Abstract
Sarcalumenin (SAR) is a luminal Ca2+ buffer protein with high capacity but low affinity for calcium binding found predominantly in the longitudinal sarcoplasmic reticulum (SR) of fast- and slow-twitch skeletal muscles and the heart. Together with other luminal Ca2+ buffer proteins, SAR plays a critical role in modulation of Ca2+ uptake and Ca2+ release during excitation–contraction coupling in muscle fibers. SAR appears to be important in a wide range of other physiological functions, such as Sarco-Endoplasmic Reticulum Calcium ATPase (SERCA) stabilization, Store-Operated-Calcium-Entry (SOCE) mechanisms, muscle fatigue resistance and muscle development. The function and structural features of SAR are very similar to those of calsequestrin (CSQ), the most abundant and well-characterized Ca2+ buffer protein of junctional SR. Despite the structural and functional similarity, very few targeted studies are available in the literature. The present review provides an overview of the role of SAR in skeletal muscle physiology, as well as of its possible involvement and dysfunction in muscle wasting disorders, in order to summarize the current knowledge on SAR and drive attention to this important but still underinvestigated/neglected protein.
1. Introduction
Ca2+ ions are intracellular messengers essential for signal transduction. In muscle physiology, the main resources of Ca2+ for proper functioning of the intracellular Ca2+ signals derive from two Ca2+ pools: Ca2+ sequestered in stores (endoplasmic/sarcoplasmic reticulum (ER/SR)) and extracellular Ca2+. The movements of contractile proteins require the correct intracellular level of Ca2+ ions to be released from the SR, a specialized membrane system and a component of the cellular reticular network. The presence of these pools guarantees the correct amount of calcium essential for the muscle to perform its pivotal function, i.e., the well-understood excitation–contraction (EC) coupling. EC coupling is a process mediated by mechanical coupling between the dihydropyridine receptor (DHPR) located on the transverse tubule membrane (invaginations of the plasma membrane) and ryanodine receptor type 1 (RyR1) on the SR membrane. Following conduction of impulses through motor neurons (neuromuscular transmission) and the activation of the nicotinic acetylcholine receptor at neuromuscular junctions, muscle membrane depolarization (via propagated action potential) induces a conformational change in the DHPR required for the RyR1 interaction. The latter opens to release Ca2+ ions from the Ca2+ SR. Subsequently, Ca2+ uptake in the SR via Ca2+-Mg2+ ATPase (SERCA) leads back to a resting level with the replenishment of the SR calcium content [1,2]. Therefore, the maintenance of intracellular Ca2+ homeostasis in muscle cells and the correct Ca2+ sequestration in the SR are essential requirements for proper muscle contraction. During muscle contraction, resting intracellular calcium (nM range) increases up to the μM range [3]. In addition to the Ca2+ released from the SR, this Ca2+ increase is obtained through processes involving different molecules and Ca2+ transport channels. Among these, it is worth mentioning the entry of extracellular calcium into the cell through store-operated calcium entry (SOCE) via Orai1 and/or the transient receptor potential canonical (TRPC) channels [4,5] or through excitation-coupled Ca2+ entry (ECCE). Calcium entry into the cell via SOCE is important for Ca2+ replenishment of the SR in order to maintain SR Ca2+ content for maximal muscle performance [6], whereas ECCE is a context-specific mechanism contributing to Ca2+ entry during muscle contraction [7]. However, free calcium concentration must be rigorously and rapidly buffered for proper calcium signaling and muscle performance. Different Ca2+ buffer proteins residing in the cytoplasm (i.e., regucalcin and calmodulin) or in the lumen of the longitudinal and junctional SR of skeletal muscle are believed to regulate EC coupling, SOCE and ECCE processes by binding Ca2+ ions (Table 1).

Table 1.
Summary of Ca2+- binding proteins residing in the cytoplasm or in the lumen of sarcoplasmic reticulum of skeletal muscle and their molecular mass and Ca2+-dissociation constant. SMP, senescence marker protein; CLP, calsequestrin-like protein; HRC, histidine-rich calcium-binding protein; N.A., not available.
Regucalcin (also called senescence marker protein 30) is a 34 kDa cytosolic multifunctional Ca2+-binding protein that lacks the typical Ca2+-binding EF motif. It is a marker of aging that principally regulates intracellular Ca2+ homeostasis by modulating the activity of several proteins involved in intracellular signaling pathways, such as Ca2+ ATPases, calmodulin kinase and PKC [16]. Calmodulin is the best-studied and most highly expressed muscle cytosolic Ca2+-binding protein containing two canonical EF-hand motifs that bind up to four Ca2+ ions. It mediates Ca2+ regulation in a broad range of physiological processes and has numerous downstream targets that are either calmodulin-dependent (i.e., calcineurin, CAMKII, RyR1 and DHPR) or calmodulin-regulated (genes encoding proteins involved in oxidative metabolism, muscle repair and plasticity) [17]. Instead, parvalbumin is a small cytosolic Ca2+ buffer protein expressed primarily in fast skeletal muscle fibers. It binds Mg2+ when the muscle is in a resting state and dissociates from it and binds Ca2+ ions after SR Ca2+ release, thereby contributing to muscle relaxation [18].
In regard to the Ca2+-buffer proteins residing in the SR, the most abundantly expressed and well-studied Ca2+ buffer located in the junctional SR is calsequestrin (CSQ), followed by the calsequestrin-like proteins CLP-150, CLP-170 and CLP-220, as well as HRC, junctate and calreticulin. The most abundant Ca2+ buffer protein located in the longitudinal SR is SAR [19]. To the best of our knowledge, most investigations have focused on the main calcium buffer protein, CSQ, while the role of SAR has not yet been fully determined. Nevertheless, the study of the physiological function of SAR and of the potential contribution of its dysfunction to skeletal muscle diseases represents an appealing research field that can reveal hidden biological functions, as well as new therapeutic targets. The present review aims to provide an overview of the currently available research about SAR, focusing on structure, physiological function and involvement in skeletal muscle diseases.
2. Overview of Sarcoplasmic Reticulum Structure and the Junctional Ca2+ Buffer Proteins: Calsequestrin, HRC, Junctate and Calreticulin
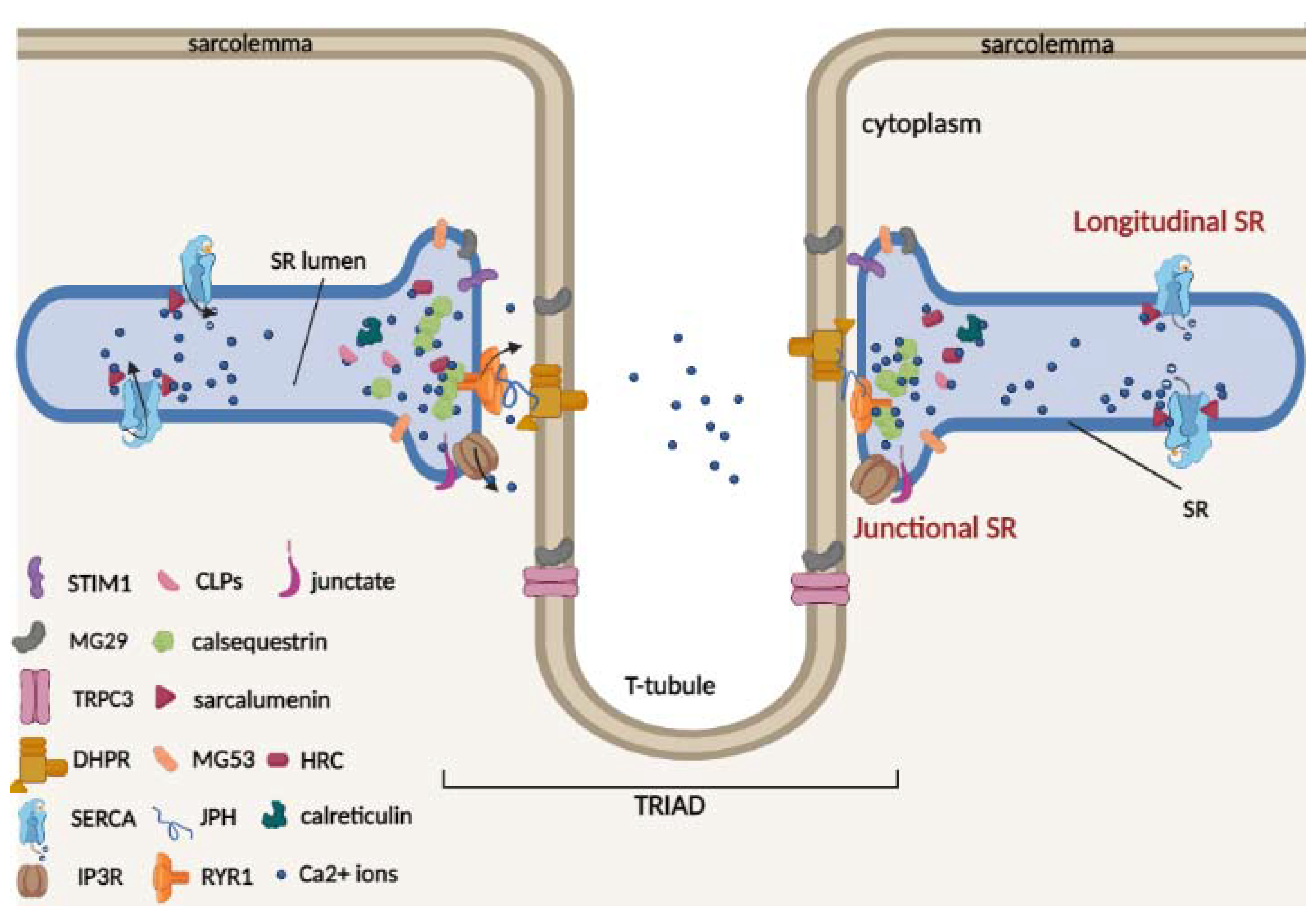
The sarcoplasmic reticulum (SR) is a specialized membrane system and a component of the cellular reticular network of striated muscle cells that surrounds each myofibril. Two well-defined structural and functional SR regions can be distinguished (Figure 1), namely the longitudinal and junctional SR [20].
