Potential Role of Low-Molecular-Weight Dioxolanes as Adjuvants for Glyphosate-Based Herbicides Using Photosystem II as an Early Post-Treatment Determinant
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Growth Condition
2.3. Experimental Design, Treatments, and Evaluation
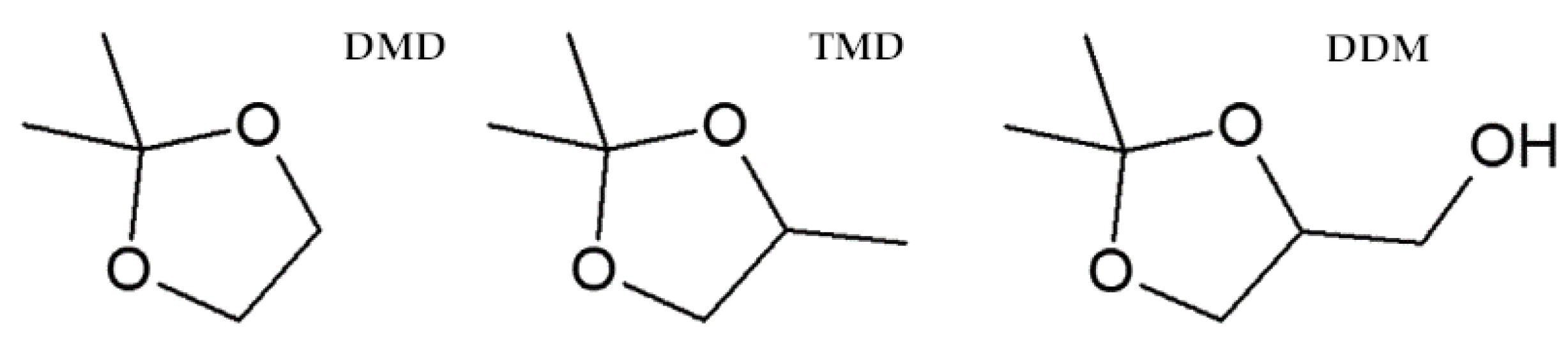
2.3.1. Effect of 1,3-Dioxolanes on Plants
2.3.2. Evaluation of the Efficacy of Dioxolane-Assisted Herbicide
2.4. Statistical Analysis
3. Results
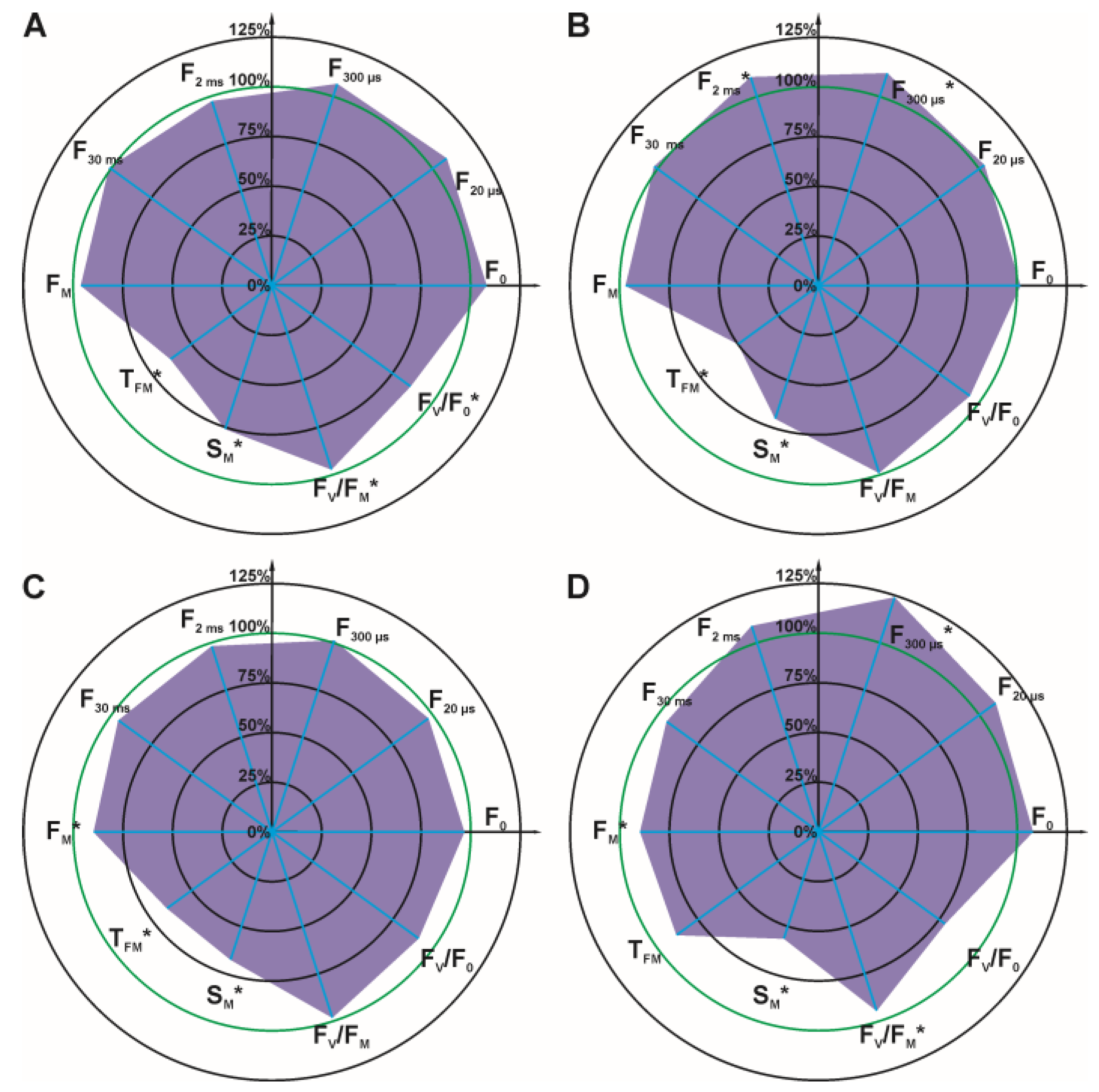
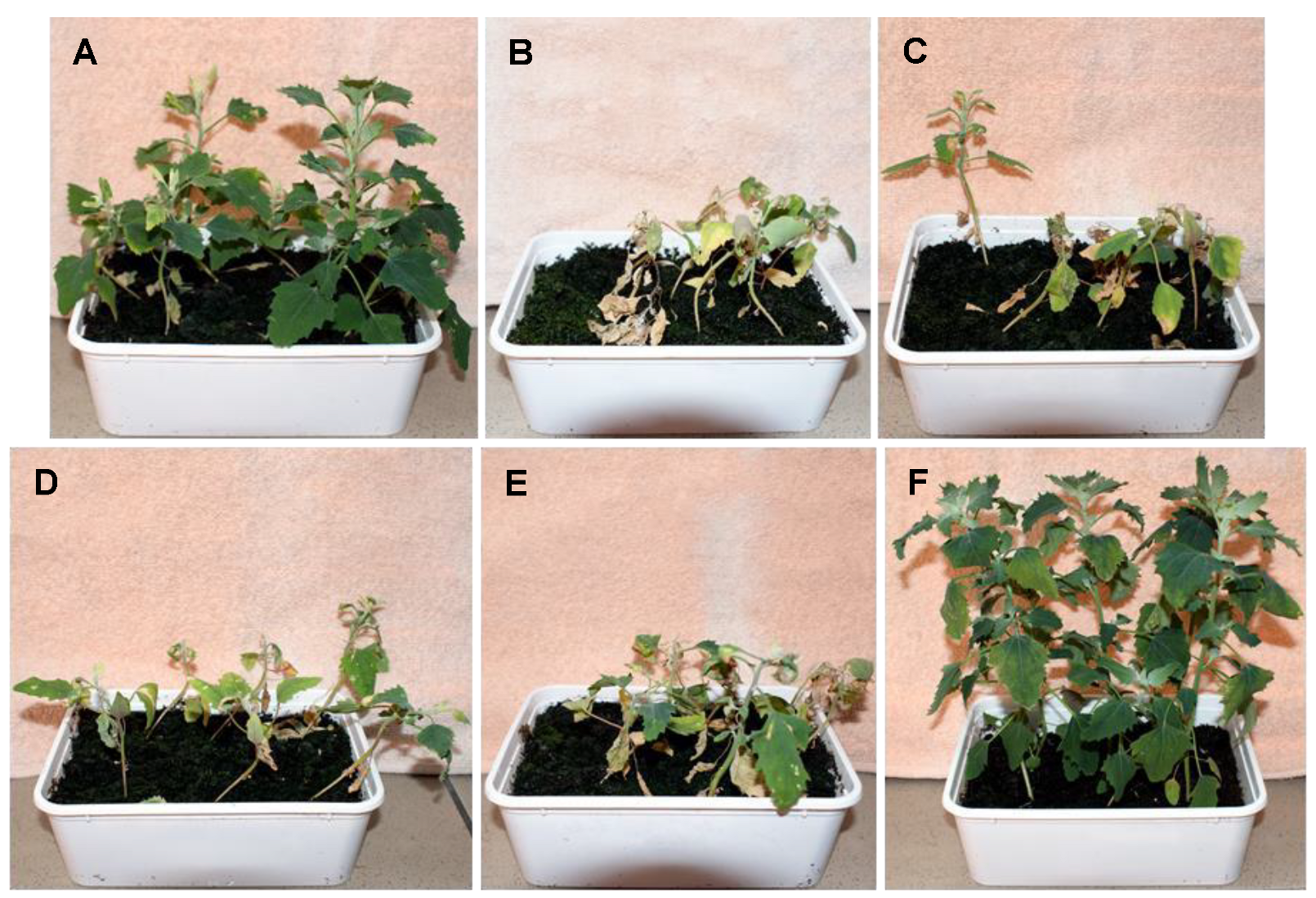
3.1. Effect of 1,3-Dioxolane on Chenopodium Album
3.2. Effect of Glyphosate on ChlF Induction Curve
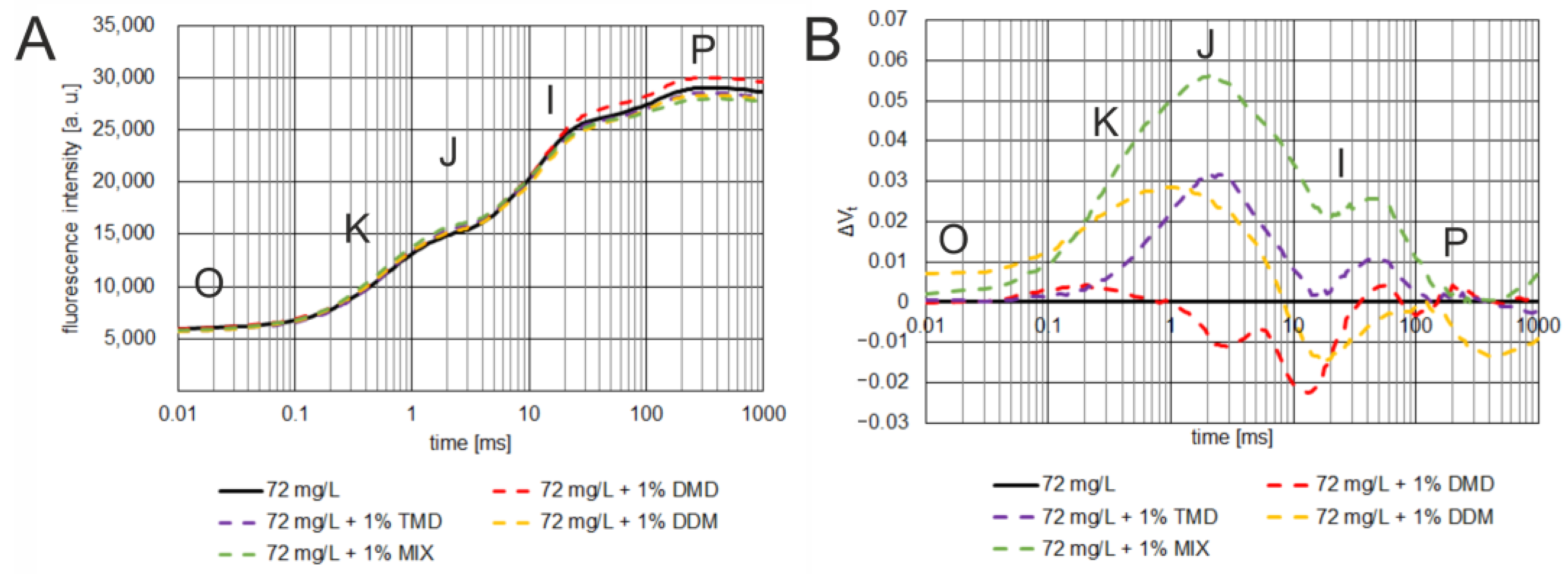
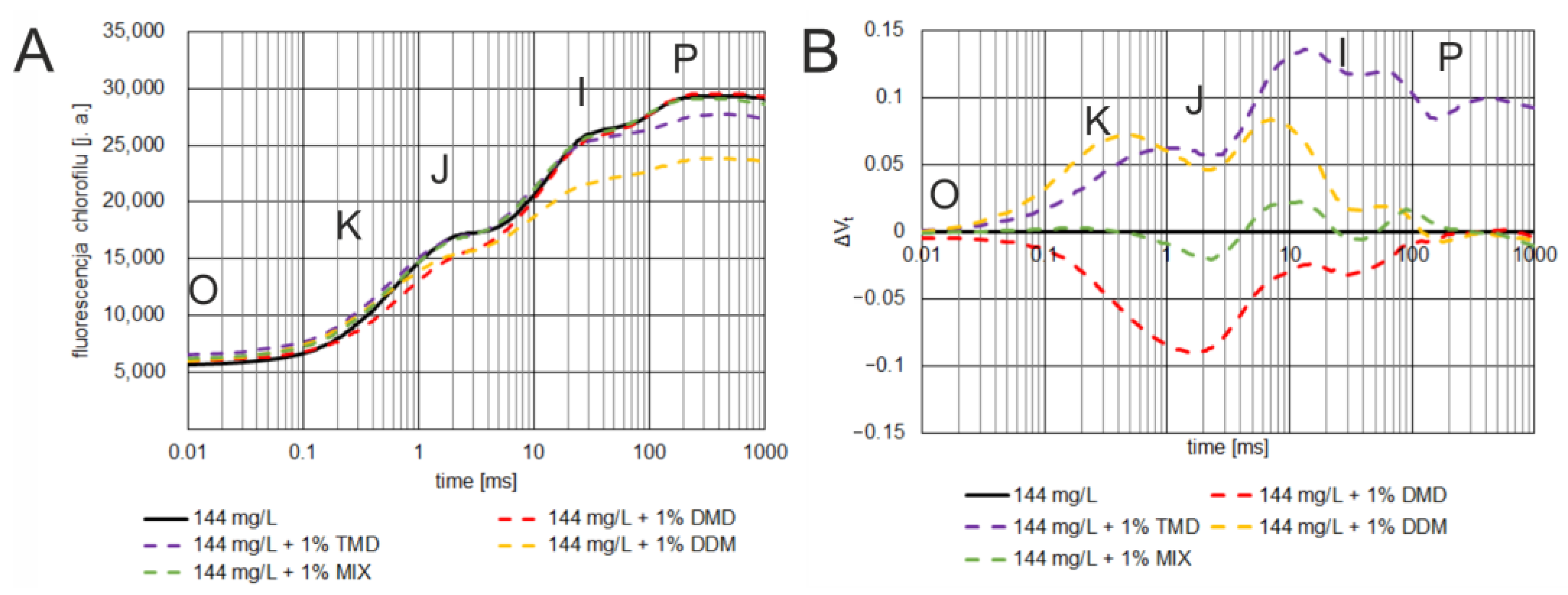
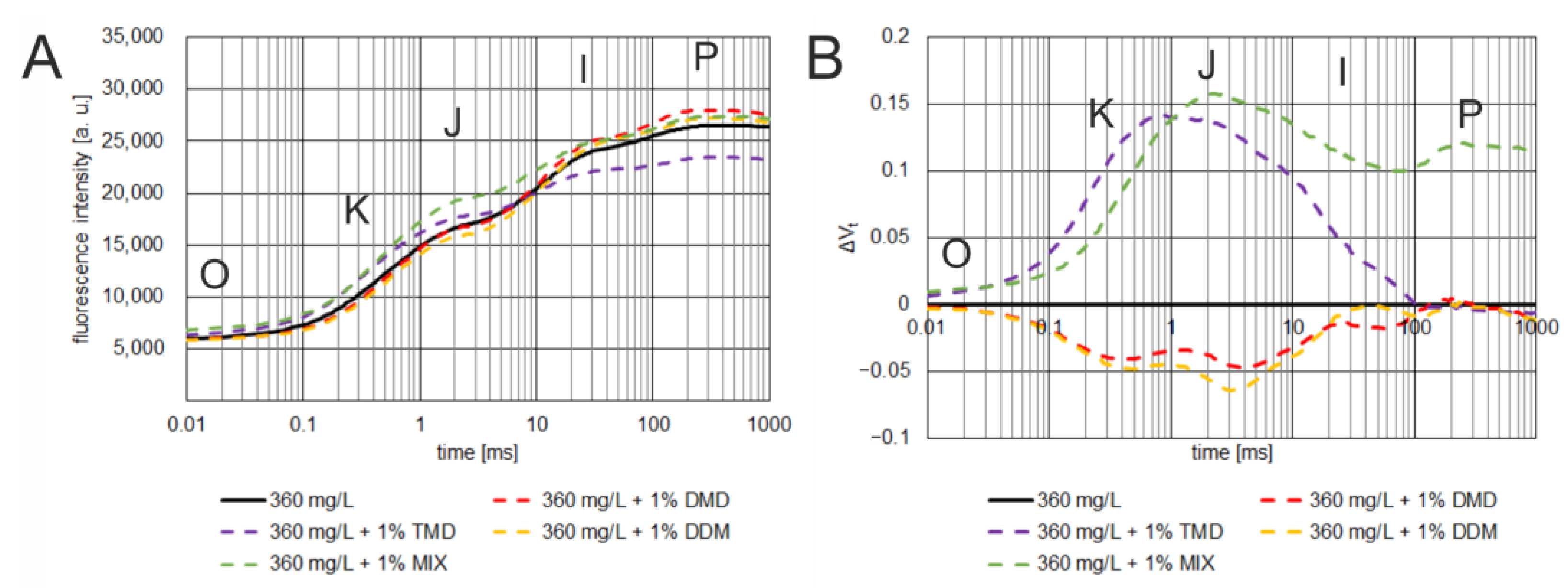
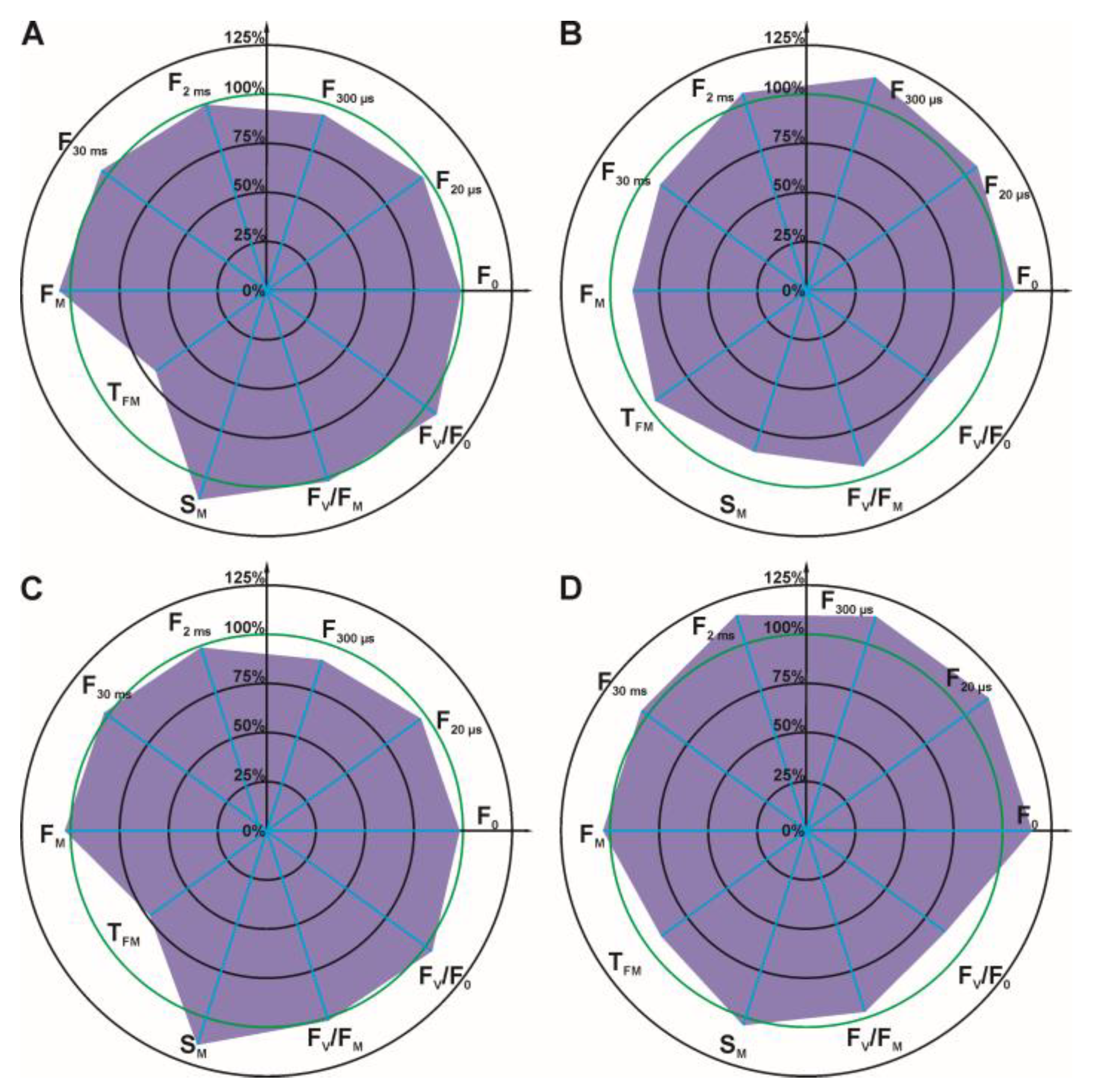
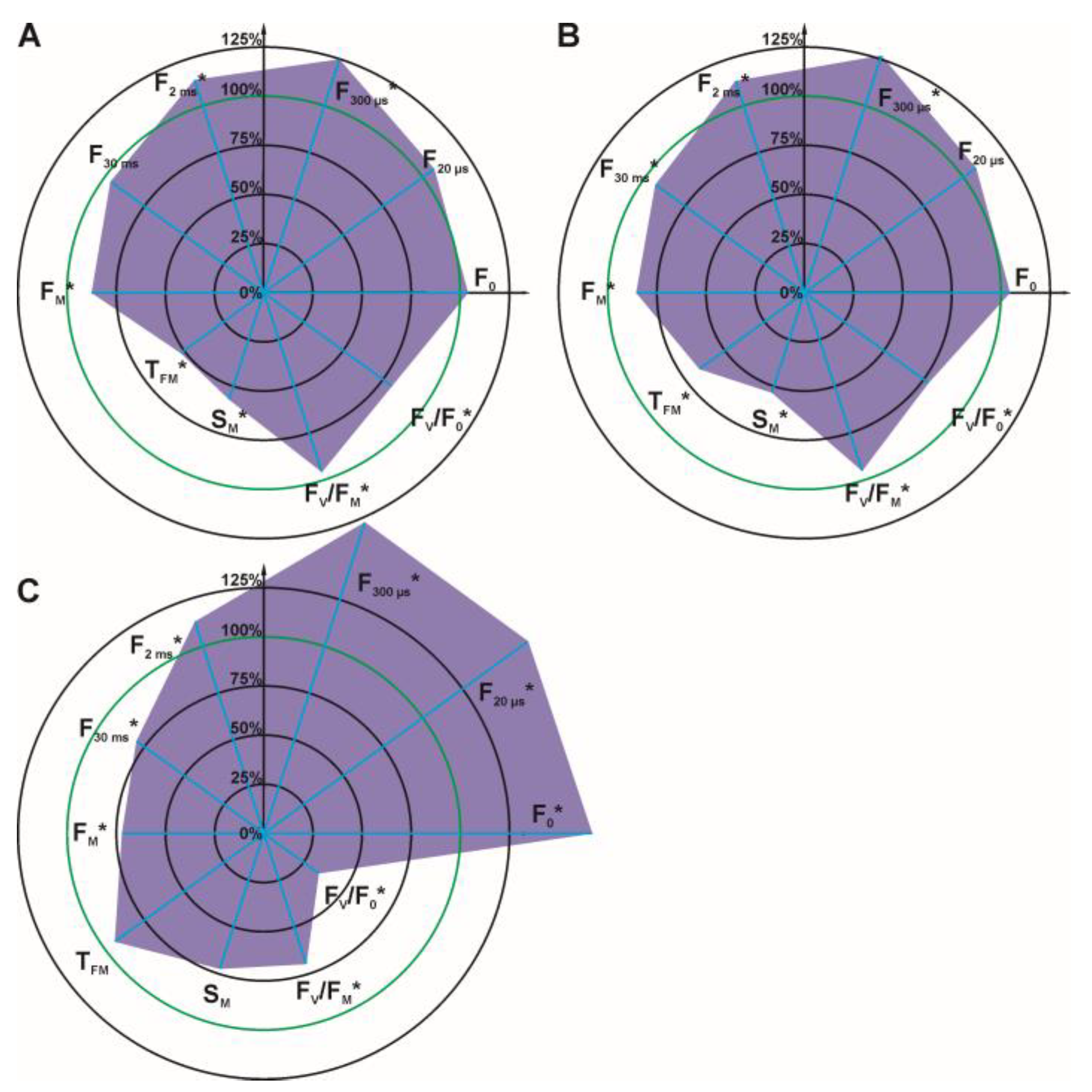
3.3. Effect of Dioxolane-Assisted Glyphosate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Roundup® 360 Plus Volume [μL] | Glyphosate Mass [mg] | Glyphosate Concentration [mg/L] |
|---|---|---|
| 1 | 0.36 | 72 |
| 2 | 0.72 | 144 |
| 4 | 1.44 | 288 |
| 5 | 1.80 | 360 |
| 6 | 2.16 | 432 |
| 8 | 2.88 | 576 |
| 10 | 3.60 | 720 |
| 25 | 9 | 1800 |






References
- Jaggard, K.W.; Qi, A.; Ober, S. Possible Changes to Arable Crop Yields by 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2835–2851. [Google Scholar] [CrossRef] [PubMed]
- Adamczewski, K.; Dobrzański, A. Future for Weed Sciences in Changing. Prog. Plant Prot. 2012, 52, 867–878. [Google Scholar]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Parween, T.; Jan, S. Ecophysiology of Pesticides: Interface Between Pesticide Chemistry and Plant Physiology; Academic Press: Cambridge, MA, USA, 2019; p. 316. [Google Scholar]
