Sarcoplasmic Reticulum Ca2+ Buffer Proteins: A Focus on the Yet-To-Be-Explored Role of Sarcalumenin in Skeletal Muscle Health and Disease
Abstract
:1. Introduction
2. Overview of Sarcoplasmic Reticulum Structure and the Junctional Ca2+ Buffer Proteins: Calsequestrin, HRC, Junctate and Calreticulin
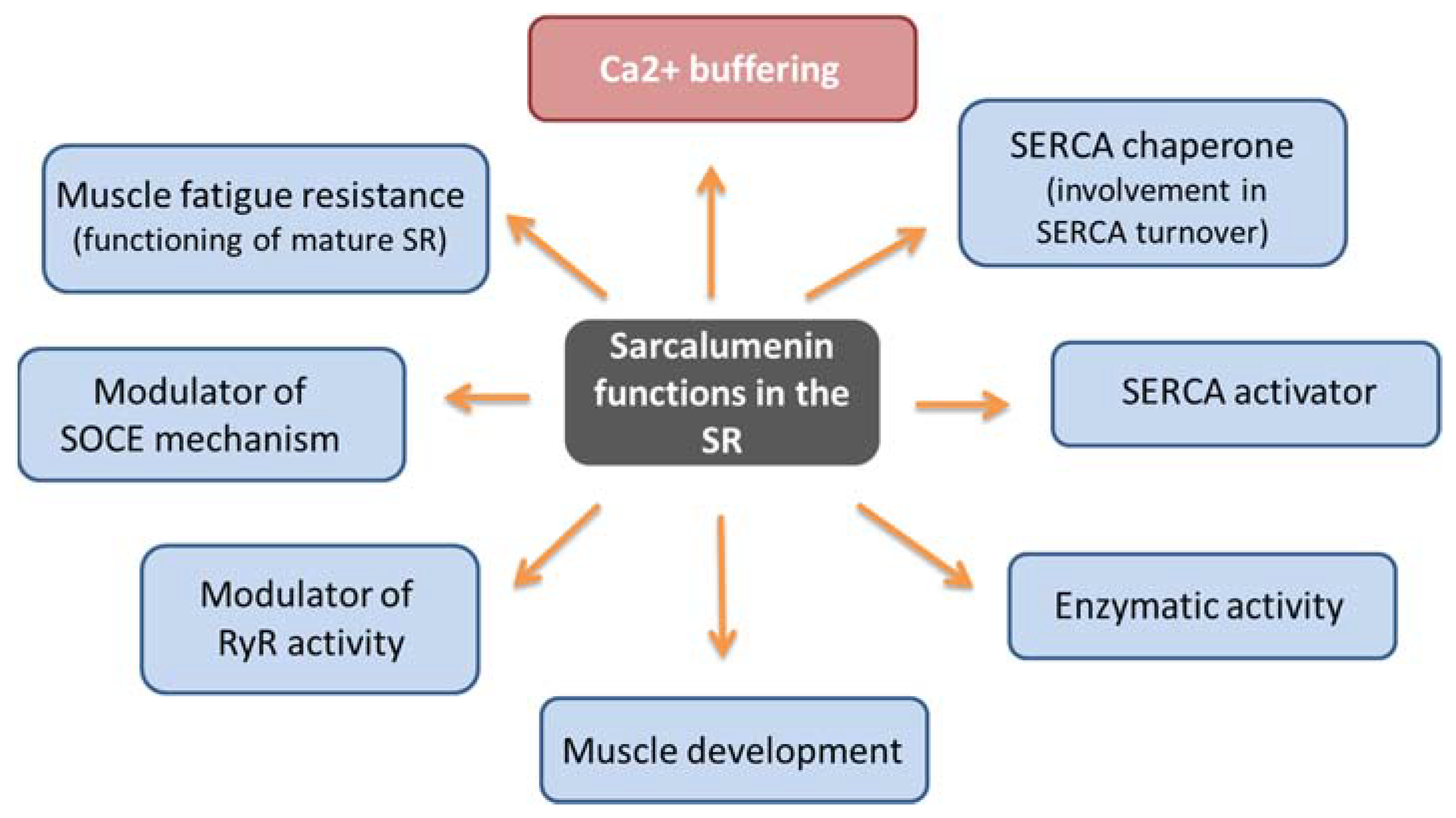
3. Sarcalumenin Structure and Physiological Functions in Skeletal Muscle
4. Skeletal Muscle Disorders Involving Sarcalumenin-Mediated Luminal Ca2+-Handling Alteration
4.1. Duchenne Muscular Dystrophy
4.2. Sarcopenia
4.3. Malignant Hypertermia
5. Sarcalumenin Ca2+ Buffer in Cardiac Muscle
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAMKII | Calmodulin kinase II |
| CLPs | Calsequestrin-like proteins |
| CSQ | Calsequestrin |
| DHPR | Dihydropyridine receptor |
| DMD | Duchenne muscular dystrophy |
| EC coupling | Excitation–contraction coupling |
| ECCE | Excitation-coupled Ca2+ entry |
| ER/SR | Endoplasmic/sarcoplasmic reticulum |
| HRC | Histidine-rich Ca2+-binding protein |
| IP3R | Inositol 1,4,5-triphosphate receptor |
| JPH | Junctophilin |
| MG29 | Mitsugumin 29 |
| MG53 | Mitsugumin 53 |
| MH | Malignant hyperthermia |
| RYR1 | Ryanodine receptor type 1 |
| SAR | Sarcalumenin |
| SERCA | Sarco-/endoplasmic reticular calcium ATPase |
| SMP30 | Senescence marker protein 30 |
| SOCE | Store-operated Ca2+ entry |
| STIM1 | Stromal-interacting molecule-1 |
| TRPCs | Transient receptor potential canonical channels |
References
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation–contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef] [Green Version]
- Dayal, A.; Perni, S.; Franzini-Armstrong, C.; Beam, K.G.; Grabner, M. The distal C terminus of the dihydropyridine receptor β1a subunit is essential for tetrad formation in skeletal muscle. Proc. Natl. Acad. Sci. USA 2022, 119, e2201136119. [Google Scholar] [CrossRef]
- Baylor, S.M.; Hollingworth, S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J. Physiol. 2003, 551, 125–138. [Google Scholar] [CrossRef]
- Ahn, M.K.; Lee, K.J.; Cai, C.; Huang, M.; Cho, C.H.; Ma, J.; Lee, E.H. Mitsugumin 53 regulates extracellular Ca(2+) entry and intracellular Ca(2+) release via Orai1 and RyR1 in skeletal muscle. Sci. Rep. 2016, 6, 36909. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Jeong, S.Y.; Oh, M.R.; Allen, P.D.; Lee, E.H. TRPCs: Influential mediators in skeletal muscle. Cells 2020, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Sztretye, M.; Geyer, N.; Vincze, J.; Al-Gaadi, D.; Olah, T.; Szentesi, P.; Kis, G.; Antal, M.; Balatoni, I.; Csernoch, L.; et al. SOCE Is Important for Maintaining Sarcoplasmic Calcium Content and Release in Skeletal Muscle Fibers. Biophys. J. 2017, 113, 2496–2507. [Google Scholar] [CrossRef] [Green Version]
- Cherednichenko, G.; Hurne, A.M.; Fessenden, J.D.; Lee, E.H.; Allen, P.D.; Beam, K.G.; Pessah, I.N. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc. Natl. Acad. Sci. USA 2004, 101, 15793–15798. [Google Scholar] [CrossRef] [Green Version]
- Park, I.Y.; Kim, E.; Park, H.; Fields, K.; Dunker, K.A.; Kang, C. Interaction between cardiac calsequestrin and drugs with known cardiotoxicity. Mol. Pharmacol. 2004, 67, 97–104. [Google Scholar] [CrossRef]
- Leberer, E.; Timms, B.G.; Campbell, K.P.; MacLennan, D.H. Purification, calcium binding properties, and ultrastructural localization of the 53,000- and 160,000 (sarcalumenin)-dalton glycoproteins of the sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 10118–10124. [Google Scholar] [CrossRef]
- Picello, E.; Damiani, E.; Margreth, A. Low-affinity Ca(2+)-binding sites versus Zn(2+)-binding sites in histidine-rich Ca(2+)-binding protein of skeletal muscle sarcoplasmic reticulum. Biochem. Biophys. Res. Commun. 1992, 186, 659–667. [Google Scholar] [CrossRef]