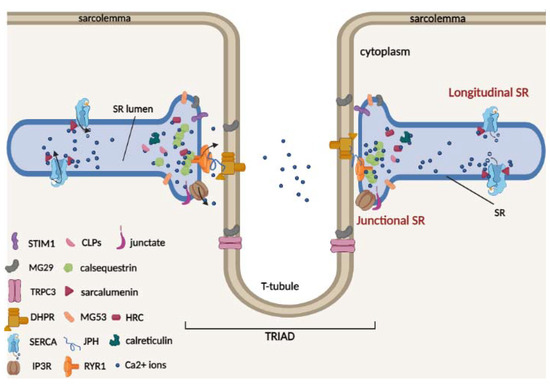
Figure 1.
Schematic model of two terminal cisternae on the opposite sides of a central t-tubule (triad) and of the luminal Ca2+-binding proteins of skeletal muscle. In the triad, the voltage-activated L-type Ca2+ channel dihydropyridine receptor (DHPR) is located on the t-tubule, and the ryanodine receptor Ca2+ release type 1 channel (RyR1) is located on the SR. The triad is stabilized by junctophilin (JPH) proteins, which act as structural bridges between the t-tubule and the SR membrane. TRPC3 protein is located on the sarcolemma and can interact with mitsugumin 29 (MG29). The SR/ER Ca2+ ATPase (SERCA) pumps are located on the longitudinal SR. RyR1, stromal interaction molecule 1 (Stim1), inositol-trisphosphate receptor (InsP3R), and mitsugumin 29 (MG29) and 53 (MG53) are located in the junctional SR. The Ca2+ buffer proteins calsequestrin (CSQ), histidine-rich Ca2+ (HRC)-binding protein and the calsequestrin-like proteins (CLPs) are located in the lumen of the junctional SR, while sarcalumenin (SAR) protein is located in the lumen of longitudinal SR.
The longitudinal SR represents the largest part of the SR in which is located the SR Ca2+ ATPases (SERCAs), which pump Ca2+ from the cytosol to the lumen of the SR [21,22,23]. It runs along the entire myofibril and connects two terminal cisternae located in proximity of the transverse t-tubules (invaginations of the sarcolemma), forming the “triad”; here, DHPR, which is located on the t-tubules, and RyR1, which is located on the SR, interact physically and functionally. Triad formation is also mediated by junctophilin isoforms (JPH1 and JPH2), which act as structural bridges between the t-tubule and SR membrane, allowing for maintenance of a close and parallel position in the triad junction [24]. JPHs are also able to interact with SR proteins, in particular with RyR1 for JPH1 [25] and DHPR for JPH2 [26], and regulate Ca2+ movements in skeletal muscle. Therefore, in the triad, upon membrane depolarization, the DHPR channels undergo a conformational change necessary for RyR1 interaction and activation for Ca2+ release into the cytosol and for myofilament contraction. In contrast, junctional SR represents the region of the terminal membrane cisternae that faces the t-tubule/SR membrane [22,24], where RyR1 and other SR proteins involved in Ca2+ released from the SR, such as Stim1, inositol-trisphosphate receptor (InsP3R) and mitsugumin 53 (MG53), are located. Furthermore, during muscle differentiation, the formation of triad junctions can be favored by mitsugumin 29 (MG29), a structural protein exclusively expressed in skeletal muscle and localized both on t-tubules and SR terminal cisternae [27,28]. It has also been proposed that in skeletal muscle, the interaction between MG29 and the membrane protein TRPC3 could contribute to regulating Ca2+ transients [29].
Luminal Ca2+ is bound to high-capacity Ca2+ binding proteins within the terminal cisternae and longitudinal tubules [30]. The most abundant skeletal and cardiac muscle Ca2+ buffer protein of junctional SR is CSQ, which belongs to the class of high-capacity and medium-affinity Ca2+ buffer proteins [31,32,33]. Two CSQ genes are present in striated muscle: Casq1 and Casq2 genes encoding for calsequestrin-1 and 2, respectively. Under physiological conditions, CSQ interacts with triadin and junctin proteins and appears as a monomer [34]. Following Ca2+ binding, CSQ monomers polymerize to form large polymers, increasing Ca2+ binding ability [35]. In addition to the buffer role of Ca2+, both calsequestrin-1 and 2 are important for regulation of Ca2+ release during muscle contraction, interacting with ryanodine receptors (RyRs) and contributing to the regulation of Ca2+ homeostasis in muscle cells [36,37,38]. Furthermore, CSQ is able to directly interact with muscular STIM1, a key protein involved in the SOCE mechanism, altering STIM1/Orai1 interaction and reducing the refilling of depleted intracellular reticulum Ca2+ stores [39,40].
Another minor SR Ca2+ buffer protein is HRC, which has structural similarities to CSQ and, like CSQ, binds Ca2+ with high capacity and low affinity. It is located within the SR lumen as a multimer [41,42], and unlike CSQ, in the presence of high Ca2+ levels, it dissociates from pentamers to trimers and dimers and is less closely folded and more sensitive to trypsin digestion [41]. HRC binds Ca2+ directly and could interact with triadin, mediating RyRs activity [42,43,44]. Altered HRC expression is particularly involved in cardiovascular disease [45] and in the onset of gastric and lung cancer [45,46,47]; on the contrary, the increased activity of HRC provides protection against heart damage induced by ischemia/reperfusion [48].
With respect to Ca2+ buffer proteins, it is worth mentioning junctate protein, an integral SR membrane protein of 33 kDa. Due to the presence of a negative luminal C-terminal Ca2+ binding domain, junctate is able to bind approximately 21 mol Ca2+/mol protein [49]. Thus, like CSQ and HRC, junctate contributes to SR calcium storage with a high-Ca2+ capacity but low Ca2+ affinity. Instead, unlike CSQ and HRC, it has been shown that in HEK cells, junctate is associated with IP3 receptors and TRPC channels, contributing to the SOCE mechanism with its N-terminal domain [50].
Lastly, calreticulin is an ubiquitous 46 kDa Ca2+ binding protein located in the lumen of the SR [51]. The structure of calreticulin contains a C-terminal region, which is critical for Ca2+ buffering, with an amino acid sequence very similar to that of CSQ [11]. Its conformation is highly dependent on Ca2+ concentrations; normally, it is globular, while Ca2+ binding causes a change to an α-helical mode [52,53]. Calreticulin mainly functions as a Ca2+ buffer protein due to its high binding capacity (20–30 mol Ca2+/ mol protein) and as a molecular chaperone, participating in different folding processes of the protein sequence in association with calnexin. The overexpression of calreticulin leads to calcium accumulation in cellular deposits and influences the SOCE mechanism [54,55,56].
