Cytological Samples: An Asset for the Diagnosis and Therapeutic Management of Patients with Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
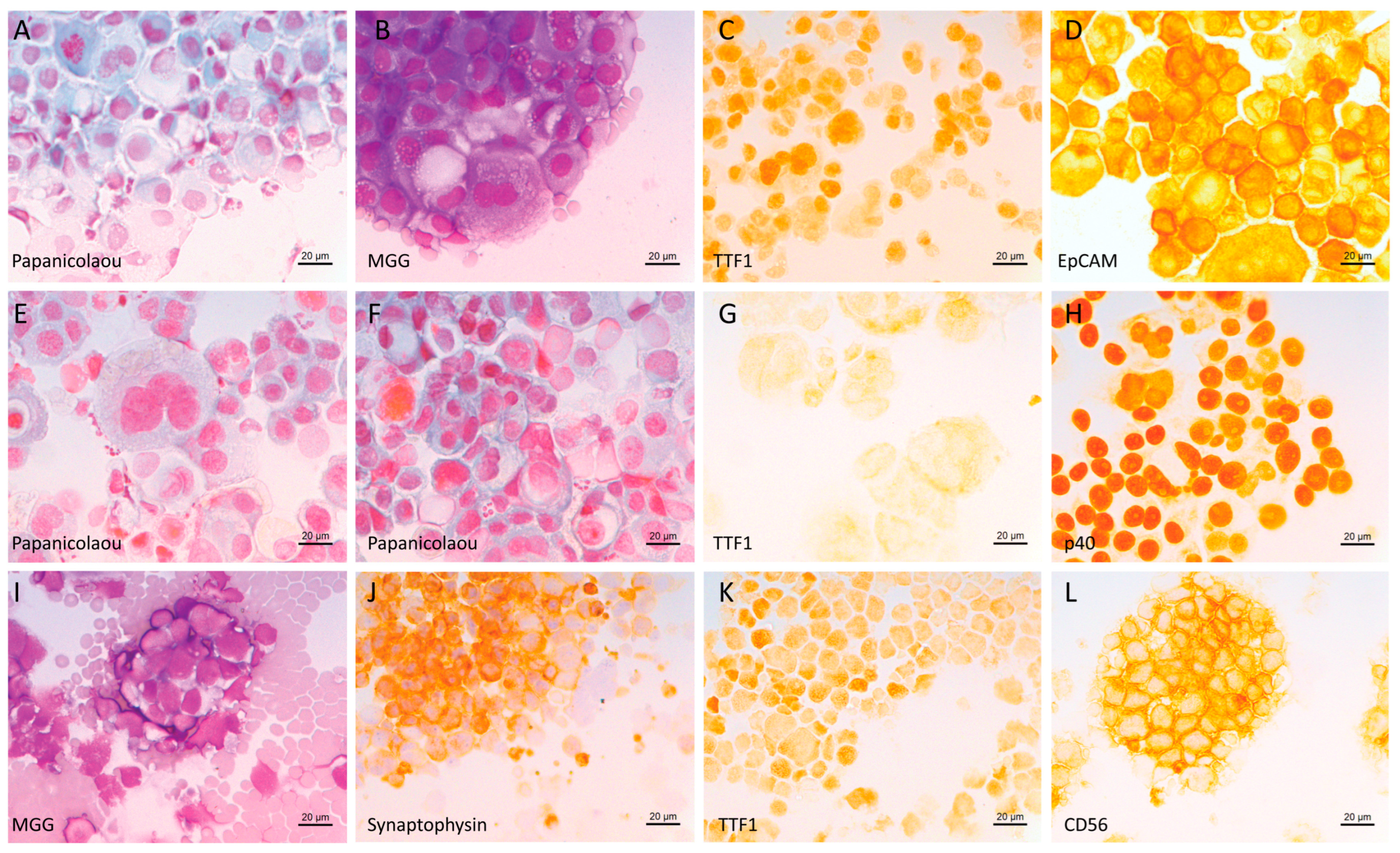
2.2. Immunocytochemistry on Cytospins to Phenotype Tumor Cells