- Nakamura, K.; Zuppini, A.; Arnaudeau, S.; Lynch, J.; Ahsan, I.; Krause, R.; Papp, S.; De Smedt, H.; Parys, J.B.; Muller-Esterl, W.; et al. Functional specialization of calreticulin domains. J. Cell Biol. 2001, 154, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.S.; Kwak, Y.G.; Ji, J.H.; Chae, S.W.; Han Kim, D. Molecular cloning and characterization of mouse cardiac junctate isoforms. Biochem. Biophys. Res. Commun. 2001, 289, 882–887. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bahnson, B.J. Crystal structure of human senescence marker protein 30: Insights linking structural, enzymatic, and physiological functions. Biochemistry 2010, 49, 3436–3444. [Google Scholar] [CrossRef] [Green Version]
- Schwaller, B. Cytosolic Ca2+ Buffers Are Inherently Ca2+ Signal Modulators. Cold Spring Harb. Perspect. Biol. 2020, 12, a035543. [Google Scholar] [CrossRef]
- Wu, X.; Reid, R.E. Structure/calcium affinity relationships of site III of calmodulin: Testing the acid pair hypothesis using calmodulin mutants. Biochemistry 1997, 36, 8649–8656. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of regucalcin in maintaining cell homeostasis and function (review). Int. J. Mol. Med. 2005, 15, 371–389. [Google Scholar] [CrossRef]
- Moradi, F.; Copeland, E.N.; Baranowski, R.W.; Scholey, A.E.; Stuart, J.A.; Fajardo, V.A. Calmodulin-Binding Proteins in Muscle: A Minireview on Nuclear Receptor Interacting Protein, Neurogranin, and Growth-Associated Protein 43. Int. J. Mol. Sci. 2020, 21, 1016. [Google Scholar] [CrossRef] [Green Version]
- Gillis, J.M.; Thomason, D.; Lefèvre, J.; Kretsinger, R.H.; Schwaller, B.; Dick, J.; Dhoot, G.; Carroll, S.; Vrbova, G.; Nicotera, P. Parvalbumins and muscle relaxation: A computer simulation study. J. Muscle Res. Cell Motil. 1982, 3, 377–398. [Google Scholar] [CrossRef]
- Yoshida, M.; Minamisawa, S.; Shimura, M.; Komazaki, S.; Kume, H.; Zhang, M.; Matsumura, K.; Nishi, M.; Saito, M.; Saeki, Y.; et al. Impaired Ca2+ store functions in skeletal and cardiac muscle cells from sarcalumenin-deficient mice. J. Biol. Chem. 2005, 280, 3500–3506. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.; Pierantozzi, E.; Amadsun, D.O.; Buonocore, S.; Rubino, E.M.; Sorrentino, V. The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites. Biomolecules 2022, 12, 488. [Google Scholar] [CrossRef]
- Rossi, A.E.; Dirksen, R.T. Sarcoplasmic reticulum: The dynamic calcium governor of muscle. Muscle Nerve 2006, 33, 715–731. [Google Scholar] [CrossRef]
- Wray, S.; Burdyga, T. Sarcoplasmic reticulum function in smooth muscle. Physiol. Rev. 2010, 90, 113–178. [Google Scholar] [CrossRef]
- Bublitz, M.; Musgaard, M.; Poulsen, H.; Thøgersen, L.; Olesen, C.; Schiøtt, B.; Morth, J.P.; Møller, J.V.; Nissen, P. Ion pathways in the sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 2013, 288, 10759–10765. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar] [CrossRef]
- Phimister, A.J.; Lango, J.; Lee, E.H.; Ernst-Russell, M.A.; Takeshima, H.; Ma, J.; Allen, P.D.; Pessah, I.N. Conformation-dependent stability of junctophilin 1 (JP1) and ryanodine receptor type 1 (RyR1) channel complex is mediated by their hyper-reactive thiols. J. Biol. Chem. 2007, 282, 8667–8677. [Google Scholar] [CrossRef] [Green Version]
- Golini, L.; Chouabe, C.; Berthier, C.; Cusimano, V.; Fornaro, M.; Bonvallet, R.; Formoso, L.; Giacomello, E.; Jacquemond, V.; Sorrentino, V. Junctophilin 1 and 2 proteins interact with the L-type Ca2+ channel dihydropyridine receptors (DHPRs) in skeletal muscle. J. Biol. Chem. 2011, 286, 43717–43725. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, H.; Shimuta, M.; Komazaki, S.; Ohmi, K.; Nishi, M.; Iino, M.; Miyata, A.; Kangawa, K. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem. J. 1998, 331 Pt 1, 317–322. [Google Scholar] [CrossRef]