3. Sarcalumenin Structure and Physiological Functions in Skeletal Muscle
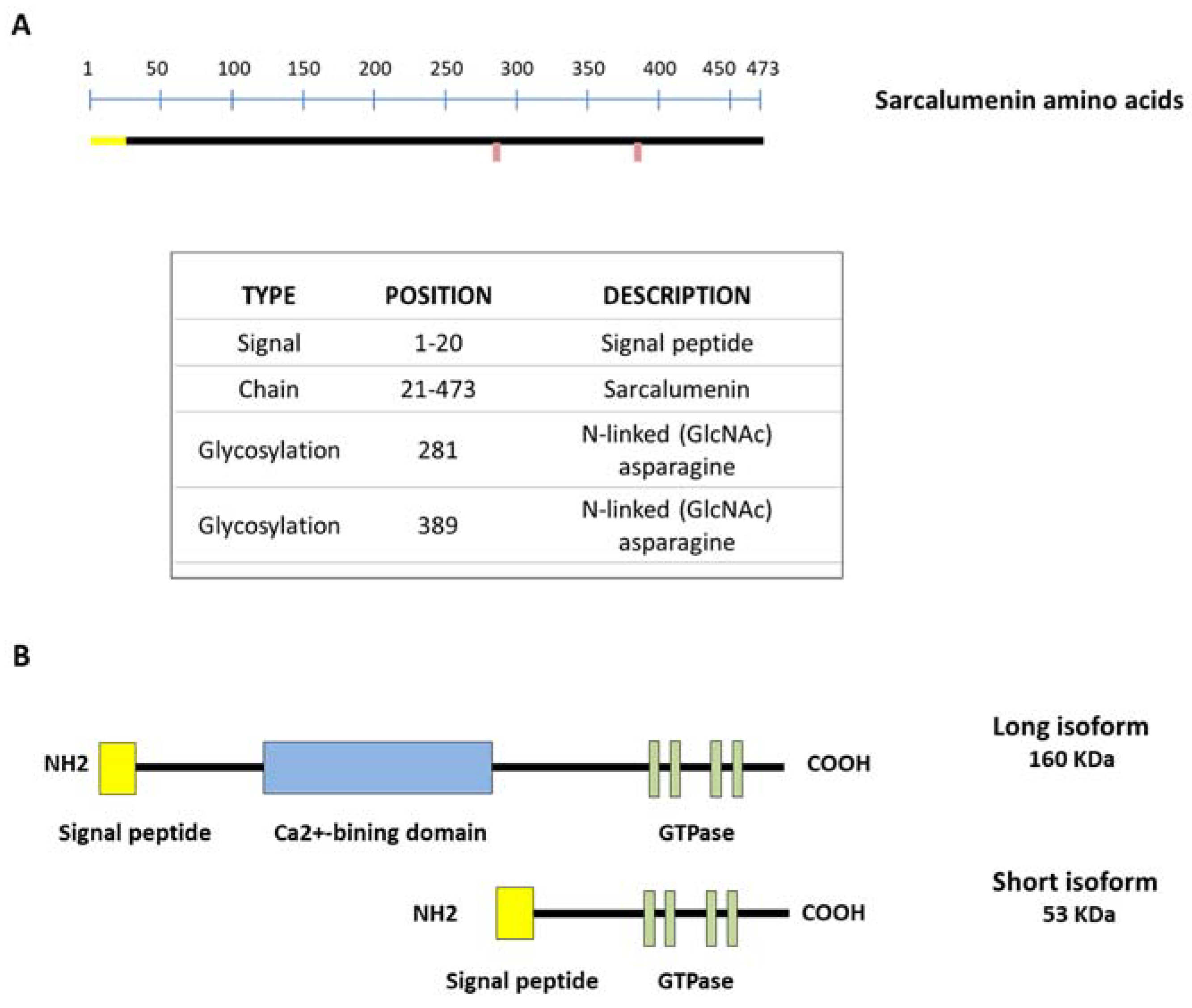
SAR is a Ca2+-binding glycoprotein composed of 473 acidic amino acids with a molecular weight of 160 KDa (long isoform) first isolated from skeletal muscle by Leberer et al. in 1989 (Figure 2A). However, the SAR gene also encodes a variant of the 53 kDa glycoprotein (short isoform) through alternative splicing of the primary transcript, identical to the COOH-terminal half of SAR and unable to bind Ca2+, with a function that currently remains unclear. Both isoforms are predominantly located in the lumen of the longitudinal SR bounding the inner side of the membrane through a Ca2+-dependent mechanism. Furthermore, both SAR and the 53 kDa glycoprotein variant represent the major non-junctional SR Ca2+-binding protein of striated muscles [9,57]. Only SAR includes a Ca2+-binding domain inserted between the N-terminal and C-terminal region, where several nucleotide-binding motifs for P-loop-containing ATPase/GTPase are located [19,57] (Figure 2B). Interestingly, it has been shown that the amount of luminal SAR and 53 kDa glycoprotein variant varies in skeletal muscle depending on the muscle fiber type. In particular, their relative density is lower in slow-twitch versus fast fibers and comparable in gastrocnemius, extensor digitorum longus (EDL) and tibialis anterior (TA) muscles [58]. This different protein expression suggests an adaptation to different physiological Ca2+-binding requirements in fast versus slow muscles.
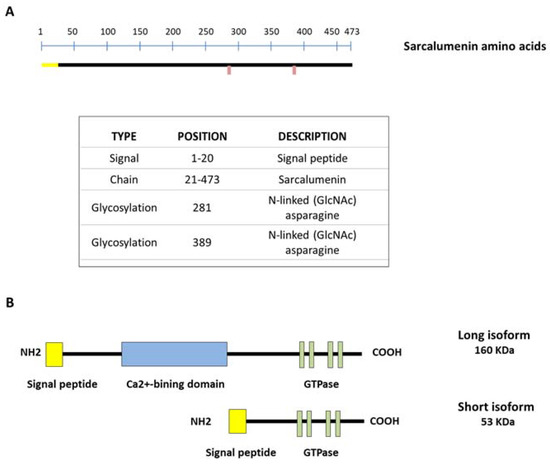
Figure 2.
Schematic representation of sarcalumenin (SAR) structure in skeletal muscle. (A) Amino acid sequencing of SAR; (B) picture showing short and long isoforms of SAR. The long SAR isoform includes a Ca2+-binding domain inserted between the N-terminal and C-terminal region, with several nucleotide-binding motifs for P-loop-containing ATPase/GTPase.
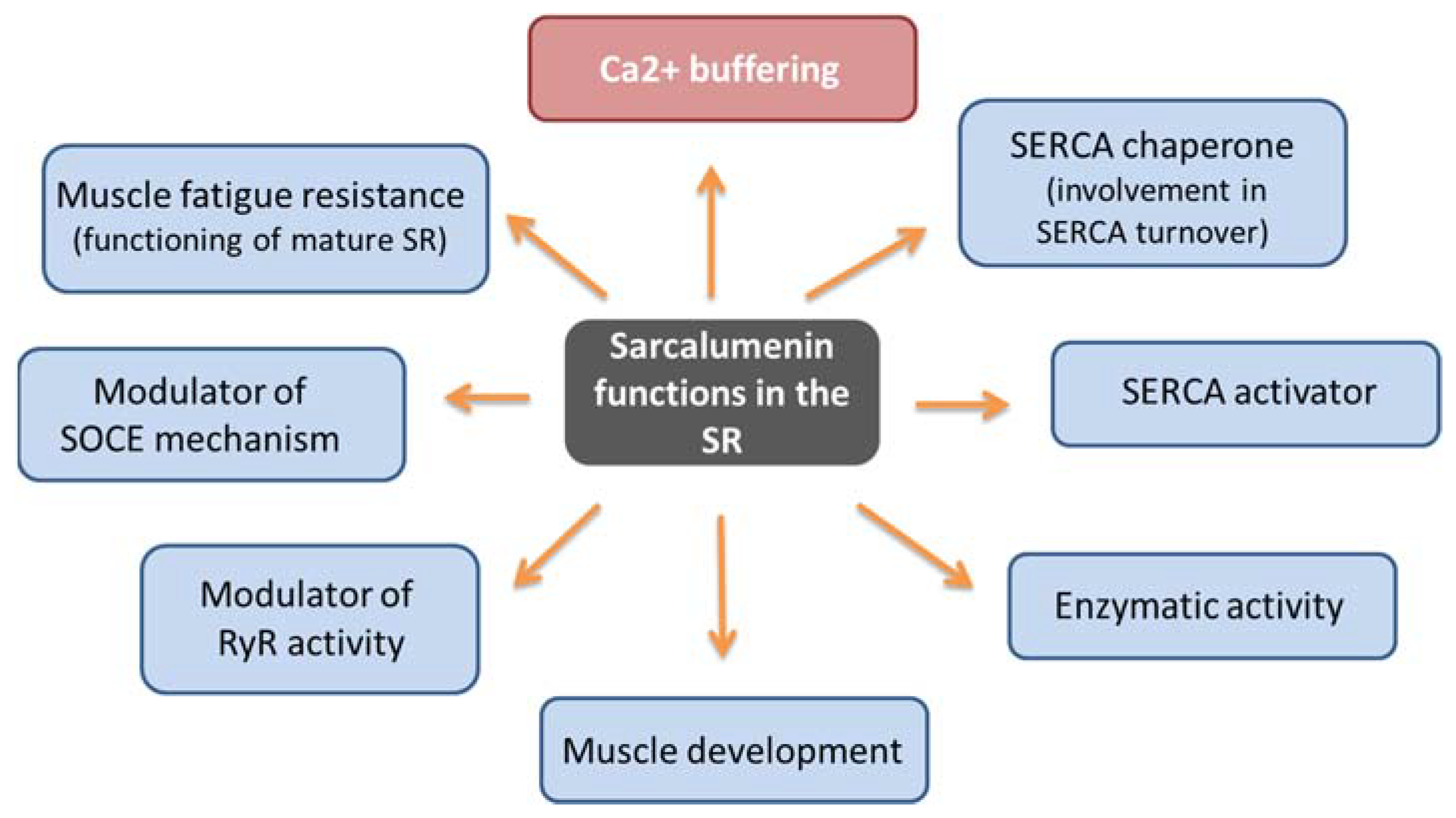
Similarly to CSQ, SAR has a high capacity to bind calcium (35 mol Ca2+/mol protein) and moderate affinity (Kd 0.6 mM) [57,59]. It represents the major non-junctional SR protein responsible for Ca2+ buffering by acting in the release and uptake of Ca2+ and favoring the excitation–contraction–relaxation cycle [19,21,60]. In addition, it has been hypothesized that SAR may have multiple context-dependent functions (Figure 3). First, by performing differential coimmunoprecipitation and chemical crosslinking experiments, it has been demonstrated that SAR colocalizes and interacts directly with SERCA Ca2+ ATPase located on the SR [61], suggesting that it performs a maintenance function of the pump itself, also contributing to SERCA turnover by functioning as a SERCA chaperone. Using SAR knockout mice, Yoshida et al. showed that the absence of SAR was paralleled by a reduction in SERCA activity with an unchanged SERCA1 mRNA expression compared with control muscle [19]. The same study also underlined that SAR could exhibit enzymatic activity in SR, considering the presence of the presumed nucleotide-binding motifs for the P-loop-containing ATPase/GTPase in the carboxyl-terminal region. Furthermore, it has been suggested that SAR may play a role in the functioning of mature SR due to increased SAR expression during muscle development [19,62]. It has been also reported that cycles of phosphorylation and dephosphorylation of SAR and HRC by a casein kinase II type could modulate the RyR activity as part of the Ca2+-mobilizing machinery during EC coupling [63]. Lastly, SAR could have a role in the SOCE mechanism and muscle fatigue resistance. In particular, using exercised knockout mice for SAR (Sar -/-), Zhao et al. demonstrated that SAR ablation improved both SOCE and the fatigability of exercised skeletal muscles, in correlation with an increased expression level of MG29, a synaptophysin-related membrane protein located in the triad junction of skeletal muscle fibers [59]. SAR could play a role in the SOCE mechanism because the loss of SAR could favor SR depletion, thereby leading to a greater activation of SOCE, an event also favored by the concomitant elevated expression of MG29. Furthermore, it cannot be ruled out that the enhanced resistance to muscle fatigue shown in these mutated mice could be related to compensatory changes in Ca2+ regulatory proteins that impact the SOCE mechanism. Thus, in addition to the Ca2+ buffer function, SAR can have different and context-dependent functions. In consideration of the limited findings reported on this topic to date, new investigations are needed to confirm and uncover molecular mechanisms underlying SAR function.
