Upregulation of TLR4-Dependent ATP Production Is Critical for Glaesserella parasuis LPS-Mediated Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Cell Culture
2.2. Cell Viability Assay
2.3. LPS Extraction and Quantification
2.4. EdU (5-ethynyl-2′-deoxyuridine) Incorporation Assay
2.5. ATP Assays
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. RNA Isolation and cDNA Synthesis
2.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot
2.10. Immunofluorescence and Imaging Analysis
2.11. Plasmids and Transfection
2.12. Plasmids and Transfection
3. Results
3.1. G. parasuis LPS Enhanced the Mortality and the ATP Level of PAM Cells
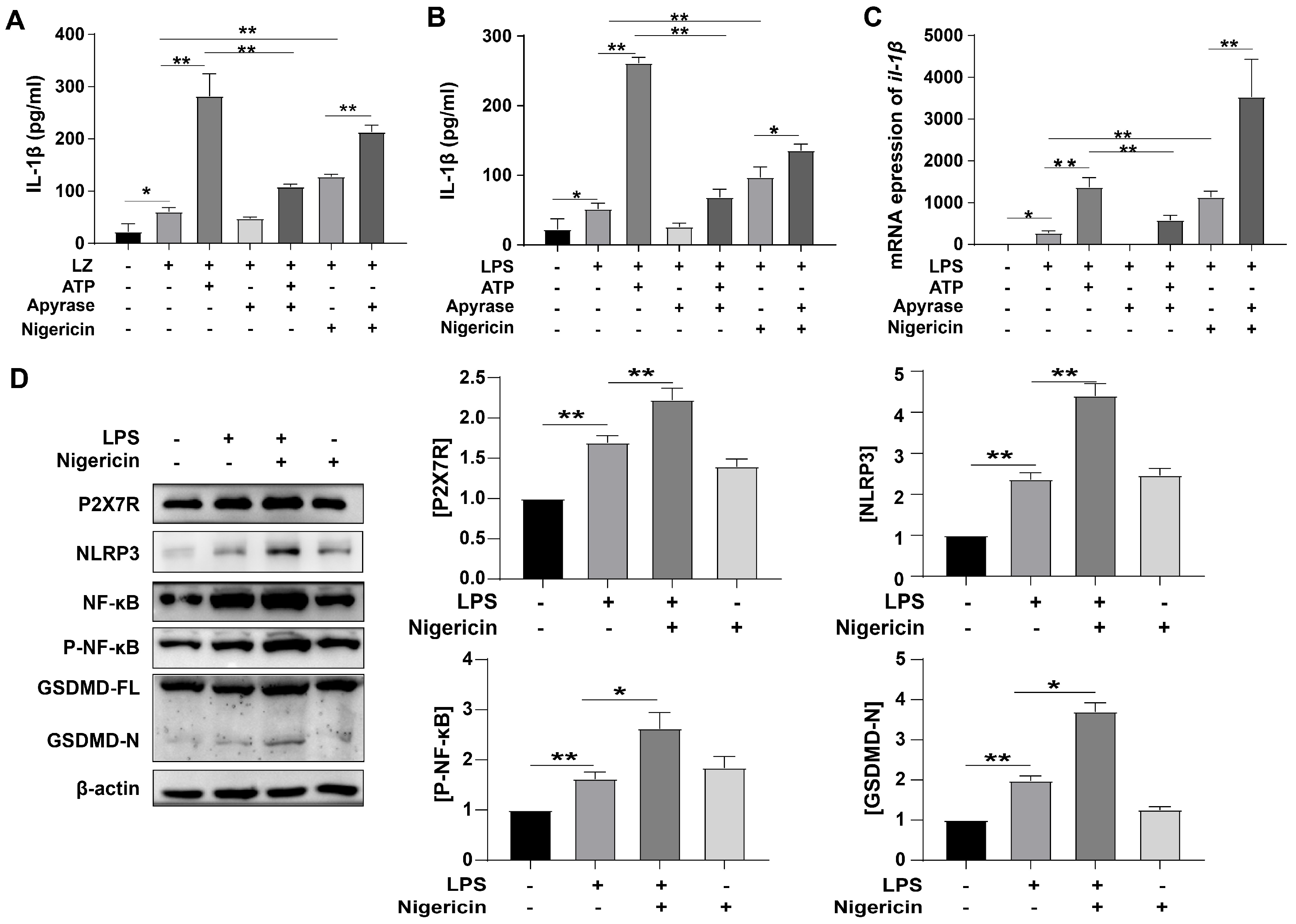
3.2. ATP-Induced Pyroptosis and Activated P2X7R Pathway
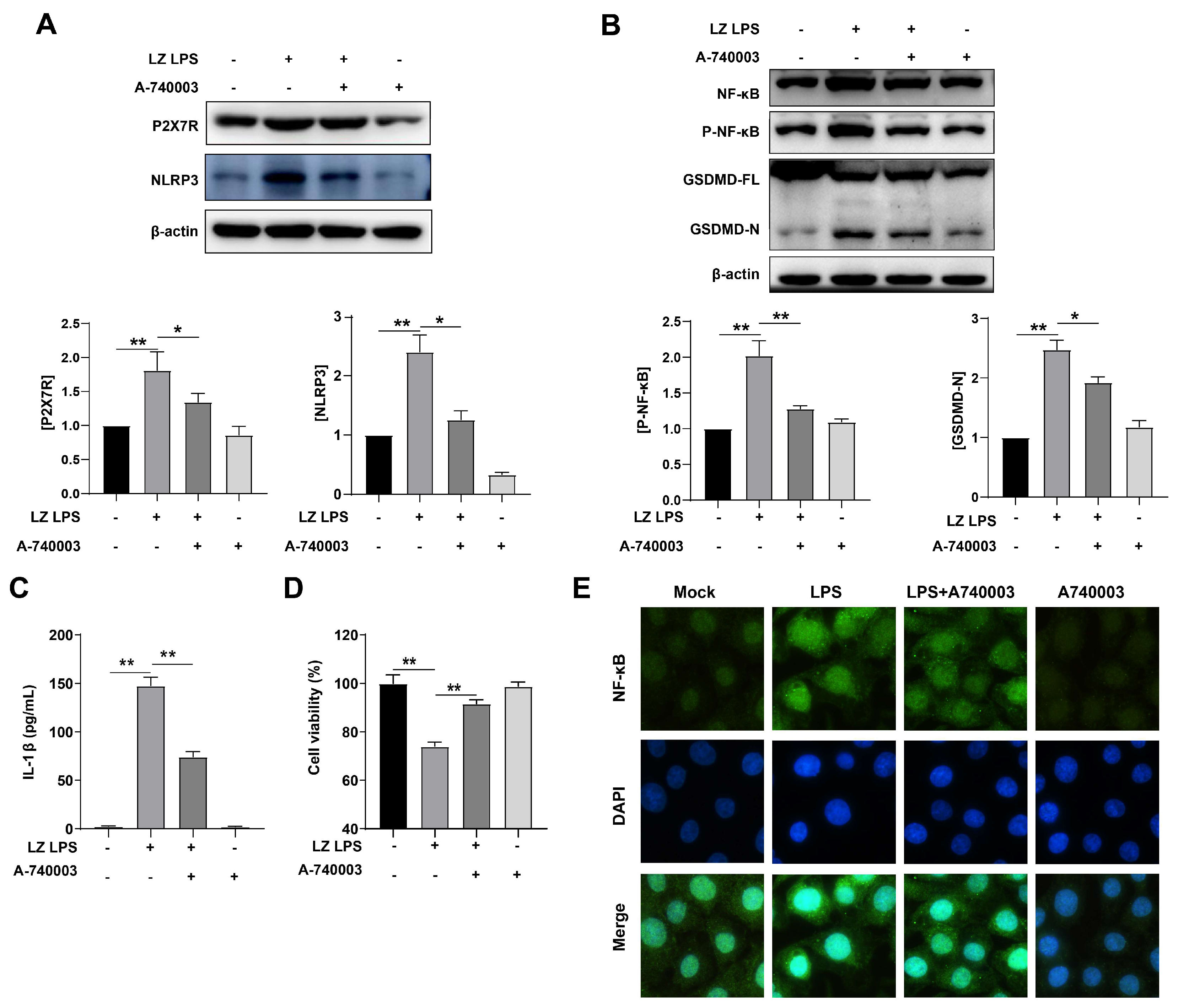
3.3. LPS-Induced Pyroptosis through Activated P2X7R Pathway
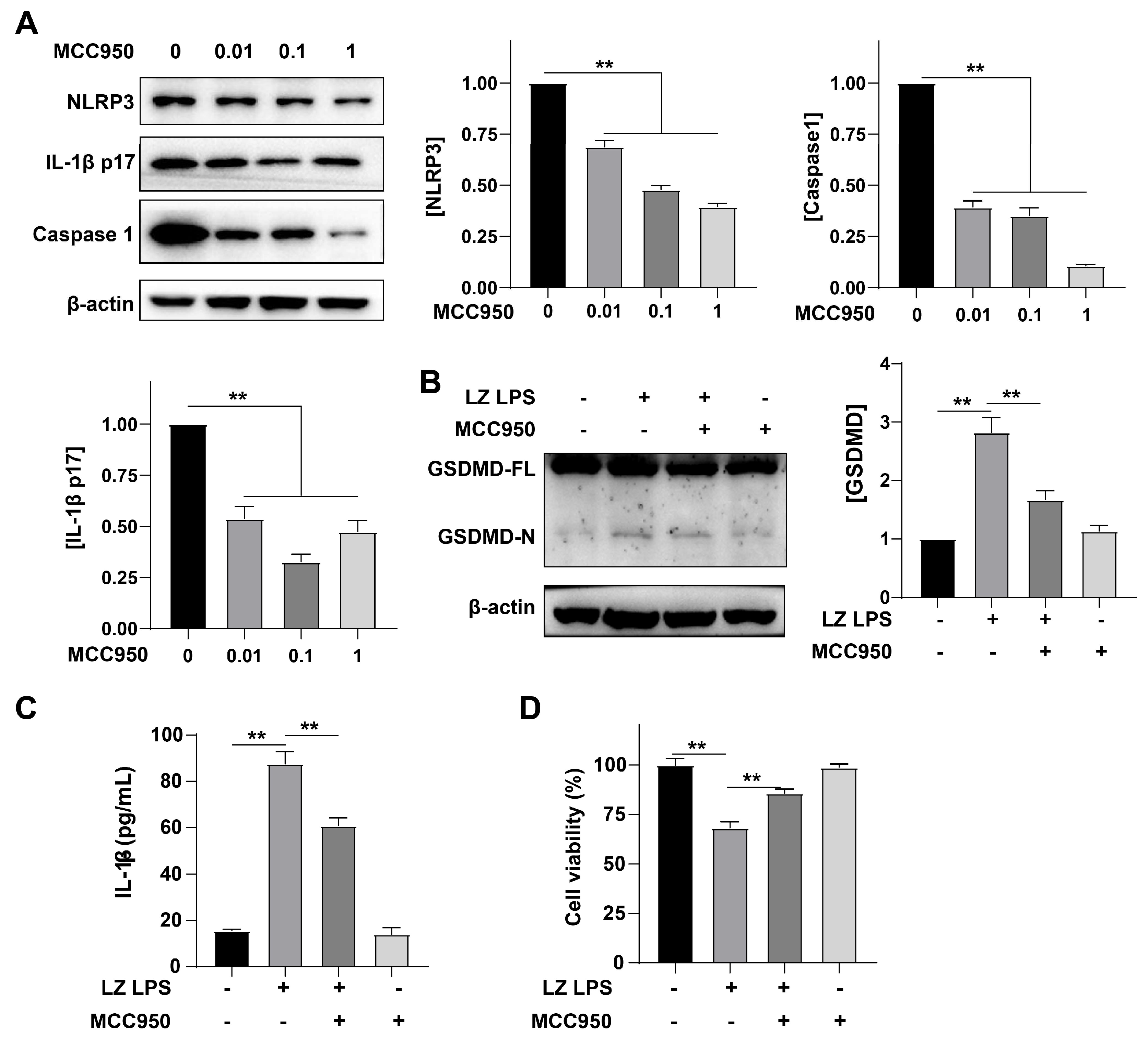
3.4. NLRP3 Was Involved in the Formation of Inflammation
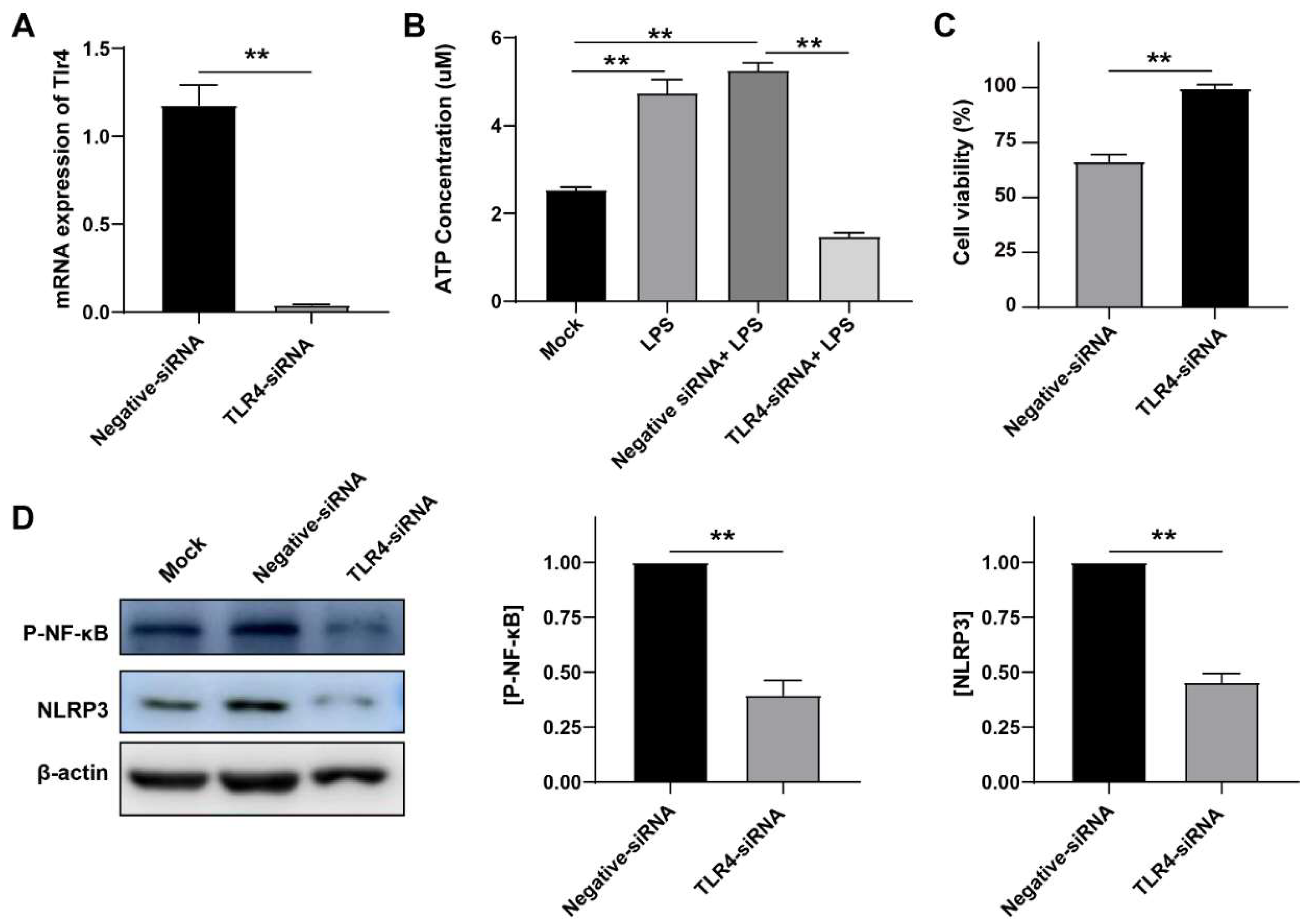
3.5. LPS Induced Inflammation in a TLR4-Dependent Manner
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, S.; Pijoan, C. Haemophilus parasuis: New trends on diagnosis, epidemiology and control. Vet. Microbiol. 2004, 99, 1–12. [Google Scholar] [CrossRef]
- Ni, H.B.; Gong, Q.L.; Zhao, Q.; Li, X.Y.; Zhang, X.X. Prevalence of Haemophilus parasuis "Glaesserella parasuis" in pigs in China: A systematic review and meta-analysis. Prev. Vet. Med. 2020, 182, 105083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, C.; Aragon, V.; Zhou, X.; Zou, M.; Wu, C.; Shen, Z. Investigation of Haemophilus parasuis from healthy pigs in China. Vet. Microbiol. 