- Nishi, M.; Komazaki, S.; Kurebayashi, N.; Ogawa, Y.; Noda, T.; Iino, M.; Takeshima, H. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J. Cell Biol. 1999, 147, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.S.; Hwang, J.H.; Huang, M.; Ahn, M.K.; Cho, C.H.; Ma, J.; Lee, E.H. Interaction between mitsugumin 29 and TRPC3 partic-ipates in regulating Ca2+ transients in skeletal muscle. Biochem. Biophys. Res. Commun. 2015, 464, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Cala, S.E.; Scott, B.T.; Jones, L.R. Intralumenal sarcoplasmic reticulum Ca2+-binding proteins. Semin. Cell Biol. 1990, 1, 265–275. [Google Scholar]
- Franzini-Armstrong, C.; Kennery, L.J.; Varriano-Martson, E. The structure of calsequestrin in triads of vertebrate skeletal muscle: A deep-etch study. J. Cell Biol. 1987, 105, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pape, P.C.; Fénelon, K.; Lamboley, C.R.; Stachura, D. Role of calsequestrin evaluated from changes in free and total calcium concentrations in the sarcoplasmic reticulum of frog cut skeletal muscle fibres. J. Physiol. 2007, 15, 319–367. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Randazzo, D.; Del Re, V.; Sorrentino, V.; Rossi, D. Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers. J. Muscle Res. Cell Motil. 2015, 36, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Close, M.; Franzini-Armstrong, C. Novel details of calsequestrin gel conformation in situ. J. Biol. Chem. 2013, 25, 31358–31362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Wu, S.; Dunker, A.K.; Kang, C. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 2003, 2, 16176–16182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Kelley, J.; Schmeisser, G.; Kobayashi, Y.M.; Jones, L.R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997, 12, 23389–23397. [Google Scholar] [CrossRef] [Green Version]
- Glover, L.; Quinn, S.; Ryan, M.; Pette, D.; Ohlendieck, K. Supramolecular calsequestrin complex. Eur. J. Biochem. 2002, 269, 4607–4616. [Google Scholar] [CrossRef]
- Wei, L.; Gallant, E.M.; Dulhunty, A.F.; Beard, N.A. Junctin and triadin each activate skeletal ryanodine receptors but junctin alone mediates functional interactions with calsequestrin. Int. J. Biochem. Cell Biol. 2009, 41, 2214–2224. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, L.; Li, S.; Zheng, Y.; Yan, X.; Chen, M.; Wang, H.; Putney, J.W.; Luo, D. Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci. Rep. 2015, 18, 11349. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, L.; Li, S.; Xue, J.; Luo, D. Calsequestrin-1 regulates store-operated Ca2+ entry by inhibiting STIM1 aggregation. Cell. Physiol. Biochem. 2016, 8, 2183–2193. [Google Scholar] [CrossRef]
- Suk, J.Y.; Kim, Y.S.; Park, W.J. HRC (histidine-rich Ca2+ binding protein) resides in the lumen of sarcoplasmic reticulum as a multimer. Biochem. Biophys. Res. Commun. 1999, 263, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kang, H.; Kim, D.H.; Park, W.J. Interaction of HRC (histidine-rich Ca(2+)-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J. Biol. Chem. 2001, 276, 39533–39538. [Google Scholar] [CrossRef] [Green Version]
- Sacchetto, R.; Turcato, F.; Damiani, E.; Margreth, A. Interaction of triadin with histidine-rich Ca(2+)-binding protein at the triadic junction in skeletal muscle fibers. J. Muscle Res. Cell Motil. 1999, 20, 403–415. [Google Scholar] [CrossRef]