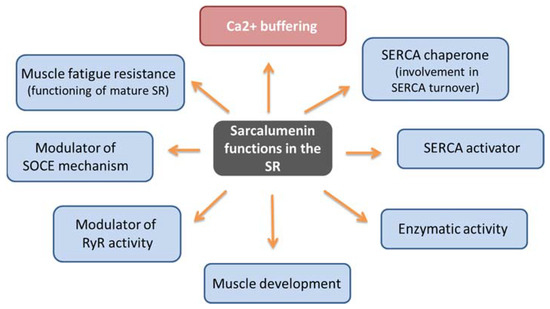
Figure 3.
Schematic representation of sarcalumenin functions.
4. Skeletal Muscle Disorders Involving Sarcalumenin-Mediated Luminal Ca2+-Handling Alteration
The elaborate mechanism of Ca2+ regulation in muscle cells works with precision. High levels of cytosolic Ca2+ are essential for muscle contraction onset, and the reuptake of Ca2+ ions is pivotal to trigger muscle relaxation. Defects in the proteins that make up this system and modifications of Ca2+ cycling often represent the main cause of neuromuscular pathologies. Importantly, it is necessary to stress that Ca2+ SR binding proteins are not simply ion traps that facilitate Ca2+ reuptake and increase luminal Ca2+ storage capacity but are also multifunctional SR proteins that act as endogenous regulators of Ca2+ SR channels and as luminal chaperones [64]. Thus, even small changes in the expression levels of SAR and other Ca2+ binding proteins may play an important role in altering the cyclic Ca2+ system in skeletal muscle diseases. Here, we discuss current knowledge of the involvement of altered SAR expression and activity in neuromuscular diseases such as Duchenne muscular dystrophy, sarcopenia and malignant hyperthermia.
4.1. Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is an X-linked lethal disease of childhood that affects approximately 1 in 5000 live births and represents the most frequent neuromuscular disorder in humans [65]. DMD is caused by mutations in the DMD gene encoding the membrane cytoskeletal protein dystrophin [66,67,68]. These mutations cause a loss of dystrophin and lead to progressive muscle degeneration. To date, there are no effective long-term therapies able to provide a lasting abolition of progressive muscle atrophy in humans, although several promising therapeutic strategies have been suggested to counteract the muscle wasting symptoms associated with DMD (i.e., pharmacological, cellular or gene-based therapy approaches) [69,70,71]. The most popular animal model for studying DMD is represented by mdx mice (X-chromosome-linked muscular dystrophy) [72,73], which have a mutation in the dystrophin gene itself, like DMD patients. In this animal model, as well as in DMD patients, the lack of dystrophin results in mechanical instability caused by chronic muscle degeneration and regeneration and a destabilization of sarcolemma [74,75,76] with a high accumulation of macrophages that favor muscle fibrosis [77]. Furthermore, although it is known that the primary abnormality is the loss of dystrophin, several studies have suggested that a rise in intracellular Ca2+ due to augmented extracellular Ca2+ entry could be an important initiating event in dystrophic muscle and could play a central role in the pathophysiological mechanisms leading to muscle weakness [78,79,80,81,82,83,84]. Increased Ca2+ levels may contribute to a cycle of activated protease and Ca2+-leak channel activation [85,86]. In addition to the increased cytosolic Ca2+ concentration, the Ca2+ buffering capacity of the dystrophic SR is also significantly altered [79]. In particular, depending on the analyzed muscle, an increase or a reduction in CSQ levels has been reported [87,88,89]. Interestingly, skeletal muscles of dystrophin-deficient mdx mice showed approximately 70% lower levels of SAR protein relative to wild-type mice [61,87]. A concomitant drastic reduction in SAR expression was also detected in dystrophic cardiac muscle [90]. Therefore, modified Ca2+ homeostasis and impaired luminal Ca2+ buffering are considered the major downstream effects of sarcolemmal rupture, eventually leading to muscle weakness and accelerating the protein degradation process in dystrophic muscles. All these results corroborate the fact that not only CSQ but also SAR can be considered an important luminal calcium buffer that may play a role in the dystrophic phenotype. Therefore, comprehension of the mechanisms underlying SAR alteration in dystrophic settings could potentially lead to the identification of novel therapeutic targets for DMD.
4.2. Sarcopenia
Sarcopenia is an age-related condition characterized by the presence of muscle atrophy and a progressive and generalized decline in muscle strength [91], leading to muscle fragility, loss of muscle mass and augmented fatigue. While loss of muscle mass has a fundamental impact on this condition, progressive muscle weakness is ultimately the primary cause of sarcopenia-associated morbidity and mortality, reducing quality of life in older adults. By understanding the molecular processes that lead to the aging phenotype, it is possible to propose treatments and therapies to reduce the functional impact of muscle weakness and slow down its progression. Multiple factors are involved in the molecular mechanisms underlying age-related sarcopenia, some of which have still not been exhaustively described. Specifically, increased muscle proteolysis, cellular autophagy, altered activation of Ca2+-activated proteases/proteasomes and dysfunction of satellite cells have been proposed to be involved [92,93]. Furthermore, several studies propose that impaired Ca2+ homeostasis can play a key role in sarcopenia and age-related muscle weakness [94,95,96,97]. Indeed, age-induced uncoupling between DHPR and RYR1 proteins and the consequent decoupling in the excitation–contraction mechanism lead to a reduced Ca2+ supply to the contractile apparatus and to a reduction in contractile force [94,98]. Several studies have also shown that a drastic reduction in the Ca2+ buffer proteins of the longitudinal SR, in particular SAR protein, occur in sarcopenic skeletal muscle [60,99,100,101]. In particular, O’Connel and colleagues suggest that the significant reduction in SAR expression leads to a reduced capacity of the longitudinal SR shuttle system, which could negatively influence the number of available ions for fast Ca2+ release mechanisms, ultimately contributing to a significant decline in contractile force and related muscle function during normal aging [60]. It has been proposed that SAR may play a role in muscle development because a gradual increase in protein expression has been shown during fiber maturation [21]. Therefore, its age-related reduction may have a major impact on muscle progression of the sarcopenic phenotype. Indeed, studies on SAR knockout mice highlight that these mice exhibit phenotypic changes similar to those observed in aged skeletal muscle, including diminished Ca2+ uptake into the SR lumen and altered Ca2+ handling properties [59], with the only difference being an enhanced SOCE, possibly resulting from chronic adaptation to SAR ablation [19]. Taking these findings into account, it can be suggested that age-induced changes in SAR levels may be due to mechanisms secondary to muscle wasting and that abnormal Ca2+ handling related to SAR reduction may contribute to the multifactorial etiology of sarcopenia and could be directly involved in contractile weakness. Unfortunately, very few studies focusing on the evaluation of the role of SAR in sarcopenia are currently available in the literature, and new evidence is needed to confirm preliminary results and to gain more insights.