2019, 231, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Matiaskova, K.; Kavanova, L.; Kulich, P.; Gebauer, J.; Nedbalcova, K.; Kudlackova, H.; Tesarik, R.; Faldyna, M. The Role of Antibodies Against the Crude Capsular Extract in the Immune Response of Porcine Alveolar Macrophages to in Vitro Infection of Various Serovars of Glaesserella (Haemophilus) parasuis. Front. Immunol. 2021, 12, 635097. [Google Scholar] [CrossRef]
- Galofre-Mila, N.; Correa-Fiz, F.; Lacouture, S.; Gottschalk, M.; Strutzberg-Minder, K.; Bensaid, A.; Pina-Pedrero, S.; Aragon, V. A robust PCR for the differentiation of potential virulent strains of Haemophilus parasuis. BMC Vet. Res. 2017, 13, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Guo, J.; Li, R.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; Guo, L.; Hou, Y.; Hu, C.A. Transcriptional Profiling of Host Cell Responses to Virulent Haemophilus parasuis: New Insights into Pathogenesis. Int. J. Mol. Sci. 2018, 19, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielstein, P.; Rapp-Gabrielson, V.J. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 1992, 30, 862–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef]
- Correa-Fiz, F.; Galofre-Mila, N.; Costa-Hurtado, M.; Aragon, V. Identification of a surface epitope specific of virulent strains of Haemophilus parasuis. Vet. Microbiol. 2017, 198, 116–120. [Google Scholar] [CrossRef]
- Olvera, A.; Ballester, M.; Nofrarias, M.; Sibila, M.; Aragon, V. Differences in phagocytosis susceptibility in Haemophilus parasuis strains. Vet. Res. 2009, 40, 24. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, C.; Fang, Y.; Liu, X.; Li, W.; Liu, S.; Liu, Y.; Liu, Y.; Charreyre, C.; Audonnet, J.C.; et al. Transcription analysis on response of porcine alveolar macrophages to Haemophilus parasuis. BMC Genom. 