- Sacchetto, R.; Damiani, E.; Turcato, F.; Nori, A.; Margreth, A. Ca(2+)-dependent interaction of triadin with histidine-rich Ca(2+)-binding protein carboxyl-terminal region. Biochem. Biophys. Res. Commun. 2001, 289, 1125–1134. [Google Scholar] [CrossRef]
- Singh, V.P.; Rubinstein, J.; Arvanitis, D.A.; Ren, X.; Gao, X.; Haghighi, K.; Gilber, T.M.; Iyer, V.R.; Kim, D.H.; Cho, C.; et al. Abnormal calcium cycling and cardiac arrhythmias associated with the human Ser96Ala genetic variant of histidine-rich calcium-binding protein. J. Am. Heart Assoc. 2013, 2, e000460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Li, X.; Fu, Y.; Li, Y.; Lu, W.; Pan, Y.; Yang, J.; Kong, J. Inhibition of lung cancer by vitamin D depends on downregulation of histidine-rich calcium-binding protein. J. Adv. Res. 2020, 29, 13–22. [Google Scholar] [CrossRef]
- Wang, C.; Ren, C.; Hu, Q.; Shen, X.; Wang, M.; Yang, Z.; Xu, E.; Wang, X.; Li, Z.; Yu, H.; et al. Histidine-rich calcium binding protein promotes gastric cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition through Raf/MEK/ERK signaling. J. Cancer 2022, 13, 1073–1085. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, G.C.; Ren, X.; Waggoner, J.R.; Gregory, K.N.; Chen, G.; Jones, W.K.; Kranias, E.G. Overexpression of histidine-rich Ca-binding protein protects against ischemia/reperfusion-induced cardiac injury. Cardiovasc. Res. 2007, 75, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Treves, S.; Feriotto, G.; Moccagatta, L.; Gambari, R.; Zorzato, F. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sarco(endo)plasmic reticulum membrane. J. Biol. Chem. 2000, 275, 39555–39568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treves, S.; Franzini-Armstrong, C.; Moccagatta, L.; Arnoult, C.; Grasso, C.; Schrum, A.; Ducreux, S.; Zhu, M.; Mikoshiba, K.; Girard, T.; et al. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J. Cell Biol. 2004, 166, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLennan, D.H.; Reithmeier, R.A. Ion tamers. Nat. Struct. Mol. 1998, 5, 409–411. [Google Scholar] [CrossRef]
- Corbett, E.F.; Michalak, K.M.; Oikawa, K.; Johnson, S.; Campbell, I.D.; Eggleton, P.; Kay, C.; Michalak, M. The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J. Biol. Chem. 2000, 275, 27177–27185. [Google Scholar] [CrossRef]
- Nørgaard Toft, K.; Larsen, N.; Jørgensen, F.S.; Højrup, P.; Houen, G.; Vestergaard, B. Small angle X-ray scattering study of calreticulin reveals conformational plasticity. Biochim. Biophys. Acta. 2008, 1784, 1265–1270. [Google Scholar] [CrossRef]
- Bastianutto, C.; Clementi, E.; Codazzi, F.; Podini, P.; De Giorgi, F.; Rizzuto, R.; Meldolesi, J.; Pozzan, T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their luminal microenvironment and funcion. J. Cell Biol. 1995, 130, 847–855. [Google Scholar] [CrossRef]
- Mery, L.; Mesaeli, N.; Michalak, M.; Opas, M.; Lew, D.P.; Krause, K.H. Overexpression of calreticulin increasea intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J. Biol. Chem. 1996, 271, 9332–9339. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Longo, F.J.; Wintermantel, M.R.; Jiang, X.; Clark, R.A.; deLisle, S. Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-triphosphate-induced Ca2+ store depletion. J. Biol. Chem. 2000, 275, 36676–36682. [Google Scholar] [CrossRef] [Green Version]
- Leberer, E.; Charuk, J.H.; Green, N.M.; MacLennan, D.H. Molecular cloning and expression of cDNA encoding a lumenal calcium binding glycoprotein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1989, 86, 6047–6051. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, D.; Donoghue, P.; O’Reilly, C.; Ohlendieck, K. Role of Calsequestrin and Related Luminal Ca2+-Binding Proteins as Mediators of Excitation-Contraction Coupling. Basic Appl. Myol. 2004, 14, 313–322. [Google Scholar]