4.3. Malignant Hypertermia
Malignant hyperthermia (MH) is a potentially fatal inherited severe myopathy characterized by a fulminant hypermetabolic state to inhalational anesthetics used during invasive procedures in predisposed individuals [102]. Symptoms of an MH episode are related to an uncontrolled elevation of intracellular Ca2+ [103] and include hypoxia, acidosis, hyperthermia, tachycardia, CO2 production, hyperkalemia, muscle rigidity and rhabdomyolysis. Mutations in the RyR1 and CACNA1S genes encoding the RyR1 isoform and DHPR, respectively, have been associated with MH [104]. Several dominant RyR1 mutations have been found in MH patients [105], which increase protein opening in the resting state, with a consequent increase in cytosolic Ca2+ levels during muscle excitation. These defects favor the onset of the typical signs of MH, i.e., glycogenolysis, ATP depletion, mitochondrial oxidation, lactic acid production, electrolyte imbalance and muscle damage [103]. In addition to mutations in RyR1 and CACNA1S, activated SOCE may contribute to increased intracellular Ca2+ levels in the skeletal muscles of MH patients [106]. Furthermore, it was supposed that abnormalities in CSQ and/or SAR might be involved in this disease [58]. Few studies on the involvement of Ca2+ buffer proteins in MH pathology are available in the literature, and almost all are focused on the role of CSQ. To date, no differences in the expression of SR luminal Ca2+ binding proteins have been found for either CSQ or SAR levels [107]. However, after halothane treatment, premature priming of CSQ for Ca2+ release was shown, and considering that RyR1 is CSQ-interdependent, this event favors RyR1 opening in MH muscle [108]. Interestingly, both human patients with mutations in CSQ [109] and CSQ knockouts mice [110] show symptoms similar to those observed in patients with MH, suggesting that the CSQ gene could be considered for genetic screening in MH patients without a mutation in the RyR1 or CACNA1S genes. Although no in-depth study has been published in the literature, the structural and functional similarities with CSQ suggest that SAR may contribute to or favor the onset of MH, suggesting a need for focused investigations in this field.
5. Sarcalumenin Ca2+ Buffer in Cardiac Muscle
Sarcalumenin is also expressed in the cardiac SR and exerts the same functions described for skeletal muscle, i.e., the regulation of intracellular Ca2+ [111]. Importantly, cardiac and skeletal muscle SAR are structurally different. Cardiac SAR shows a distinct electrophoretic mobility, immunological analysis and amino acid sequencing, suggesting that it is a different isoform with respect to skeletal muscle SAR [112]. Furthermore, similarly to skeletal muscle, SAR is able to interact with SERCA2a, improving its stability and modulating its function in the heart [113]. Studies performed using a SAR knockout mouse model have demonstrated that cardiac SAR is pivotal in maintaining the function of the heart by regulating Ca2+ transport activity into the SR, even when the heart is subjected to stress. Specifically, biomechanical stress, such as pressure overload, was shown to promote progressive heart failure in this SAR-deficient mouse model, suggesting that cardiac SAR expression is essential for heart adaptation to this stress [113]. Likewise, it was demonstrated that a physiological stress, such as resistance training, was able to reduce the expression of SERCA2a and to alter cardiac function and the maximal exercise capacity of this SAR-deficient mouse model [114]. This evidence suggests that, similarly to skeletal muscle, SAR may play an important role in maintaining cardiac function, particularly under physiological stress, and may be considered an important target to open alternative avenues for potential therapeutic approaches against heart disease.
6. Conclusions and Perspectives
Although SAR was discovered 15 years ago as a Ca2+ buffer protein located in the longitudinal part of SR muscle, dedicated studies have focused mainly on CSQ, likely due to its higher expression in SR. However, physiologically, SAR represents the major non-junctional SR protein responsible for Ca2+ buffering to support and regulate the muscle excitation–contraction cycle. In addition to this function, new roles of SAR have been recently revealed, with multiple context-dependent functions important for the stability of the SR membrane network. Importantly, even small changes in the expression levels of SAR may play an important role in altering the cyclic Ca2+ system in skeletal muscle diseases, corroborating the idea of the potential role of SAR as a pharmacological target. It is not clear whether and how the different Ca2+ buffer proteins interact with each other or whether protein–protein interactions can occur. Furthermore, the lack of SAR modulators represents a strong limitation. Our current understanding of the involvement of SAR and other Ca2+ buffer proteins in skeletal muscle diseases suggests that there may be a relationship between SAR and other SR Ca2+ buffer proteins through an adaptive or compensatory response of cells to the alteration of the expression of specific Ca2+ buffer proteins by enhancing or inhibiting other Ca2+ buffer proteins in a complementary manner. For this reason, it is important to characterize not only CSQ but also SAR, which continues to be underestimated. Once a complete map of the real role and involvement of the two main Ca2+ buffer proteins, SAR and CSQ (and others), in various pathophysiological conditions has been established, it may be possible to compose a three-dimensional map of each possible protein interaction and fully understand their complexity and involvement in skeletal muscle diseases. Furthermore, studying the interactions between Ca2+ buffer proteins and other proteins could pave the way for the identification of ligands and their relative binding sites.
The number of patients with skeletal muscle diseases continues to increase, and improved understanding of their underlying mechanisms is fundamental for the development of new therapies. Investigation of the roles of SAR in skeletal muscle diseases could offer a great opportunity to meet currently unmet needs.
Author Contributions
E.C. conceived the original idea; E.C. and A.L. wrote the paper; G.D. prepared figures and tables; A.D.L. and P.I. reviewed and edited the text. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the PRIN-MIUR 2020 (Research Projects of Relevant National Interest-Ministry of Education, University and Research) Prot. 20202YAY9B_004 granted to A.L.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CAMKII | Calmodulin kinase II |
| CLPs | Calsequestrin-like proteins |
| CSQ | Calsequestrin |
| DHPR | Dihydropyridine receptor |
| DMD | Duchenne muscular dystrophy |
| EC coupling | Excitation–contraction coupling |
| ECCE | Excitation-coupled Ca2+ entry |
| ER/SR | Endoplasmic/sarcoplasmic reticulum |
| HRC | Histidine-rich Ca2+-binding protein |
| IP3R | Inositol 1,4,5-triphosphate receptor |
| JPH | Junctophilin |
| MG29 | Mitsugumin 29 |
| MG53 | Mitsugumin 53 |
| MH | Malignant hyperthermia |
| RYR1 | Ryanodine receptor type 1 |
| SAR | Sarcalumenin |
| SERCA | Sarco-/endoplasmic reticular calcium ATPase |
| SMP30 | Senescence marker protein 30 |
| SOCE | Store-operated Ca2+ entry |
| STIM1 | Stromal-interacting molecule-1 |
| TRPCs | Transient receptor potential canonical channels |
References
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation–contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef]
- Dayal, A.; Perni, S.; Franzini-Armstrong, C.; Beam, K.G.; Grabner, M. The distal C terminus of the dihydropyridine receptor β1a subunit is essential for tetrad formation in skeletal muscle. Proc. Natl. Acad. Sci. USA 2022, 119, e2201136119. [Google Scholar] [CrossRef]
- Baylor, S.M.; Hollingworth, S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J. Physiol. 2003, 551, 125–138. [Google Scholar] [CrossRef]
- Ahn, M.K.; Lee, K.J.; Cai, C.; Huang, M.; Cho, C.H.; Ma, J.; Lee, E.H. Mitsugumin 53 regulates extracellular Ca(2+) entry and intracellular Ca(2+) release via Orai1 and RyR1 in skeletal muscle. Sci. Rep. 2016, 6, 36909. [Google Scholar] [CrossRef]