2012, 13, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Hua, K.; Zhang, S.; Wan, Y.; Gong, H.; Ma, B.; Luo, R.; Zhou, R.; Jin, H. COX-2 mediated crosstalk between Wnt/beta-catenin and the NF-kappaB signaling pathway during inflammatory responses induced by Haemophilus parasuis in PK-15 and NPTr cells. Dev. Comp. Immunol. 2020, 105, 103588. [Google Scholar] [CrossRef] [PubMed]
- Macedo, N.; Rovira, A.; Torremorell, M. Haemophilus parasuis: Infection, immunity and enrofloxacin. Vet. Res. 2015, 46, 128. [Google Scholar] [CrossRef] [Green Version]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Boughaba, J.A.; Chojnowski, G.; Christensen, M.E.; Waterhouse, N.J. Dead Cert: Measuring Cell Death. Cold Spring Harb. Protoc. 2016, 2016, pdb-top070318. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Hornung, V. The NLRP3 Inflammasome Renders Cell Death Pro-inflammatory. J. Mol. Biol. 2018, 430, 133–141. [Google Scholar] [CrossRef]
- Zheng, M.; Williams, E.P.; Malireddi, R.K.S.; Karki, R.; Banoth, B.; Burton, A.; Webby, R.; Channappanavar, R.; Jonsson, C.B.; Kanneganti, T.D. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J. Biol. Chem. 2020, 295, 14040–14052. [Google Scholar] [CrossRef]
- Xing, Y.; Yao, X.; Li, H.; Xue, G.; Guo, Q.; Yang, G.; An, L.; Zhang, Y.; Meng, G. Cutting Edge: TRAF6 Mediates TLR/IL-1R Signaling-Induced Nontranscriptional Priming of the NLRP3 Inflammasome. J. Immunol. 2017, 199, 1561–1566. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.M.; Hu, W.; Troutman, T.D.; Jennings, M.; Brewer, T.; Li, X.; Nanda, S.; Cohen, P.; Thomas, J.A.; Pasare, C. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 2014, 111, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Gram, A.; Kowalewski, M.P. Molecular Mechanisms of Lipopolysaccharide (LPS) Induced Inflammation in an Immortalized Ovine Luteal Endothelial Cell Line (OLENDO). Vet. Sci. 2022, 9, 99. [Google Scholar] [CrossRef]