- Zhao, X.; Yoshida, M.; Brotto, L.; Takeshima, H.; Weisleder, N.; Hirata, Y.; Nosek, T.M.; Ma, J.; Brotto, M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol. Genom. 2005, 23, 72–78. [Google Scholar] [CrossRef]
- O’Connell, K.; Gannon, J.; Doran, P.; Ohlendieck, K. Reduced expression of sarcalumenin and related Ca2+-regulatory proteins in aged rat skeletal muscle. Exp. Gerontol. 2008, 43, 958–961. [Google Scholar] [CrossRef] [Green Version]
- Dowling, P.; Doran, P.; Ohlendieck, K. Drastic reduction of sarcalumenin in Dp427 (dystrophin of 427 kDa)-deficient fibres indicates that abnormal calcium handling plays a key role in muscular dystrophy. Biochem. J. 2004, 379 Pt 2, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Raeymaekers, L.; Verbist, J.; Wuytack, F.; Plessers, L.; Casteels, R. Expression of Ca2+ binding proteins of the sarcoplasmic reticulum of striated muscle in the endoplasmic reticulum of pig smooth muscles. Cell Calcium 1993, 14, 581–589. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Orr, I.; Weil, S.; Meyer, H.; Varsanyi, M.; Heilmeyer, L.M. The identification of the phosphorylated 150/160-kDa proteins of sarcoplasmic reticulum, their kinase and their association with the ryanodine receptor. Biochim. Biophys. Acta. 1996, 1283, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, C.; Donoso, P. Luminal calcium regulation of calcium release from the sarcoplasmic reticulum. Biosci. Rep. 1995, 15, 387–397. [Google Scholar] [CrossRef]
- McNally, E.M.; Kaltman, J.R.; Benson, D.W.; Canter, C.E. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation 2015, 131, 1590–1598. [Google Scholar] [CrossRef] [Green Version]
- Koenig, M.; Hoffman, E.P.; Bertelson, C.J.; Monaco, A.P.; Feener, C.; Kunkel, L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef]
- Bladen, C.L.; Salgado, D.; Monges, S.; Foncuberta, M.E.; Kekou, K.; Kosma, K.; Dawkins, H.; Lamont, L.; Roy, A.J.; Chamova, T.; et al. The TREAT-NMD DMD Global Database: Analysis of more than 7000 duchenne muscular dystrophy mutations. Hum. Mutat. 2015, 36, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Carter, J.C.; Sheehan, D.W.; Prochoroff, A.; Birnkrant, D.J. Muscular dystrophies. Clin. Chest Med. 2018, 39, 377–389. [Google Scholar] [CrossRef]
- Nelson, C.E.; Wu, Y.; Gemberling, M.P.; Oliver, M.L.; Waller, M.A.; Bohning, J.D.; Robinson-Hamm, J.N.; Bulaklak, K.; Castellanos Rivera, R.M.; Collier, J.H.; et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 427–432. [Google Scholar] [CrossRef]
- Soblechero-Martín, P.; López-Martínez, A.; de la Puente-Ovejero, L.; Vallejo-Illarramendi, A.; Arechavala-Gomeza, V. Utrophin modulator drugs as potential therapies for Duchenne and Becker muscular dystrophies. Neuropathol. Appl. Neurobiol. 2021, 47, 711–723. [Google Scholar] [CrossRef]
- Angelini, G.; Mura, G.; Messina, G. Therapeutic approaches to preserve the musculature in Duchenne Muscular Dystrophy: The importance of the secondary therapies. Exp. Cell Res. 2022, 410, 112968. [Google Scholar] [CrossRef]
- Bulfield, G.; Siller, W.G.; Wight, P.A.; Moore, K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 1984, 81, 1189–1192. [Google Scholar] [CrossRef] [Green Version]