- Choi, J.H.; Jeong, S.Y.; Oh, M.R.; Allen, P.D.; Lee, E.H. TRPCs: Influential mediators in skeletal muscle. Cells 2020, 9, 850. [Google Scholar] [CrossRef]
- Sztretye, M.; Geyer, N.; Vincze, J.; Al-Gaadi, D.; Olah, T.; Szentesi, P.; Kis, G.; Antal, M.; Balatoni, I.; Csernoch, L.; et al. SOCE Is Important for Maintaining Sarcoplasmic Calcium Content and Release in Skeletal Muscle Fibers. Biophys. J. 2017, 113, 2496–2507. [Google Scholar] [CrossRef]
- Cherednichenko, G.; Hurne, A.M.; Fessenden, J.D.; Lee, E.H.; Allen, P.D.; Beam, K.G.; Pessah, I.N. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc. Natl. Acad. Sci. USA 2004, 101, 15793–15798. [Google Scholar] [CrossRef]
- Park, I.Y.; Kim, E.; Park, H.; Fields, K.; Dunker, K.A.; Kang, C. Interaction between cardiac calsequestrin and drugs with known cardiotoxicity. Mol. Pharmacol. 2004, 67, 97–104. [Google Scholar] [CrossRef]
- Leberer, E.; Timms, B.G.; Campbell, K.P.; MacLennan, D.H. Purification, calcium binding properties, and ultrastructural localization of the 53,000- and 160,000 (sarcalumenin)-dalton glycoproteins of the sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 10118–10124. [Google Scholar] [CrossRef]
- Picello, E.; Damiani, E.; Margreth, A. Low-affinity Ca(2+)-binding sites versus Zn(2+)-binding sites in histidine-rich Ca(2+)-binding protein of skeletal muscle sarcoplasmic reticulum. Biochem. Biophys. Res. Commun. 1992, 186, 659–667. [Google Scholar] [CrossRef]
- Nakamura, K.; Zuppini, A.; Arnaudeau, S.; Lynch, J.; Ahsan, I.; Krause, R.; Papp, S.; De Smedt, H.; Parys, J.B.; Muller-Esterl, W.; et al. Functional specialization of calreticulin domains. J. Cell Biol. 2001, 154, 961–972. [Google Scholar] [CrossRef]
- Hong, C.S.; Kwak, Y.G.; Ji, J.H.; Chae, S.W.; Han Kim, D. Molecular cloning and characterization of mouse cardiac junctate isoforms. Biochem. Biophys. Res. Commun. 2001, 289, 882–887. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bahnson, B.J. Crystal structure of human senescence marker protein 30: Insights linking structural, enzymatic, and physiological functions. Biochemistry 2010, 49, 3436–3444. [Google Scholar] [CrossRef]
- Schwaller, B. Cytosolic Ca2+ Buffers Are Inherently Ca2+ Signal Modulators. Cold Spring Harb. Perspect. Biol. 2020, 12, a035543. [Google Scholar] [CrossRef]
- Wu, X.; Reid, R.E. Structure/calcium affinity relationships of site III of calmodulin: Testing the acid pair hypothesis using calmodulin mutants. Biochemistry 1997, 36, 8649–8656. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of regucalcin in maintaining cell homeostasis and function (review). Int. J. Mol. Med. 2005, 15, 371–389. [Google Scholar] [CrossRef]
- Moradi, F.; Copeland, E.N.; Baranowski, R.W.; Scholey, A.E.; Stuart, J.A.; Fajardo, V.A. Calmodulin-Binding Proteins in Muscle: A Minireview on Nuclear Receptor Interacting Protein, Neurogranin, and Growth-Associated Protein 43. Int. J. Mol. Sci. 2020, 21, 1016. [Google Scholar] [CrossRef]
- Gillis, J.M.; Thomason, D.; Lefèvre, J.; Kretsinger, R.H.; Schwaller, B.; Dick, J.; Dhoot, G.; Carroll, S.; Vrbova, G.; Nicotera, P. Parvalbumins and muscle relaxation: A computer simulation study. J. Muscle Res. Cell Motil. 1982, 3, 377–398. [Google Scholar] [CrossRef]
- Yoshida, M.; Minamisawa, S.; Shimura, M.; Komazaki, S.; Kume, H.; Zhang, M.; Matsumura, K.; Nishi, M.; Saito, M.; Saeki, Y.; et al. Impaired Ca2+ store functions in skeletal and cardiac muscle cells from sarcalumenin-deficient mice. J. Biol. Chem. 2005, 280, 3500–3506. [Google Scholar] [CrossRef]
- Rossi, D.; Pierantozzi, E.; Amadsun, D.O.; Buonocore, S.; Rubino, E.M.; Sorrentino, V. The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites. Biomolecules 2022, 12, 488. [Google Scholar] [CrossRef]
- Rossi, A.E.; Dirksen, R.T. Sarcoplasmic reticulum: The dynamic calcium governor of muscle. Muscle Nerve 2006, 33, 715–731. [Google Scholar] [CrossRef]
- Wray, S.; Burdyga, T. Sarcoplasmic reticulum function in smooth muscle. Physiol. Rev. 2010, 90, 113–178. [Google Scholar] [CrossRef]
- Bublitz, M.; Musgaard, M.; Poulsen, H.; Thøgersen, L.; Olesen, C.; Schiøtt, B.; Morth, J.P.; Møller, J.V.; Nissen, P. Ion pathways in the sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 2013, 288, 10759–10765. [Google Scholar] [CrossRef]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar] [CrossRef]
- Phimister, A.J.; Lango, J.; Lee, E.H.; Ernst-Russell, M.A.; Takeshima, H.; Ma, J.; Allen, P.D.; Pessah, I.N. Conformation-dependent stability of junctophilin 1 (JP1) and ryanodine receptor type 1 (RyR1) channel complex is mediated by their hyper-reactive thiols. J. Biol. Chem. 2007, 282, 8667–8677. [Google Scholar] [CrossRef]
- Golini, L.; Chouabe, C.; Berthier, C.; Cusimano, V.; Fornaro, M.; Bonvallet, R.; Formoso, L.; Giacomello, E.; Jacquemond, V.; Sorrentino, V. Junctophilin 1 and 2 proteins interact with the L-type Ca2+ channel dihydropyridine receptors (DHPRs) in skeletal muscle. J. Biol. Chem. 2011, 286, 43717–43725. [Google Scholar] [CrossRef]
- Takeshima, H.; Shimuta, M.; Komazaki, S.; Ohmi, K.; Nishi, M.; Iino, M.; Miyata, A.; Kangawa, K. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem. J. 1998, 331 Pt 1, 317–322. [Google Scholar] [CrossRef]
- Nishi, M.; Komazaki, S.; Kurebayashi, N.; Ogawa, Y.; Noda, T.; Iino, M.; Takeshima, H. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J. Cell Biol. 1999, 147, 1473–1480. [Google Scholar] [CrossRef]
- Woo, J.S.; Hwang, J.H.; Huang, M.; Ahn, M.K.; Cho, C.H.; Ma, J.; Lee, E.H. Interaction between mitsugumin 29 and TRPC3 partic-ipates in regulating Ca2+ transients in skeletal muscle. Biochem. Biophys. Res. Commun. 2015, 464, 133–139. [Google Scholar] [CrossRef]
- Cala, S.E.; Scott, B.T.; Jones, L.R. Intralumenal sarcoplasmic reticulum Ca2+-binding proteins. Semin. Cell Biol. 1990, 1, 265–275. [Google Scholar]
- Franzini-Armstrong, C.; Kennery, L.J.; Varriano-Martson, E. The structure of calsequestrin in triads of vertebrate skeletal muscle: A deep-etch study. J. Cell Biol. 1987, 105, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pape, P.C.; Fénelon, K.; Lamboley, C.R.; Stachura, D. Role of calsequestrin evaluated from changes in free and total calcium concentrations in the sarcoplasmic reticulum of frog cut skeletal muscle fibres. J. Physiol. 2007, 15, 319–367. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Randazzo, D.; Del Re, V.; Sorrentino, V.; Rossi, D. Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers. J. Muscle Res. Cell Motil. 2015, 36, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Close, M.; Franzini-Armstrong, C. Novel details of calsequestrin gel conformation in situ. J. Biol. Chem. 2013, 25, 31358–31362. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Wu, S.; Dunker, A.K.; Kang, C. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 2003, 2, 16176–16182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kelley, J.; Schmeisser, G.; Kobayashi, Y.M.; Jones, L.R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997, 12, 23389–23397. [Google Scholar] [CrossRef]
- Glover, L.; Quinn, S.; Ryan, M.; Pette, D.; Ohlendieck, K. Supramolecular calsequestrin complex. Eur. J. Biochem. 2002, 269, 4607–4616. [Google Scholar] [CrossRef]
- Wei, L.; Gallant, E.M.; Dulhunty, A.F.; Beard, N.A. Junctin and triadin each activate skeletal ryanodine receptors but junctin alone mediates functional interactions with calsequestrin. Int. J. Biochem. Cell Biol. 2009, 41, 2214–2224. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, S.; Zheng, Y.; Yan, X.; Chen, M.; Wang, H.; Putney, J.W.; Luo, D. Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci. Rep. 2015, 18, 11349. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Li, S.; Xue, J.; Luo, D. Calsequestrin-1 regulates store-operated Ca2+ entry by inhibiting STIM1 aggregation. Cell. Physiol. Biochem. 2016, 8, 2183–2193. [Google Scholar] [CrossRef]
- Suk, J.Y.; Kim, Y.S.; Park, W.J. HRC (histidine-rich Ca2+ binding protein) resides in the lumen of sarcoplasmic reticulum as a multimer. Biochem. Biophys. Res. Commun. 1999, 263, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kang, H.; Kim, D.H.; Park, W.J. Interaction of HRC (histidine-rich Ca(2+)-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J. Biol. Chem. 2001, 276, 39533–39538. [Google Scholar] [CrossRef]