- Haziak, K.; Herman, A.P.; Wojtulewicz, K.; Pawlina, B.; Paczesna, K.; Bochenek, J.; Tomaszewska-Zaremba, D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intracerebroventricular administration of LPS. J. Anim. Sci. Biotechnol. 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.K.; Zhu, X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells 2020, 9, 1808. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Yao, Y.; Teng, F.; Li, Y.; Wu, L.; Yan, W.; Lin, N. The role of P2X7 receptor in infection and metabolism: Based on inflammation and immunity. Int. Immunopharmacol. 2021, 101, 108297. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; Sun, Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genetos, D.C.; Karin, N.J.; Geist, D.J.; Donahue, H.J.; Duncan, R.L. Purinergic signaling is required for fluid shear stress-induced NF-kappaB translocation in osteoblasts. Exp. Cell Res. 2011, 317, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, M.; Minns, M.; Greenberg, E.N.; Diaz-Aponte, J.; Pestonjamasp, K.; Johnson, J.L.; Rathkey, J.K.; Abbott, D.W.; Wang, K.; Shao, F.; et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat. Commun. 2020, 11, 2212. [Google Scholar] [CrossRef]
- Luo, X.; Chang, X.; Zhou, H.; Lin, H.; Fan, H. Glaesserella parasuis induces inflammatory response in 3D4/21 cells through activation of NLRP3 inflammasome signaling pathway via ROS. Vet. Microbiol. 2021, 256, 109057. [Google Scholar] [CrossRef]
- Whitfield, C.; Trent, M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef]