- Green, J.M. Current State of Herbicides in Herbicide-Resistant Crops. Pest. Manag. Sci. 2014, 70, 1351–1357. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Bhuiyan, T.F.; Anee, T.I.; Masud, A.A.C.; Nahar, K. Phytotoxicity, environmental and health hazards of herbicides: Challenges and ways forward. In Agrochemicals Detection, Treatment and Remediation; Vara Prasad, M.N., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 55–99. [Google Scholar]
- Duke, S.O.; Dayan, F.E. Herbicides. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 376–384. [Google Scholar] [CrossRef]
- Zaller, J.G.; Brühl, C.A. Editorial: Non-Target Effects of Pesticides on Organisms Inhabiting Agroecosystems. Front. Environ. Sci. 2019, 7, 75. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Mushtaq, W.; Siddiqui, S.A.; Ayadi, S.; Kaur, P.; Yeboah, S.; Mazraedoost, S.; Al-Taey, D.K.A.; Tampubolon, K. Herbicide Residues in Agroecosystems: Fate, Detection, and Effect on Non-Target Plants. Rev. Agric. Sci. 2021, 9, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Zaller, J.G. Herbicides: A necessary evil? An integrative overview. In Herbicides: Chemistry, Efficacy, Toxicology, and Environmental Impacts; Mesnage, R., Zaller, J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–333. [Google Scholar]
- Choudhury, P.P. Transformation of herbicides in the environment. In Herbicide Residue Research in North–Western India; Singh, S.B., Singh, N., Banerjee, T., Eds.; Springer: Singapore, 2019; pp. 415–442. [Google Scholar]