- Sacchetto, R.; Turcato, F.; Damiani, E.; Margreth, A. Interaction of triadin with histidine-rich Ca(2+)-binding protein at the triadic junction in skeletal muscle fibers. J. Muscle Res. Cell Motil. 1999, 20, 403–415. [Google Scholar] [CrossRef]
- Sacchetto, R.; Damiani, E.; Turcato, F.; Nori, A.; Margreth, A. Ca(2+)-dependent interaction of triadin with histidine-rich Ca(2+)-binding protein carboxyl-terminal region. Biochem. Biophys. Res. Commun. 2001, 289, 1125–1134. [Google Scholar] [CrossRef]
- Singh, V.P.; Rubinstein, J.; Arvanitis, D.A.; Ren, X.; Gao, X.; Haghighi, K.; Gilber, T.M.; Iyer, V.R.; Kim, D.H.; Cho, C.; et al. Abnormal calcium cycling and cardiac arrhythmias associated with the human Ser96Ala genetic variant of histidine-rich calcium-binding protein. J. Am. Heart Assoc. 2013, 2, e000460. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, X.; Fu, Y.; Li, Y.; Lu, W.; Pan, Y.; Yang, J.; Kong, J. Inhibition of lung cancer by vitamin D depends on downregulation of histidine-rich calcium-binding protein. J. Adv. Res. 2020, 29, 13–22. [Google Scholar] [CrossRef]
- Wang, C.; Ren, C.; Hu, Q.; Shen, X.; Wang, M.; Yang, Z.; Xu, E.; Wang, X.; Li, Z.; Yu, H.; et al. Histidine-rich calcium binding protein promotes gastric cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition through Raf/MEK/ERK signaling. J. Cancer 2022, 13, 1073–1085. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, G.C.; Ren, X.; Waggoner, J.R.; Gregory, K.N.; Chen, G.; Jones, W.K.; Kranias, E.G. Overexpression of histidine-rich Ca-binding protein protects against ischemia/reperfusion-induced cardiac injury. Cardiovasc. Res. 2007, 75, 487–497. [Google Scholar] [CrossRef]
- Treves, S.; Feriotto, G.; Moccagatta, L.; Gambari, R.; Zorzato, F. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sarco(endo)plasmic reticulum membrane. J. Biol. Chem. 2000, 275, 39555–39568. [Google Scholar] [CrossRef] [PubMed]
- Treves, S.; Franzini-Armstrong, C.; Moccagatta, L.; Arnoult, C.; Grasso, C.; Schrum, A.; Ducreux, S.; Zhu, M.; Mikoshiba, K.; Girard, T.; et al. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J. Cell Biol. 2004, 166, 537–548. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Reithmeier, R.A. Ion tamers. Nat. Struct. Mol. 1998, 5, 409–411. [Google Scholar] [CrossRef]
- Corbett, E.F.; Michalak, K.M.; Oikawa, K.; Johnson, S.; Campbell, I.D.; Eggleton, P.; Kay, C.; Michalak, M. The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J. Biol. Chem. 2000, 275, 27177–27185. [Google Scholar] [CrossRef]
- Nørgaard Toft, K.; Larsen, N.; Jørgensen, F.S.; Højrup, P.; Houen, G.; Vestergaard, B. Small angle X-ray scattering study of calreticulin reveals conformational plasticity. Biochim. Biophys. Acta. 2008, 1784, 1265–1270. [Google Scholar] [CrossRef]
- Bastianutto, C.; Clementi, E.; Codazzi, F.; Podini, P.; De Giorgi, F.; Rizzuto, R.; Meldolesi, J.; Pozzan, T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their luminal microenvironment and funcion. J. Cell Biol. 1995, 130, 847–855. [Google Scholar] [CrossRef]
- Mery, L.; Mesaeli, N.; Michalak, M.; Opas, M.; Lew, D.P.; Krause, K.H. Overexpression of calreticulin increasea intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J. Biol. Chem. 1996, 271, 9332–9339. [Google Scholar] [CrossRef]
- Xu, W.; Longo, F.J.; Wintermantel, M.R.; Jiang, X.; Clark, R.A.; deLisle, S. Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-triphosphate-induced Ca2+ store depletion. J. Biol. Chem. 2000, 275, 36676–36682. [Google Scholar] [CrossRef]
- Leberer, E.; Charuk, J.H.; Green, N.M.; MacLennan, D.H. Molecular cloning and expression of cDNA encoding a lumenal calcium binding glycoprotein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1989, 86, 6047–6051. [Google Scholar] [CrossRef]
- Schreiber, D.; Donoghue, P.; O’Reilly, C.; Ohlendieck, K. Role of Calsequestrin and Related Luminal Ca2+-Binding Proteins as Mediators of Excitation-Contraction Coupling. Basic Appl. Myol. 2004, 14, 313–322. [Google Scholar]
- Zhao, X.; Yoshida, M.; Brotto, L.; Takeshima, H.; Weisleder, N.; Hirata, Y.; Nosek, T.M.; Ma, J.; Brotto, M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol. Genom. 2005, 23, 72–78. [Google Scholar] [CrossRef]
- O’Connell, K.; Gannon, J.; Doran, P.; Ohlendieck, K. Reduced expression of sarcalumenin and related Ca2+-regulatory proteins in aged rat skeletal muscle. Exp. Gerontol. 2008, 43, 958–961. [Google Scholar] [CrossRef]
- Dowling, P.; Doran, P.; Ohlendieck, K. Drastic reduction of sarcalumenin in Dp427 (dystrophin of 427 kDa)-deficient fibres indicates that abnormal calcium handling plays a key role in muscular dystrophy. Biochem. J. 2004, 379 Pt 2, 479–488. [Google Scholar] [CrossRef]
- Raeymaekers, L.; Verbist, J.; Wuytack, F.; Plessers, L.; Casteels, R. Expression of Ca2+ binding proteins of the sarcoplasmic reticulum of striated muscle in the endoplasmic reticulum of pig smooth muscles. Cell Calcium 1993, 14, 581–589. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Orr, I.; Weil, S.; Meyer, H.; Varsanyi, M.; Heilmeyer, L.M. The identification of the phosphorylated 150/160-kDa proteins of sarcoplasmic reticulum, their kinase and their association with the ryanodine receptor. Biochim. Biophys. Acta. 1996, 1283, 89–100. [Google Scholar] [CrossRef]
- Hidalgo, C.; Donoso, P. Luminal calcium regulation of calcium release from the sarcoplasmic reticulum. Biosci. Rep. 1995, 15, 387–397. [Google Scholar] [CrossRef]
- McNally, E.M.; Kaltman, J.R.; Benson, D.W.; Canter, C.E. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation 2015, 131, 1590–1598. [Google Scholar] [CrossRef]
- Koenig, M.; Hoffman, E.P.; Bertelson, C.J.; Monaco, A.P.; Feener, C.; Kunkel, L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef]
- Bladen, C.L.; Salgado, D.; Monges, S.; Foncuberta, M.E.; Kekou, K.; Kosma, K.; Dawkins, H.; Lamont, L.; Roy, A.J.; Chamova, T.; et al. The TREAT-NMD DMD Global Database: Analysis of more than 7000 duchenne muscular dystrophy mutations. Hum. Mutat. 2015, 36, 395–402. [Google Scholar] [CrossRef]
- Carter, J.C.; Sheehan, D.W.; Prochoroff, A.; Birnkrant, D.J. Muscular dystrophies. Clin. Chest Med. 2018, 39, 377–389. [Google Scholar] [CrossRef]
- Nelson, C.E.; Wu, Y.; Gemberling, M.P.; Oliver, M.L.; Waller, M.A.; Bohning, J.D.; Robinson-Hamm, J.N.; Bulaklak, K.; Castellanos Rivera, R.M.; Collier, J.H.; et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 427–432. [Google Scholar] [CrossRef]
- Soblechero-Martín, P.; López-Martínez, A.; de la Puente-Ovejero, L.; Vallejo-Illarramendi, A.; Arechavala-Gomeza, V. Utrophin modulator drugs as potential therapies for Duchenne and Becker muscular dystrophies. Neuropathol. Appl. Neurobiol. 2021, 47, 711–723. [Google Scholar] [CrossRef]
- Angelini, G.; Mura, G.; Messina, G. Therapeutic approaches to preserve the musculature in Duchenne Muscular Dystrophy: The importance of the secondary therapies. Exp. Cell Res. 2022, 410, 112968. [Google Scholar] [CrossRef]
- Bulfield, G.; Siller, W.G.; Wight, P.A.; Moore, K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 1984, 81, 1189–1192. [Google Scholar] [CrossRef]
- Yucel, N.; Chang, A.C.; Day, J.W.; Rosenthal, N.; Blau, H.M. Humanizing the mdx mouse model of DMD: The long and the short of it. NPJ Regen. Med. 2018, 3, 4. [Google Scholar] [CrossRef]
- Tkatchenko, A.V.; Le Cam, G.; Leger, J.J.; Dechesne, C.A. Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochim. Biophys. Acta. 2000, 1500, 17–30. [Google Scholar] [CrossRef]
- Chen, Y.W.; Zhao, P.; Borup, R.; Hoffman, E.P. Expression profiling in the muscular dystrophies: Identification of novel aspects of molecular pathophysiology. J. Cell Biol. 2000, 151, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ito, M.; Fukami, M.; Hashimoto, M.; Hirayama, M.; Ohno, K. Molecular hydrogen alleviates motor deficits and muscle degeneration in mdx mice. Redox Rep. 2017, 22, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Juban, G.; Saclier, M.; Yacoub-Youssef, H.; Kernou, A.; Arnold, L.; Boisson, C.; Ben Larbi, S.; Magnan, M.; Cuvellier, S.; Théret, M.; et al. AMPK activation regulates LTBP4-dependent TGF-beta1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep. 2018, 25, 2163–2176.e2166. [Google Scholar] [CrossRef] [PubMed]
- Mallouk, N.; Jacquemond, V.; Allard, B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibres detected with Ca2+-activated K+ channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4950–4955. [Google Scholar] [CrossRef]