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sa-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.O.; Wang, H.; Hoiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, X.; Su, E.M.; Huang, Y.; Li, L.; Matthay, M.A.; Su, X. Signals of vagal circuits engaging with AKT1 in alpha7 nAChR(+) CD11b(+) cells lessen E. coli and LPS-induced acute inflammatory injury. Cell Discov. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberhardt, M.A.; Goldberg, J.B.; Hogardt, M.; Papin, J.A. Metabolic network analysis of Pseudomonas aeruginosa during chronic cystic fibrosis lung infection. J. Bacteriol. 2010, 192, 5534–5548. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, S.; Diab, A.; Lindberg, A.; Svanborg, C. Monoclonal anti-LPS inner core antibodies protect against experimental hematogenous Haemophilus influenzae type b meningitis. Microb. Pathog. 2000, 28, 1–8. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Miller, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14, 1590–1604. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.J.; Zheng, L.; Hu, Y.W.; Wang, Q. Pyroptosis and its relationship to atherosclerosis. Clin. Chim. Acta 2018, 476, 28–37. [Google Scholar] [CrossRef]
- Chen, X.; He, W.T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, W.; Xu, X.; Xiong, Y.; Zhang, Y.; Zhang, H.; Sun, B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc. Natl. Acad. Sci.USA 2017, 114, 4483–4488. [Google Scholar] [CrossRef] [Green Version]
- Derangere, V.; Chevriaux, A.; Courtaut, F.; Bruchard, M.; Berger, H.; Chalmin, F.; Causse, S.Z.; Limagne, E.; Vegran, F.; Ladoire, S.; et al. Liver X receptor beta activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014, 21, 1914–1924. [Google Scholar] [CrossRef]