- Yucel, N.; Chang, A.C.; Day, J.W.; Rosenthal, N.; Blau, H.M. Humanizing the mdx mouse model of DMD: The long and the short of it. NPJ Regen. Med. 2018, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.V.; Le Cam, G.; Leger, J.J.; Dechesne, C.A. Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochim. Biophys. Acta. 2000, 1500, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.W.; Zhao, P.; Borup, R.; Hoffman, E.P. Expression profiling in the muscular dystrophies: Identification of novel aspects of molecular pathophysiology. J. Cell Biol. 2000, 151, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ito, M.; Fukami, M.; Hashimoto, M.; Hirayama, M.; Ohno, K. Molecular hydrogen alleviates motor deficits and muscle degeneration in mdx mice. Redox Rep. 2017, 22, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juban, G.; Saclier, M.; Yacoub-Youssef, H.; Kernou, A.; Arnold, L.; Boisson, C.; Ben Larbi, S.; Magnan, M.; Cuvellier, S.; Théret, M.; et al. AMPK activation regulates LTBP4-dependent TGF-beta1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep. 2018, 25, 2163–2176.e2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallouk, N.; Jacquemond, V.; Allard, B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibres detected with Ca2+-activated K+ channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4950–4955. [Google Scholar] [CrossRef] [Green Version]
- Culligan, K.; Ohlendieck, K. Abnormal calcium handling in muscular dystrophy. Basic Appl. Myol. 2002, 12, 147–157. [Google Scholar]
- Fraysse, B.; Liantonio, A.; Cetrone, M.; Burdi, R.; Pierno, S.; Frigeri, A.; Pisoni, M.; Camerino, C.; De Luca, A. The alteration of calcium homeostasis in adult dystrophic mdx muscle fibers is worsened by a chronic exercise in vivo. Neurobiol. Dis. 2004, 17, 144–154. [Google Scholar] [CrossRef]
- Rolland, J.F.; De Luca, A.; Burdi, R.; Andreetta, F.; Confalonieri, P.; Conte Camerino, D. Overactivity of exercise-sensitive cation channels and their impaired modulation by IGF-1 in mdx native muscle fibers: Beneficial effect of pentoxifylline. Neurobiol. Dis. 2006, 24, 466–474. [Google Scholar] [CrossRef]
- Burdi, R.; Rolland, J.F.; Fraysse, B.; Litvinova, K.; Cozzoli, A.; Giannuzzi, V.; Liantonio, A.; Camerino, G.M.; Sblendorio, V.; Capogrosso, R.F.; et al. Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: Outcome of a large array of in vivo and ex vivo tests. J. Appl. Physiol. 2009, 106, 1311–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capogrosso, R.F.; Mantuano, P.; Uaesoontrachoon, K.; Cozzoli, A.; Giustino, A.; Dow, T.; Srinivassane, S.; Filipovic, M.; Bell, C.; Vandermeulen, J.; et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: Proof-of-concept study and independent validation of efficacy. FASEB J. 2018, 32, 1025–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mareedu, S.; Million, E.D.; Duan, D.; Babu, G.J. Abnormal Calcium Handling in Duchenne Muscular Dystrophy: Mechanisms and Potential Therapies. Front. Physiol. 2021, 12, 647010. [Google Scholar] [CrossRef]
- Alderton, J.M.; Steinhardt, R.A. How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc. Med. 2000, 10, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Shanmugam, M.; Gonzalez, J.P.; Lopez, H.; Gordan, R.; Fraidenraich, D.; Babu, G.J. Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 2013, 34, 349–356. [Google Scholar] [CrossRef]
- Doran, P.; Dowling, P.; Lohan, J.; McDonnell, K.; Poetsch, S.; Ohlendieck, K. Subproteomics analysis of Ca+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle. Eur. J. Biochem. 2004, 271, 3943–3952. [Google Scholar] [CrossRef]
- Lewis, C.; Carberry, S.; Ohlendieck, K. Proteomic profiling of x-linked muscular dystrophy. J. Muscle Res. Cell Motil. 2009, 30, 267–269. [Google Scholar] [CrossRef]
- Maranhão, J.B.; de Oliveira Moreira, D.; Maurício, A.F.; de Carvalho, S.C.; Ferretti, R.; Pereira, J.A.; Santo Neto, H.; Marques, M.J. Changes in calsequestrin, TNF-α, TGF-β and MyoD levels during the progression of skeletal muscle dystrophy in mdx mice: A comparative analysis of the quadriceps, diaphragm and intrinsic laryngeal muscles. Int. J. Exp. Pathol. 2015, 96, 285–293. [Google Scholar] [CrossRef]
