| C3 activating factors | ||
| classical pathway activator, n (%) alternative pathway activator, n (%) | 2 (12), n = 17 2 (12), n = 17 | 0 (0), n = 13 0 (0), n = 13 |
| Paraproteins | ||
| Free kappa light chain in serum, mg/L (SD) | 66.9 (77.2) | 79 (80.9), n = 14 |
| Free lambda light chain in serum, mg/L (SD) | 37.4 (31.9) | 64.5 (53), n = 14 |
| Kappa/lambda light chain ratio (SD) | 1.6 (1) | 1.2 (0.4), n = 15 |
| Serum paraprotein, n (%) | 5 (22) | 6 (40), n = 15 |
| Urine paraprotein, n (%) | 0 (0), n = 16 | 3 (25), n = 12 |
| Variable | IC-MPGN, n = 37 | C3G, n = 23 |
|---|---|---|
| Immunosuppressive medication at baseline | ||
| Corticosteroids, n (%) Mycophenolate mofetil, n (%) Other, n (%) * | 3 (9), n = 34 0 (0), n = 33 1 (3) | 6 (26) 1 (5) 1 (4) |
| Immunosuppressive medication during follow-up | ||
| Corticosteroids, n (%) Mycophenolate mofetil, n (%) Other, n (%) * | 17 (46) 5 (14) 8 (22) | 13 (57) 6 (26) 7 (30) |
| Follow-up | ||
| Dialysis during follow-up, n (%) | 8 (22) | 7 (30) |
| Kidney transplantation during follow-up, n (%) | 8 (22) | 4 (17) |
| Progressive disease, n (%) | 16 (43) | 11 (48) |
| More than one kidney transplant, n (%) | 3 (8) | 1 (4) |
| Follow-up time from diagnostic biopsy, years (range) | 8.1 (0.9–39.1) | 5.9 (0.6–25.7) |
| Time from diagnostic biopsy to start of 1st dialysis, months (range) | 59 (0.2–140), n = 10 | 30 (0.2–61), n = 9 |
| Time from diagnostic biopsy to 1st transplantation, months (range) | 83 (33–165), n = 6 | 51 (34–70), n = 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovala, M.; Seppälä, M.; Räisänen-Sokolowski, A.; Meri, S.; Honkanen, E.; Kaartinen, K. Diagnostic and Prognostic Comparison of Immune-Complex-Mediated Membranoproliferative Glomerulonephritis and C3 Glomerulopathy. Cells 2023, 12, 712. https://doi.org/10.3390/cells12050712
Kovala M, Seppälä M, Räisänen-Sokolowski A, Meri S, Honkanen E, Kaartinen K. Diagnostic and Prognostic Comparison of Immune-Complex-Mediated Membranoproliferative Glomerulonephritis and C3 Glomerulopathy. Cells. 2023; 12(5):712. https://doi.org/10.3390/cells12050712
Chicago/Turabian StyleKovala, Marja, Minna Seppälä, Anne Räisänen-Sokolowski, Seppo Meri, Eero Honkanen, and Kati Kaartinen. 2023. "Diagnostic and Prognostic Comparison of Immune-Complex-Mediated Membranoproliferative Glomerulonephritis and C3 Glomerulopathy" Cells 12, no. 5: 712. https://doi.org/10.3390/cells12050712
APA StyleKovala, M., Seppälä, M., Räisänen-Sokolowski, A., Meri, S., Honkanen, E., & Kaartinen, K. (2023). Diagnostic and Prognostic Comparison of Immune-Complex-Mediated Membranoproliferative Glomerulonephritis and C3 Glomerulopathy. Cells, 12(5), 712. https://doi.org/10.3390/cells12050712