- Krawczyk, R.; Kaczmarek, S.; Mrówczyński, M. Sustainable agriculture-new technologies and the problems of weed control. Probl. Inżynierii Rol. 2008, 16, 25–32. [Google Scholar]
- Miklaszewska, K.; Kierzek, R. Efficacy of Lower Doses of Aminopyralid + Piroksysulam + Florasulam (Lancet Plus 125 WG) Applied with Adjuvant in Winter Wheat. Prog. Plant Prot. 2014, 54, 451–455. [Google Scholar] [CrossRef]
- Kieloch, R.; Gołębiowska, H. Evaluation of the Impact of Crotonic Acid Addition to the Mixture of Bromoxynil with Terbuthylazine and Nicosulfuron in Lowered Doses on Selected Weed Species Control. Prog. Plant Prot. 2020, 60, 261–265. [Google Scholar] [CrossRef]
- Foy, C.L. Adjuvants for Agrichemicals; CRC Press: Boca Raton, FL, USA, 2018; p. 735. [Google Scholar]
- Wang, C.J.; Liu, Z.Q. Foliar Uptake of Pesticides-Present Status and Future Challenge. Pestic. Biochem. Physiol. 2007, 87, 1–8. [Google Scholar] [CrossRef]
- Woźnica, Z. Herbologia. Podstawy biologii, Ekologii i Zwalczania Chwastów; PWRiL: Poznań, Poland, 2008; p. 430. [Google Scholar]
- Kucharski, M.; Sadowski, J.; Domaradzki, K. New Solutions in the Herbicide Application System the Influence on Contamination of the Soil Environment. Soil Sci. Annu. 2012, 63, 36–38. [Google Scholar] [CrossRef]
- Menendez, J.; Rojano-Delgado, M.A.; De Prado, R. Differences in Herbicide Uptake, Translocation, and Distribution as Sources of Herbicide Resistance in Weeds. ACS Symp. Ser. 2014, 1171, 141–157. [Google Scholar] [CrossRef]