- Lohan, J.; Ohlendieck, K. Drastic reduction in the luminal Ca2+ -binding proteins calsequestrin and sarcalumenin in dystrophin-deficient cardiac muscle. Biochim. Biophys. Acta. 2004, 1689, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kim, J.H. Impact of skeletal muscle mass on metabolic health. Endocrinol. Metab. 2020, 35, 1–6. [Google Scholar] [CrossRef]
- Teixeira Vde, O.; Filippin, L.I.; Xavier, R.M. Mechanisms of muscle wasting in sarcopenia. Rev. Bras. Reumatol. 2012, 52, 252–259. [Google Scholar] [CrossRef]
- Romanick, M.; Thompson, L.V.; Brown-Borg, H.M. Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle. Biochim. Biophys. Acta. 2013, 1832, 1410–1420. [Google Scholar] [CrossRef] [Green Version]
- Delbono, O.; O’Rourke, K.S.; Ettinger, W.H. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J. Membr. Biol. 1995, 148, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, B.; Desaphy, J.F.; Rolland, J.F.; Pierno, S.; Liantonio, A.; Giannuzzi, V.; Camerino, C.; Didonna, M.P.; Cocchi, D.; De Luca, A.; et al. Fiber type-related changes in rat skeletal muscle calcium homeostasis during aging and restoration by growth hormone. Neurobiol. Dis. 2006, 21, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mijares, A.; Allen, P.D.; Lopez, J.R. Senescence Is Associated With Elevated Intracellular Resting [Ca2+] in Mice Skeletal Muscle Fibers. An in vivo Study. Front. Physiol. 2021, 11, 601189. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Imbrici, P.; Mantuano, P.; Coppola, M.A.; Camerino, G.M.; De Luca, A.; Liantonio, A. Alteration of STIM1/Orai1-Mediated SOCE in Skeletal Muscle: Impact in Genetic Muscle Diseases and Beyond. Cells 2021, 10, 2722. [Google Scholar] [CrossRef]
- Wang, Z.M.; Messi, M.L.; Delbono, O. L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys. J. 2000, 78, 1947–1954. [Google Scholar] [CrossRef] [Green Version]
- Gueugneau, M.; Coudy-Gandilhon, C.; Gourbeyre, O.; Chambon, C.; Combaret, L.; Polge, C.; Taillandier, D.; Attaix, D.; Friguet, B.; Maier, A.B.; et al. Proteomics of muscle chronological ageing in post-menopausal women. BMC Genom. 2014, 15, 1165. [Google Scholar] [CrossRef] [Green Version]
- Mosole, S.; Zampieri, S.; Furlan, S.; Carraro, U.; Löefler, S.; Kern, H.; Volpe, P.; Nori, A. Effects of Electrical Stimulation on Skeletal Muscle of Old Sedentary People. Gerontol. Geriatr. Med. 2018, 4, 2333721418768998. [Google Scholar] [CrossRef] [Green Version]
- Kelley, R.C.; McDonagh, B.; Ferreira, L.F. Advanced aging causes diaphragm functional abnormalities, global proteome remodeling, and loss of mitochondrial cysteine redox flexibility in mice. Exp. Gerontol. 2018, 103, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.E. Malignant hyperthermia: A pharmacogenetic disease of Ca++ regulating proteins. Curr. Mol. Med. 2002, 2, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; MacLennan, D.H. Malignant hyperthermia and central core disease: Disorders of Ca2+ release channels. Am. J. Med. 1998, 104, 470–486. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Duff, C.; Zorzato, F.; Fujii, J.; Phillips, M.; Korneluk, R.G.; Frodis, W.; Britt, B.A.; Worton, R.G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature 1990, 343, 559–561. [Google Scholar] [CrossRef]
- Robinson, R.; Carpenter, D.; Shaw, M.A.; Halsall, J.; Hopkins, P. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum. Mutat. 2006, 27, 977–989. [Google Scholar] [CrossRef]