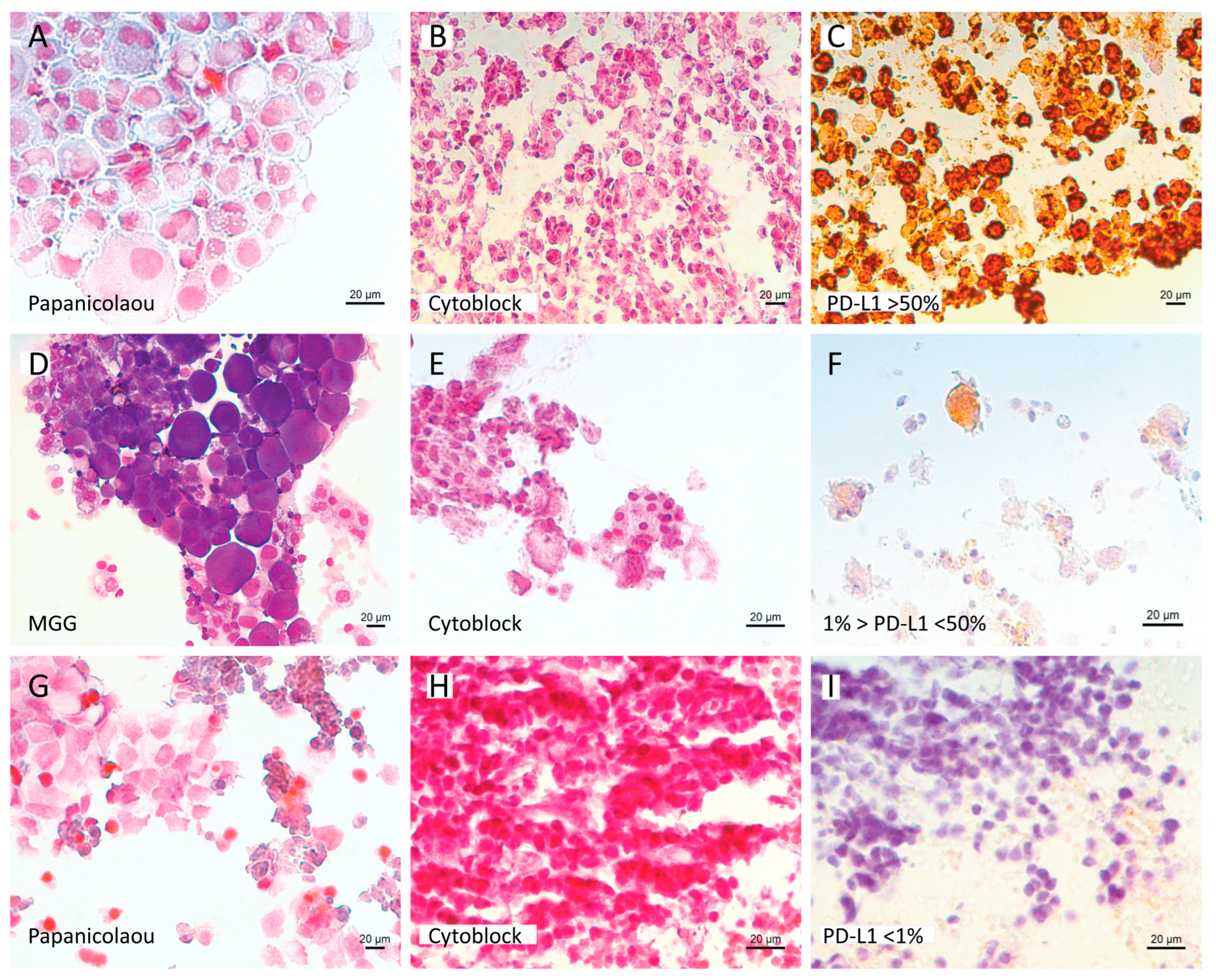
2.3. Immunohistochemistry on Cytoblock for PD-L1 Expression
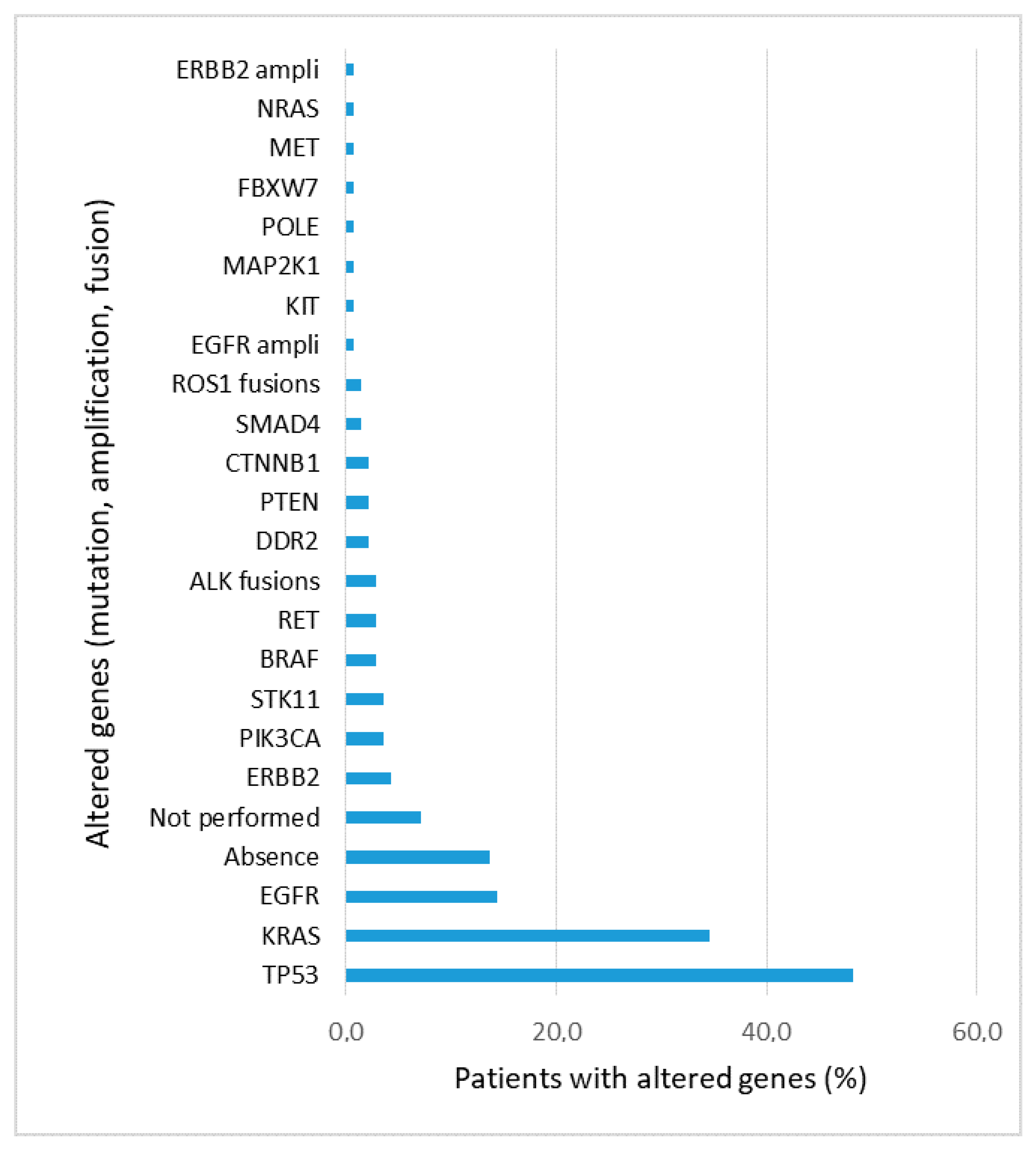
2.4. Next Generation Sequencing
3. Results
3.1. General Results
3.2. Molecular and PD-L1 Results
3.3. Improvement of Diagnosis and Therapeutic Management with Cytological Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/index.html (accessed on 21 December 2022).