- Culligan, K.; Ohlendieck, K. Abnormal calcium handling in muscular dystrophy. Basic Appl. Myol. 2002, 12, 147–157. [Google Scholar]
- Fraysse, B.; Liantonio, A.; Cetrone, M.; Burdi, R.; Pierno, S.; Frigeri, A.; Pisoni, M.; Camerino, C.; De Luca, A. The alteration of calcium homeostasis in adult dystrophic mdx muscle fibers is worsened by a chronic exercise in vivo. Neurobiol. Dis. 2004, 17, 144–154. [Google Scholar] [CrossRef]
- Rolland, J.F.; De Luca, A.; Burdi, R.; Andreetta, F.; Confalonieri, P.; Conte Camerino, D. Overactivity of exercise-sensitive cation channels and their impaired modulation by IGF-1 in mdx native muscle fibers: Beneficial effect of pentoxifylline. Neurobiol. Dis. 2006, 24, 466–474. [Google Scholar] [CrossRef]
- Burdi, R.; Rolland, J.F.; Fraysse, B.; Litvinova, K.; Cozzoli, A.; Giannuzzi, V.; Liantonio, A.; Camerino, G.M.; Sblendorio, V.; Capogrosso, R.F.; et al. Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: Outcome of a large array of in vivo and ex vivo tests. J. Appl. Physiol. 2009, 106, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, R.F.; Mantuano, P.; Uaesoontrachoon, K.; Cozzoli, A.; Giustino, A.; Dow, T.; Srinivassane, S.; Filipovic, M.; Bell, C.; Vandermeulen, J.; et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: Proof-of-concept study and independent validation of efficacy. FASEB J. 2018, 32, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Mareedu, S.; Million, E.D.; Duan, D.; Babu, G.J. Abnormal Calcium Handling in Duchenne Muscular Dystrophy: Mechanisms and Potential Therapies. Front. Physiol. 2021, 12, 647010. [Google Scholar] [CrossRef]
- Alderton, J.M.; Steinhardt, R.A. How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc. Med. 2000, 10, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Shanmugam, M.; Gonzalez, J.P.; Lopez, H.; Gordan, R.; Fraidenraich, D.; Babu, G.J. Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 2013, 34, 349–356. [Google Scholar] [CrossRef]
- Doran, P.; Dowling, P.; Lohan, J.; McDonnell, K.; Poetsch, S.; Ohlendieck, K. Subproteomics analysis of Ca+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle. Eur. J. Biochem. 2004, 271, 3943–3952. [Google Scholar] [CrossRef]
- Lewis, C.; Carberry, S.; Ohlendieck, K. Proteomic profiling of x-linked muscular dystrophy. J. Muscle Res. Cell Motil. 2009, 30, 267–269. [Google Scholar] [CrossRef]
- Maranhão, J.B.; de Oliveira Moreira, D.; Maurício, A.F.; de Carvalho, S.C.; Ferretti, R.; Pereira, J.A.; Santo Neto, H.; Marques, M.J. Changes in calsequestrin, TNF-α, TGF-β and MyoD levels during the progression of skeletal muscle dystrophy in mdx mice: A comparative analysis of the quadriceps, diaphragm and intrinsic laryngeal muscles. Int. J. Exp. Pathol. 2015, 96, 285–293. [Google Scholar] [CrossRef]
- Lohan, J.; Ohlendieck, K. Drastic reduction in the luminal Ca2+ -binding proteins calsequestrin and sarcalumenin in dystrophin-deficient cardiac muscle. Biochim. Biophys. Acta. 2004, 1689, 252–258. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.H. Impact of skeletal muscle mass on metabolic health. Endocrinol. Metab. 2020, 35, 1–6. [Google Scholar] [CrossRef]
- Teixeira Vde, O.; Filippin, L.I.; Xavier, R.M. Mechanisms of muscle wasting in sarcopenia. Rev. Bras. Reumatol. 2012, 52, 252–259. [Google Scholar] [CrossRef]
- Romanick, M.; Thompson, L.V.; Brown-Borg, H.M. Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochim. Biophys. Acta. 2013, 1832, 1410–1420. [Google Scholar] [CrossRef]
- Delbono, O.; O’Rourke, K.S.; Ettinger, W.H. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J. Membr. Biol. 1995, 148, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, B.; Desaphy, J.F.; Rolland, J.F.; Pierno, S.; Liantonio, A.; Giannuzzi, V.; Camerino, C.; Didonna, M.P.; Cocchi, D.; De Luca, A.; et al. Fiber type-related changes in rat skeletal muscle calcium homeostasis during aging and restoration by growth hormone. Neurobiol. Dis. 2006, 21, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mijares, A.; Allen, P.D.; Lopez, J.R. Senescence Is Associated With Elevated Intracellular Resting [Ca2+] in Mice Skeletal Muscle Fibers. An in vivo Study. Front. Physiol. 2021, 11, 601189. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Imbrici, P.; Mantuano, P.; Coppola, M.A.; Camerino, G.M.; De Luca, A.; Liantonio, A. Alteration of STIM1/Orai1-Mediated SOCE in Skeletal Muscle: Impact in Genetic Muscle Diseases and Beyond. Cells 2021, 10, 2722. [Google Scholar] [CrossRef]
- Wang, Z.M.; Messi, M.L.; Delbono, O. L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys. J. 2000, 78, 1947–1954. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Gourbeyre, O.; Chambon, C.; Combaret, L.; Polge, C.; Taillandier, D.; Attaix, D.; Friguet, B.; Maier, A.B.; et al. Proteomics of muscle chronological ageing in post-menopausal women. BMC Genom. 2014, 15, 1165. [Google Scholar] [CrossRef]
- Mosole, S.; Zampieri, S.; Furlan, S.; Carraro, U.; Löefler, S.; Kern, H.; Volpe, P.; Nori, A. Effects of Electrical Stimulation on Skeletal Muscle of Old Sedentary People. Gerontol. Geriatr. Med. 2018, 4, 2333721418768998. [Google Scholar] [CrossRef]
- Kelley, R.C.; McDonagh, B.; Ferreira, L.F. Advanced aging causes diaphragm functional abnormalities, global proteome remodeling, and loss of mitochondrial cysteine redox flexibility in mice. Exp. Gerontol. 2018, 103, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.E. Malignant hyperthermia: A pharmacogenetic disease of Ca++ regulating proteins. Curr. Mol. Med. 2002, 2, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; MacLennan, D.H. Malignant hyperthermia and central core disease: Disorders of Ca2+ release channels. Am. J. Med. 1998, 104, 470–486. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Duff, C.; Zorzato, F.; Fujii, J.; Phillips, M.; Korneluk, R.G.; Frodis, W.; Britt, B.A.; Worton, R.G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature 1990, 343, 559–561. [Google Scholar] [CrossRef]
- Robinson, R.; Carpenter, D.; Shaw, M.A.; Halsall, J.; Hopkins, P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum. Mutat. 2006, 27, 977–989. [Google Scholar] [CrossRef]
- Duke, A.M.; Hopkins, P.M.; Calaghan, S.C.; Halsall, J.P.; Steele, D.S. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J. Biol. Chem. 2010, 285, 25645–25653. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.; Heffron, J.J.; Ohlendieck, K. Increased sensitivity of the ryanodine receptor to halothaneinduced oligomerization in malignant hyperthermia-susceptible human skeletal muscle. J. Appl. Physiol. 2004, 96, 11–18. [Google Scholar] [CrossRef]
- O’Sullivan, G.H.; McIntosh, J.M.; Heffron, J.J. Abnormal uptake and release of Ca2+ ions from human malignant hyperthermia-susceptible sarcoplasmic reticulum. Biochem. Pharmacol. 2001, 61, 1479–1485. [Google Scholar] [CrossRef]
- Lewis, K.M.; Ronish, L.A.; Rios, E.; Kang, C. Characterization of two human skeletal calsequestrin mutants implicated in malignant hyperthermia and vacuolar aggregate myopathy. J. Biol. Chem. 2015, 290, 28665–28674. [Google Scholar] [CrossRef]
- Dainese, M.; Quarta, M.; Lyfenko, A.D.; Paolini, C.; Canato, M.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice. FASEB J. 2009, 23, 1710–1720. [Google Scholar] [CrossRef]
- Ebashi, S. Excitation-contraction coupling and mechanism of muscle contraction. Annu. Rev. Physiol. 1991, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hadad, N.; Meyer, H.E.; Varsanyi, M.; Fleischer, S.; Shoshan-Barmatz, V. Cardiac sarcalumenin: Phosphorylation, comparison with the skeletal muscle sarcalumenin and modulation of ryanodine receptor. J Membr. Biol. 1999, 170, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Minamisawa, S.; Takeshima, H.; Jiao, Q.; Bai, Y.; Umemura, S.; Ishikawa, Y. Sarcalumenin alleviates stress-induced cardiac dysfunction by improving Ca2+ handling of the sarcoplasmic reticulum. Cardiovasc. Res 2008, 77, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Bai, Y.; Akaike, T.; Takeshima, H.; Ishikawa, Y.; Minamisawa, S. Sarcalumenin is essential for maintaining cardiac function during endurance exercise training. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H576–H582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).