- Kirkwood, R.C. Recent Developments in Our Understanding of the Plant Cuticle as a Barrier to the Foliar Uptake of Pesticides. Pestic. Sci. 1999, 55, 69–77. [Google Scholar] [CrossRef]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar Water and Solute Absorption: An Update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef]
- Leaper, C.; Holloway, P.J. Adjuvants and Glyphosate Activity. Pest Manag. Sci. 2000, 56, 313–319. [Google Scholar] [CrossRef]
- Hazen, J.L. Adjuvants—Terminology, Classification, and Chemistry. Weed Technol. 2000, 14, 773–784. [Google Scholar] [CrossRef]
- Beck, B.; Steurbaut, W.; Spanoghe, P. How to Define Green Adjuvants. Pest Manag. Sci. 2012, 68, 1107–1110. [Google Scholar] [CrossRef]
- Pacanoski, Z. Role of Adjuvants on Herbicide Behavior: A Review of Different Experiences. Herbologia 2010, 11, 67–79. [Google Scholar]
- Pacanoski, Z. Herbicides and adjuvants. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen Limited: London, UK, 2015; pp. 125–147. [Google Scholar] [CrossRef]
- Castro, E.B.; Carbonari, C.A.; Velini, E.D.; Gomes, G.L.G.C.; Belapart, D. Influence of Adjuvants on the Surface Tension, Deposition and Effectiveness of Herbicides on Fleabane Plants. Planta Daninha 2018, 36, 1–12. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Wesołowski, M.; Drabowicz, M.; Misztal-Majewska, B. The Effect of Adjuvants and Reduced Rates of Crop Protection Agents on the Occurrence of Agricultural Pests and on Winter Wheat Productivity. Agron. Sci. 2012, 67, 12–21. [Google Scholar] [CrossRef]
- Idziak, R.; Woźnica, Z. Efficacy of nicosulfuron, rimsulfuron and dicamba mixture applied in maize. Prog. Plant Prot. 2013, 53, 735–739. [Google Scholar]