- BlueBooksOnline. Available online: https://tumourclassification.iarc.who.int/home (accessed on 19 December 2022).
- Frankel, D.; Kaspi, E.; Roll, P. Immunocytochemical Detection of ALK and ROS1 Rearrangements in Lung Cancer Cytological Samples. Methods Mol. Biol. 2021, 2279, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Frankel, D.; Nanni, I.; Ouafik, L.; Camilla, C.; Pellegrino, E.; Beaufils, N.; Greillier, L.; Dutau, H.; Astoul, P.; Kaspi, E.; et al. Comparison between Immunocytochemistry, FISH and NGS for ALK and ROS1 Rearrangement Detection in Cytological Samples. Int. J. Mol. Sci. 2022, 23, 10556. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Nambirajan, A.; Borczuk, A.; Chen, G.; Minami, Y.; Moreira, A.L.; Motoi, N.; Papotti, M.; Rekhtman, N.; Russell, P.A.; et al. Immunocytochemistry for Predictive Biomarker Testing in Lung Cancer Cytology. Cancer Cytopathol. 2019, 127, 325–339. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef]
- Proietti, A.; Boldrini, L.; Alì, G.; Servadio, A.; Lupi, C.; Sensi, E.; Miccoli, M.; Ribechini, A.; Chella, A.; Lucchi, M.; et al. Histo-Cytological Diagnostic Accuracy in Lung Cancer. Cytopathology 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Rekhtman, N.; Brandt, S.M.; Sigel, C.S.; Friedlander, M.A.; Riely, G.J.; Travis, W.D.; Zakowski, M.F.; Moreira, A.L. Suitability of Thoracic Cytology for New Therapeutic Paradigms in Non-Small Cell Lung Carcinoma: High Accuracy of Tumor Subtyping and Feasibility of EGFR and KRAS Molecular Testing. J. Thorac. Oncol. 2011, 6, 451–458. [Google Scholar] [CrossRef]
- Metovic, J.; Righi, L.; Delsedime, L.; Volante, M.; Papotti, M. Role of Immunocytochemistry in the Cytological Diagnosis of Pulmonary Tumors. Acta Cytol. 2020, 64, 16–29. [Google Scholar] [CrossRef]
- Arnold, D.T.; De Fonseka, D.; Perry, S.; Morley, A.; Harvey, J.E.; Medford, A.; Brett, M.; Maskell, N.A. Investigating Unilateral Pleural Effusions: The Role of Cytology. Eur. Respir. J. 2018, 52, 1801254. [Google Scholar] [CrossRef]
- Shojaee, S.; Roy-Chowdhuri, S.; Safi, J.; Grosu, H.B. Cytologic Investigations for the Diagnosis of Malignant Pleural Effusion in Non-Small Cell Lung Cancer: State-of-the-Art Review for Pulmonologists. J. Bronchol. Interv. Pulmonol. 2021, 28, 310–321. [Google Scholar] [CrossRef]
- Fatkin, D.; MacRae, C.; Sasaki, T.; Wolff, M.R.; Porcu, M.; Frenneaux, M.; Atherton, J.; Vidaillet, H.J.; Spudich, S.; De Girolami, U.; et al. Missense Mutations in the Rod Domain of the Lamin A/C Gene as Causes of Dilated Cardiomyopathy and Conduction-System Disease. N. Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef]
- Grosu, H.B.; Kazzaz, F.; Vakil, E.; Molina, S.; Ost, D. Sensitivity of Initial Thoracentesis for Malignant Pleural Effusion Stratified by Tumor Type in Patients with Strong Evidence of Metastatic Disease. Respiration 2018, 96, 363–369. [Google Scholar] [CrossRef]