- Schuwerk, L.; Hoeltig, D.; Waldmann, K.H.; Strutzberg-Minder, K.; Valentin-Weigand, P.; Rohde, J. Serotyping and pathotyping of Glaesserella parasuis isolated 2012-2019 in Germany comparing different PCR-based methods. Vet. Res. 2020, 51, 137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Li, C.; Wang, C.; Liu, Y.; Wang, G.; He, X.; Hu, L.; Liu, Y.; Cui, M.; et al. Secondary Haemophilus parasuis infection enhances highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection-mediated inflammatory responses. Vet. Microbiol. 2017, 204, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, S.; Liu, Q.; Li, Y.; Xu, L.; Zhang, Z.; Cai, X.; He, X. Porcine alveolar macrophage polarization is involved in inhibition of porcine reproductive and respiratory syndrome virus (PRRSV) replication. J. Vet. Med. Sci. 2017, 79, 1906–1915. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Gao, Y.; Jiao, J.; Zhang, Y.; Li, J.; Ding, L.; Zhang, L.; Chen, Z.; Song, X.; Yang, G.; et al. Upregulation of TLR4-Dependent ATP Production Is Critical for Glaesserella parasuis LPS-Mediated Inflammation. Cells 2023, 12, 751. https://doi.org/10.3390/cells12050751
Liu F, Gao Y, Jiao J, Zhang Y, Li J, Ding L, Zhang L, Chen Z, Song X, Yang G, et al. Upregulation of TLR4-Dependent ATP Production Is Critical for Glaesserella parasuis LPS-Mediated Inflammation. Cells. 2023; 12(5):751. https://doi.org/10.3390/cells12050751
Chicago/Turabian StyleLiu, Fei, Yidan Gao, Jian Jiao, Yuyu Zhang, Jianda Li, Luogang Ding, Lin Zhang, Zhi Chen, Xiangbin Song, Guiwen Yang, and et al. 2023. "Upregulation of TLR4-Dependent ATP Production Is Critical for Glaesserella parasuis LPS-Mediated Inflammation" Cells 12, no. 5: 751. https://doi.org/10.3390/cells12050751