- Gołębiowska, H.; Kieloch, R. Herbicidal efficiency of bromoxynil used independently and in mixtures under conditions of integrated maize cultivation. Prog. Plant Prot. 2020, 60, 157–162. [Google Scholar] [CrossRef]
- Moity, L.; Benazzouz, A.; Molinier, V.; Nardello-Rataj, V.; Elmkaddem, M.K.; De Caro, P.; Thiébaud-Roux, S.; Gerbaud, V.; Marion, P.; Aubry, J.M. Glycerol Acetals and Ketals as Bio-Based Solvents: Positioning in Hansen and COSMO-RS Spaces, Volatility and Stability towards Hydrolysis and Autoxidation. Green Chem. 2015, 17, 1779–1792. [Google Scholar] [CrossRef]
- Samoilov, V.; Ni, D.; Goncharova, A.; Zarezin, D.; Kniazeva, M.; Ladesov, A.; Kosyakov, D.; Bermeshev, M.; Maximov, A. Bio-Based Solvents and Gasoline Components from Renewable 2,3-Butanediol and 1,2-Propanediol: Synthesis and Characterization. Molecules 2020, 25, 1723. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.S.; Adrijanto, E.; Alsalme, A.; Kozhevnikov, I.V.; Cooke, D.J.; Brown, D.R.; Shiju, N.R. Glycerol Utilization: Solvent-Free Acetalisation over Niobia Catalysts. Catal. Sci. Technol. 2012, 2, 1173–1179. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef]
- Kapkowski, M.; Ludynia, M.; Rudnicka, M.; Dzida, M.; Zorębski, E.; Musiał, M.; Doležal, M.; Polanski, J. Enhancing the CO2 Capturing Ability in Leaf via Xenobiotic Auxin Uptake. Sci. Total Environ. 2020, 745, 141032. [Google Scholar] [CrossRef] [PubMed]
- Fleute-Schlachter, I.; Heldt, S.; Kempers, P.; Schörken, U.; Paetzold, E.; Kragl, E. Agrochemical Compositions Comprising Alkoxylated Glycerol Acetals and Their Derivatives. Patent No. EP 2305662 B1, 23 January 2013. [Google Scholar]