- Pai-Scherf, L.; Blumenthal, G.M.; Li, H.; Subramaniam, S.; Mishra-Kalyani, P.S.; He, K.; Zhao, H.; Yu, J.; Paciga, M.; Goldberg, K.B.; et al. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist 2017, 22, 1392–1399. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non–Small-Cell Lung Cancer. JCO 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Punekar, S.R.; Shum, E.; Grello, C.M.; Lau, S.C.; Velcheti, V. Immunotherapy in Non-Small Cell Lung Cancer: Past, Present, and Future Directions. Front. Oncol. 2022, 12, 877594. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 Expression in Lung Cancer. J. Thorac. Oncol. 2016, 11, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Lantuejoul, S.; Sound-Tsao, M.; Cooper, W.A.; Girard, N.; Hirsch, F.R.; Roden, A.C.; Lopez-Rios, F.; Jain, D.; Chou, T.-Y.; Motoi, N.; et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J. Thorac. Oncol. 2020, 15, 499–519. [Google Scholar] [CrossRef]
- Bozzetti, C.; Squadrilli, A.; Nizzoli, R.; Lagrasta, C.; Gasparro, D.; Majori, M.; Filippo, M.D.; Becchi, G.; Azzoni, C.; Campanini, N.; et al. Optimizing PD-L1 Evaluation on Cytological Samples from Advanced Non-Small-Cell Lung Cancer. Immunotherapy 2020, 12, 183–193. [Google Scholar] [CrossRef]
- Mansour, M.S.I.; Lindquist, K.E.; Seidal, T.; Mager, U.; Mohlin, R.; Tran, L.; Hejny, K.; Holmgren, B.; Violidaki, D.; Dobra, K.; et al. PD-L1 Testing in Cytological Non-Small Cell Lung Cancer Specimens: A Comparison with Biopsies and Review of the Literature. Acta Cytol. 2021, 65, 501–509. [Google Scholar] [CrossRef]
- Hara, N.; Ichihara, E.; Harada, D.; Inoue, K.; Fujiwara, K.; Hosokawa, S.; Kishino, D.; Haruyuki, K.; Ochi, N.; Oda, N.; et al. Significance of PD-L1 Expression in the Cytological Samples of Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors. J. Cancer Res. Clin. Oncol. 2021, 147, 3749–3755. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients with Lung Cancer for Treatment with Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018, 13, 323–358. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Barbareschi, M.; Barberis, M.; Buttitta, F.; Doglioni, C.; Fiorentino, M.; Fontanini, G.; Franco, R.; Marchetti, A.; Rossi, G.; Troncone, G. Predictive Markers in Lung Cancer: A Few Hints for the Practicing Pathologist. Pathologica 2018, 110, 29–38. [Google Scholar]
- Frankel, D.; Nanni-Metellus, I.; Robaglia-Schlupp, A.; Tomasini, P.; Guinde, J.; Barlesi, F.; Astoul, P.; Ouafik, L.; Amatore, F.; Secq, V.; et al. Detection of EGFR, KRAS and BRAF Mutations in Metastatic Cells from Cerebrospinal Fluid. Clin. Chem. Lab. Med. 2018, 56, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Rojo, F.; Gómez, J.; Enguita, A.B.; Abdulkader, I.; González, A.; Lozano, D.; Mancheño, N.; Salas, C.; Salido, M.; et al. Molecular Diagnosis in Non-Small-Cell Lung Cancer: Expert Opinion on ALK and ROS1 Testing. J. Clin. Pathol. 2021, 75, 145–153. [Google Scholar] [CrossRef]