- Duke, A.M.; Hopkins, P.M.; Calaghan, S.C.; Halsall, J.P.; Steele, D.S. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J. Biol. Chem. 2010, 285, 25645–25653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glover, L.; Heffron, J.J.; Ohlendieck, K. Increased sensitivity of the ryanodine receptor to halothaneinduced oligomerization in malignant hyperthermia-susceptible human skeletal muscle. J. Appl. Physiol. 2004, 96, 11–18. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, G.H.; McIntosh, J.M.; Heffron, J.J. Abnormal uptake and release of Ca2+ ions from human malignant hyperthermia-susceptible sarcoplasmic reticulum. Biochem. Pharmacol. 2001, 61, 1479–1485. [Google Scholar] [CrossRef]
- Lewis, K.M.; Ronish, L.A.; Rios, E.; Kang, C. Characterization of two human skeletal calsequestrin mutants implicated in malignant hyperthermia and vacuolar aggregate myopathy. J. Biol. Chem. 2015, 290, 28665–28674. [Google Scholar] [CrossRef] [Green Version]
- Dainese, M.; Quarta, M.; Lyfenko, A.D.; Paolini, C.; Canato, M.; Reggiani, C.; Dirksen, R.T.; Protasi, F. Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice. FASEB J. 2009, 23, 1710–1720. [Google Scholar] [CrossRef] [Green Version]
- Ebashi, S. Excitation-contraction coupling and mechanism of muscle contraction. Annu. Rev. Physiol. 1991, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hadad, N.; Meyer, H.E.; Varsanyi, M.; Fleischer, S.; Shoshan-Barmatz, V. Cardiac sarcalumenin: Phosphorylation, comparison with the skeletal muscle sarcalumenin and modulation of ryanodine receptor. J Membr. Biol. 1999, 170, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Minamisawa, S.; Takeshima, H.; Jiao, Q.; Bai, Y.; Umemura, S.; Ishikawa, Y. Sarcalumenin alleviates stress-induced cardiac dysfunction by improving Ca2+ handling of the sarcoplasmic reticulum. Cardiovasc. Res 2008, 77, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Q.; Bai, Y.; Akaike, T.; Takeshima, H.; Ishikawa, Y.; Minamisawa, S. Sarcalumenin is essential for maintaining cardiac function during endurance exercise training. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H576–H582. [Google Scholar] [CrossRef] [Green Version]
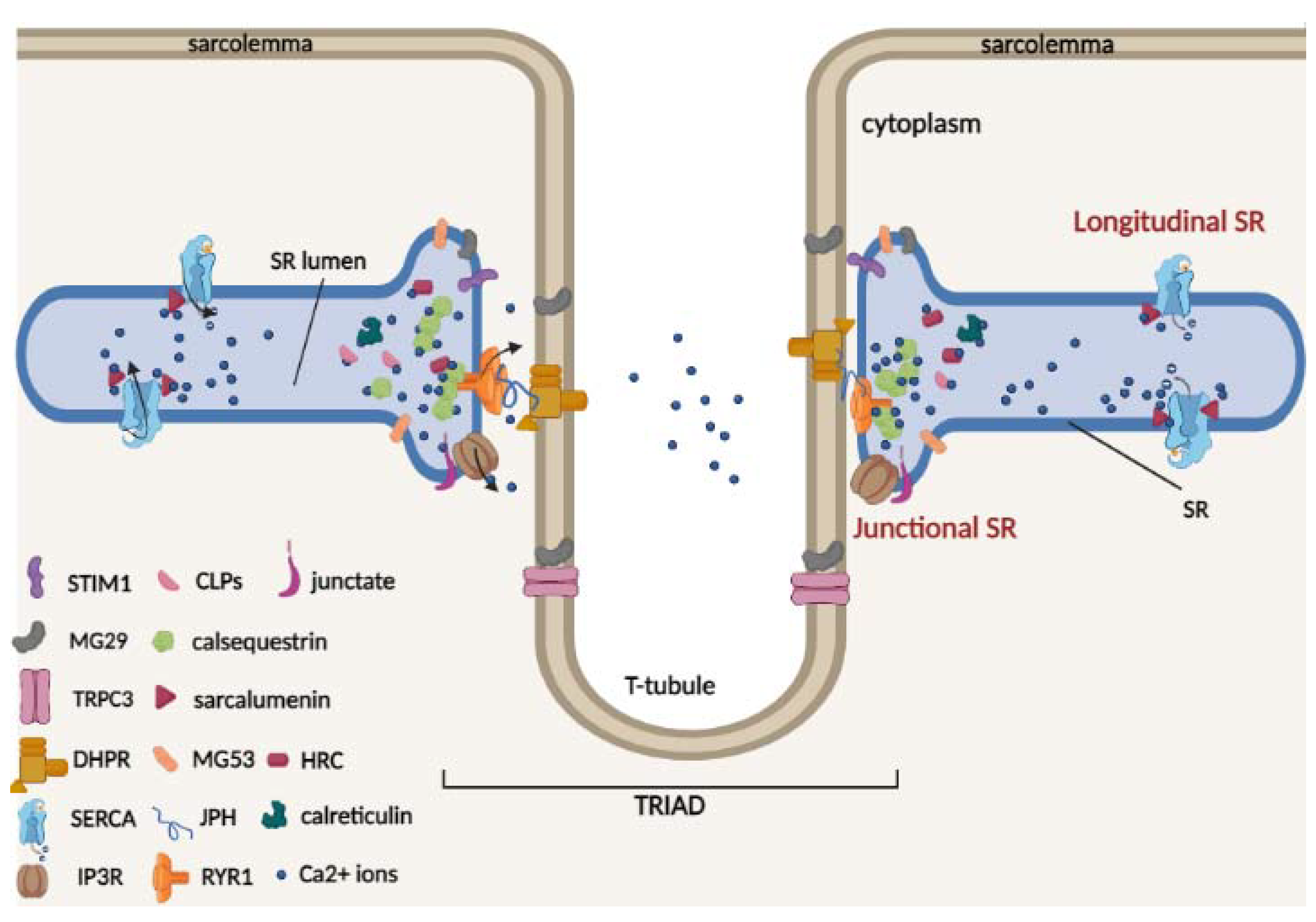

| SR Ca2+-Binding Protein | Molecular Mass | Ca2+ Dissociation Constants | Reference |
|---|---|---|---|
| Calsequestrin | 63 kDa | Kd= 1–2 × 10−3 M | [8] |
| CLP-220 | 220 kDa | N.A. | |
| CLP-170 | 170 kDa | N.A. | |
| CLP-150 | 150 kDa | N.A. | |
| Sarcalumenin | 160 kDa | Kd = 0.3–0.6 × 10−3 M | [9] |
| HRC | 170 kDa | Kd = 1.9 × 10−3 M | [10] |
| Calreticulin | 55 kDa | Kd = 2 × 10−3 M | [11] |
| Junctate | 33 kDa | Kd = 0.217 × 10−3 M | [12] |
| Cytosolic Ca2+-binding protein | |||
| Regucalcin (SMP30) | 34 kDa | Kd = 0.566 × 10−3 M | [13] |
| Parvalbumin | 12 kDa | Kd = 4–9 × 10−9 M | [14] |
| Calmodulin | 17 kDa | Kd = 1 × 10−9–0.1 × 10−3 | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, E.; Dinoi, G.; Imbrici, P.; De Luca, A.; Liantonio, A. Sarcoplasmic Reticulum Ca2+ Buffer Proteins: A Focus on the Yet-To-Be-Explored Role of Sarcalumenin in Skeletal Muscle Health and Disease. Cells 2023, 12, 715. https://doi.org/10.3390/cells12050715
Conte E, Dinoi G, Imbrici P, De Luca A, Liantonio A. Sarcoplasmic Reticulum Ca2+ Buffer Proteins: A Focus on the Yet-To-Be-Explored Role of Sarcalumenin in Skeletal Muscle Health and Disease. Cells. 2023; 12(5):715. https://doi.org/10.3390/cells12050715
Chicago/Turabian StyleConte, Elena, Giorgia Dinoi, Paola Imbrici, Annamaria De Luca, and Antonella Liantonio. 2023. "Sarcoplasmic Reticulum Ca2+ Buffer Proteins: A Focus on the Yet-To-Be-Explored Role of Sarcalumenin in Skeletal Muscle Health and Disease" Cells 12, no. 5: 715. https://doi.org/10.3390/cells12050715
APA StyleConte, E., Dinoi, G., Imbrici, P., De Luca, A., & Liantonio, A. (2023). Sarcoplasmic Reticulum Ca2+ Buffer Proteins: A Focus on the Yet-To-Be-Explored Role of Sarcalumenin in Skeletal Muscle Health and Disease. Cells, 12(5), 715. https://doi.org/10.3390/cells12050715