- Kapkowski, M.; Ludynia, M.; Polański, J.; Dzida, M.; Rudnicka, M.; Balin, K.; Doležal, M.; Kastner, P. Composition Improving the Penetration of Biologically Active Substances through the Surfaces of Plant Organs. Patent No. PL 239074 B1, 2 November 2021. [Google Scholar]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, M.H.; Rastogi, A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef]
- Zhang, J.; Jäck, O.; Menegat, A.; Li, G.; Wang, X. Chlorophyll Fluorescence Measurement: A New Method to Test the Effect of Two Adjuvants on the Efficacy of Topramezone on Weeds. IFIP Adv. Inf. Commun. Technol. 2019, 545, 206–216. [Google Scholar] [CrossRef]
- Bhatia, M.; Singh, S.; Pagare, S.; Kumar, B. A Pharmacological Comprehensive Review on Chenopodium album L. Int. J. Curr. Res. 2020, 12, 15360–15368. [Google Scholar] [CrossRef]
- Singh, V.; Gupta, S.; Singh, H.; Raghubanshi, A.S. Ecophysiological Characteristics of Five Weeds and a Wheat Crop in the Indo-Gangetic Plains, India. Weed Biol. Manag. 2015, 15, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, V.; Mahallati, M.N.; Nezami, A.; Mohassel, M.H.R. Effects of the Relative Time of Emergence and the Density of Common Lambsquarters (Chenopodium album) on Corn (Zea mays) Yield. Weed Biol. Manag. 2011, 11, 127–136. [Google Scholar] [CrossRef]
- Tehmina, A.; Rukhsana, B. Competition Losses Caused by Rumex dentatus L. and Cenopodium album L. in Wheat (Triticum aestivum L.). Philipp. Agric. Sci. 2010, 93, 365–368. [Google Scholar]