- Tafoya, M.; Judd, A.; Chiotti, K.; Dearen, K.; Jiron, K.; Chabot-Richards, D.; Broehm, C.J. Performance of a 50-Gene next Generation Sequencing Panel with Post-Centrifuge Supernatant Cytology Fluid in Non-Small-Cell Lung Cancer. Diagn Cytopathol. 2021, 49, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Abouzgheib, W.; Bartter, T.; Dagher, H.; Pratter, M.; Klump, W. A Prospective Study of the Volume of Pleural Fluid Required for Accurate Diagnosis of Malignant Pleural Effusion. Chest 2009, 135, 999–1001. [Google Scholar] [CrossRef]
- Swiderek, J.; Morcos, S.; Donthireddy, V.; Surapaneni, R.; Jackson-Thompson, V.; Schultz, L.; Kini, S.; Kvale, P. Prospective Study to Determine the Volume of Pleural Fluid Required to Diagnose Malignancy. Chest 2010, 137, 68–73. [Google Scholar] [CrossRef]
- DeMaio, A.; Clarke, J.M.; Dash, R.; Sebastian, S.; Wahidi, M.M.; Shofer, S.L.; Cheng, G.Z.; Li, X.; Wang, X.; Mahmood, K. Yield of Malignant Pleural Effusion for Detection of Oncogenic Driver Mutations in Lung Adenocarcinoma. J. Bronchol. Interv. Pulmonol. 2019, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, S.D.; Chau, K.; Sajjan, S.; Chakraborty, B.; Karam, P.; Khutti, S.; Gimenez, C.; Das, K. Adequacy of Pleural Fluid Cytology for Comprehensive Molecular Analysis of Lung Adenocarcinoma: Experience of a Large Health-Care System. Cytojournal 2022, 19, 7. [Google Scholar] [CrossRef] [PubMed]
| Parameter | n (%) | |
|---|---|---|
| Age (years) | Mean ± SD | 67 ± 11 |
| Range | 32–91 | |
| Gender | Male | 108 (57%) |
| Female | 81 (43%) | |
| Histopathological type | Lung adenocarcinoma | 106 (53%) |
| Squamous cell carcinoma | 20 (10%) | |
| NSCLC NOS | 44 (22%) | |
| Small cell lung cancer | 15 (7.5%) | |
| Large cell neuroendocrine carcinoma | 3 (1.5%) | |
| Carcinoid tumor of the lung | 1 (0.5%) | |
| Smoking status | Never | 32 (17%) |
| Current/former | 138 (73%) | |
| Unknown | 19 (10%) | |
| Stage | I | 3 (1.6%) |
| II | 6 (3.2%) | |
| III | 35 (18.5%) | |
| IV | 142 (75.1%) | |
| Unknown | 3 (1.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankel, D.; Nanni, I.; Ouafik, L.; Greillier, L.; Dutau, H.; Astoul, P.; Daniel, L.; Kaspi, E.; Roll, P. Cytological Samples: An Asset for the Diagnosis and Therapeutic Management of Patients with Lung Cancer. Cells 2023, 12, 754. https://doi.org/10.3390/cells12050754
Frankel D, Nanni I, Ouafik L, Greillier L, Dutau H, Astoul P, Daniel L, Kaspi E, Roll P. Cytological Samples: An Asset for the Diagnosis and Therapeutic Management of Patients with Lung Cancer. Cells. 2023; 12(5):754. https://doi.org/10.3390/cells12050754
Chicago/Turabian StyleFrankel, Diane, Isabelle Nanni, L’Houcine Ouafik, Laurent Greillier, Hervé Dutau, Philippe Astoul, Laurent Daniel, Elise Kaspi, and Patrice Roll. 2023. "Cytological Samples: An Asset for the Diagnosis and Therapeutic Management of Patients with Lung Cancer" Cells 12, no. 5: 754. https://doi.org/10.3390/cells12050754
APA StyleFrankel, D., Nanni, I., Ouafik, L., Greillier, L., Dutau, H., Astoul, P., Daniel, L., Kaspi, E., & Roll, P. (2023). Cytological Samples: An Asset for the Diagnosis and Therapeutic Management of Patients with Lung Cancer. Cells, 12(5), 754. https://doi.org/10.3390/cells12050754