- Siemieniuk, A.; Ludynia, M.; Rudnicka, M. Response of Two Crop Plants, Zea mays L. and Solanum lycopersicum L., to Diclofenac and Naproxen. Int. J. Mol. Sci. 2021, 22, 8856. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Buonasera, K.; Lambreva, M.; Rea, G.; Touloupakis, E.; Giardi, M.T. Technological Applications of Chlorophyll a Fluorescence for the Assessment of Environmental Pollutants. Anal. Bioanal. Chem. 2011, 401, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 5th ed.; PrenticeHall, Inc.: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Kalaji, H.; Guo, P. Chlorophyll Fluorescence: A Useful Tool in Barley Plant Breeding Programs. Photochem. Res. Prog. 2008, 29, 447–471. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Trajdos, J.; Kucharski, M.; Sadowski, J. Influence of Metamitron Dose and Surfactant on Weed Control and Yield of Sugar Beet. Prog. Plant Prot. 2014, 54, 51–55. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of Chlorophyll Fluorescence Can Improve Crop Production Strategies: An Examination of Future Possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; de Oliveira, J.R.S.; Kremer, R.J.; Constantin, J.; Bonato, C.M.; Muniz, A.S. Water Use Efficiency and Photosynthesis of Glyphosate-Resistant Soybean as Affected by Glyphosate. Pestic. Biochem. Physiol. 2010, 97, 182–193. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, F.; Zhao, Y.; Huang, Y.; Reddy, K.N.; Dong, L. Detection of the Onset of Crop Stress Induced by Glyphosate Using Chlorophyll Fluorescence Measurements. In Proceedings of the 3rd International Conference on Agro-Geoinformatics, Agro-Geoinformatics, Beijing, China, 11–14 August 2014; pp. 1–5. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Huang, Y.; Reddy, K.N.; Zhao, Y.; Molin, W.T. Detection of the Onset of Glyphosate-Induced Soybean Plant Injury through Chlorophyll Fluorescence Signal Extraction and Measurement. J. Appl. Remote Sens. 2015, 9, 097098-1. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Z.; Gao, D.; Si, P.; Yu, H.; Liu, J. Effects of Herbicide Drift on Chlorophyll Fluorescence and Antioxidant Enzyme Levels of Various Types of Fruit Trees. Pakistan J. Bot. 2021, 53, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.G.; Teicher, H.B.; Streibig, J.C. Linking Fluorescence Induction Curve and Biomass in Herbicide Screening. Pest Manag. Sci. 2003, 59, 1303–1310. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwajczak, E.; Sierka, E.; Ludynia, M. Potential Role of Low-Molecular-Weight Dioxolanes as Adjuvants for Glyphosate-Based Herbicides Using Photosystem II as an Early Post-Treatment Determinant. Cells 2023, 12, 777. https://doi.org/10.3390/cells12050777
Szwajczak E, Sierka E, Ludynia M. Potential Role of Low-Molecular-Weight Dioxolanes as Adjuvants for Glyphosate-Based Herbicides Using Photosystem II as an Early Post-Treatment Determinant. Cells. 2023; 12(5):777. https://doi.org/10.3390/cells12050777
Chicago/Turabian StyleSzwajczak, Ewa, Edyta Sierka, and Michał Ludynia. 2023. "Potential Role of Low-Molecular-Weight Dioxolanes as Adjuvants for Glyphosate-Based Herbicides Using Photosystem II as an Early Post-Treatment Determinant" Cells 12, no. 5: 777. https://doi.org/10.3390/cells12050777
APA StyleSzwajczak, E., Sierka, E., & Ludynia, M. (2023). Potential Role of Low-Molecular-Weight Dioxolanes as Adjuvants for Glyphosate-Based Herbicides Using Photosystem II as an Early Post-Treatment Determinant. Cells, 12(5), 777. https://doi.org/10.3390/cells12050777






