Micrandilactone C, a Nortriterpenoid Isolated from Roots of Schisandra chinensis, Ameliorates Huntington’s Disease by Inhibiting Microglial STAT3 Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Approval
2.2. Experimental Group, Model Induction, and Drug Treatment
2.3. Behavioral Semi-Quantitative Assessment
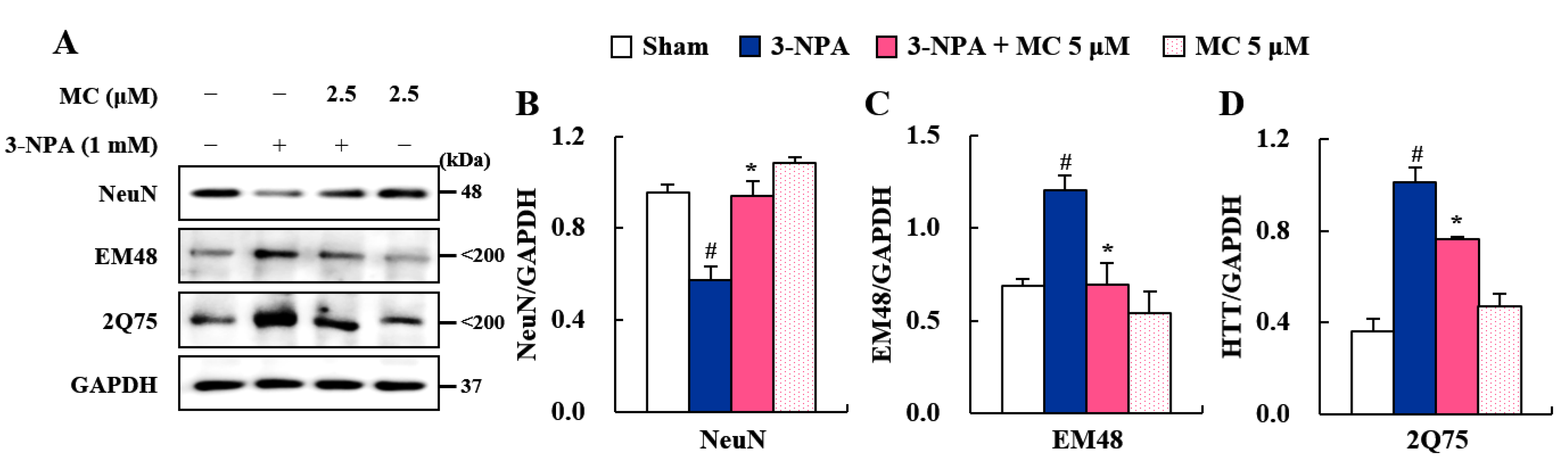
2.4. Histopathological Analysis of Striatal Damage
2.5. Fluoro-Jade C (FJC) and Cresyl Violet Stains
2.6. Immunohistochemical and Immunofluorescence Evaluation
2.7. Western Blot Analysis
2.8. Flow Cytometry
2.9. Real-Time Polymerase Chain Reaction (PCR) Analyses
2.10. STHdh Cell Culture
2.11. Preparation of Conditioned Medium (CM) from BV2 Cells and Determination of Activity of STHdh Cells
2.12. Statistical Analysis
3. Results
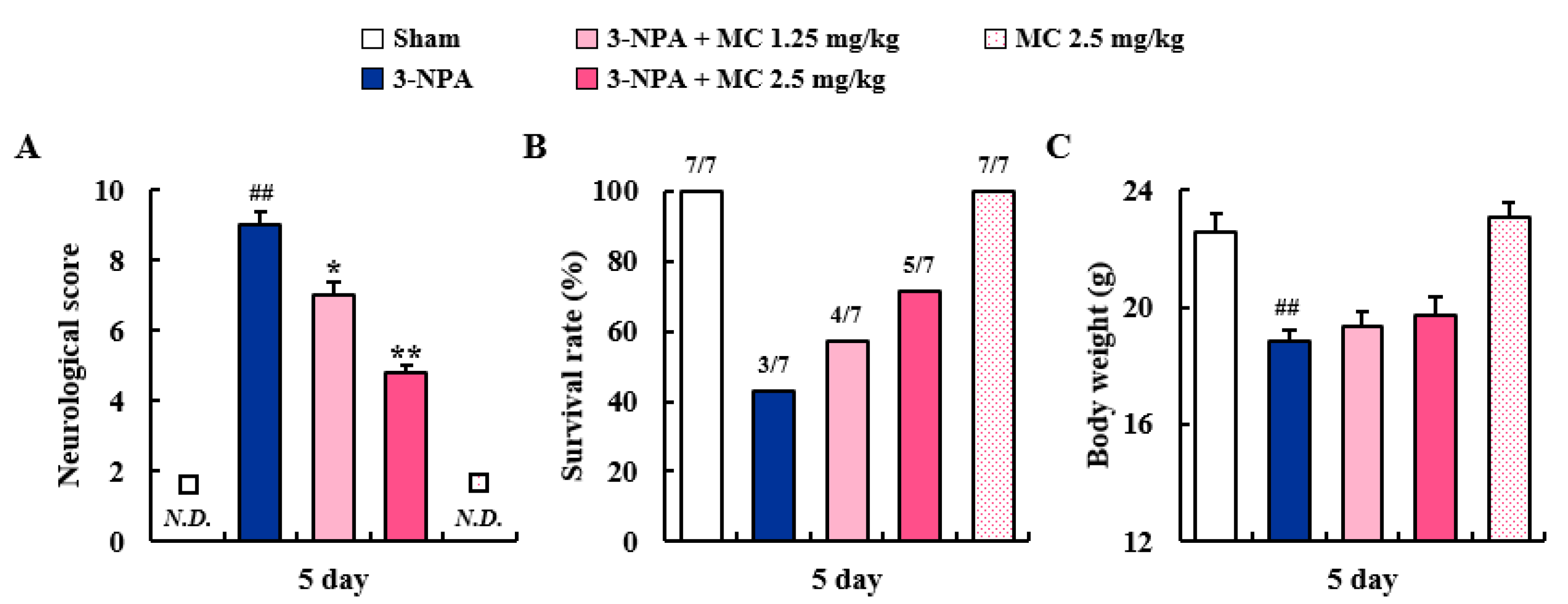
3.1. Effects of MC on Neurological Score and Survival Rate after 3-NPA Intoxication
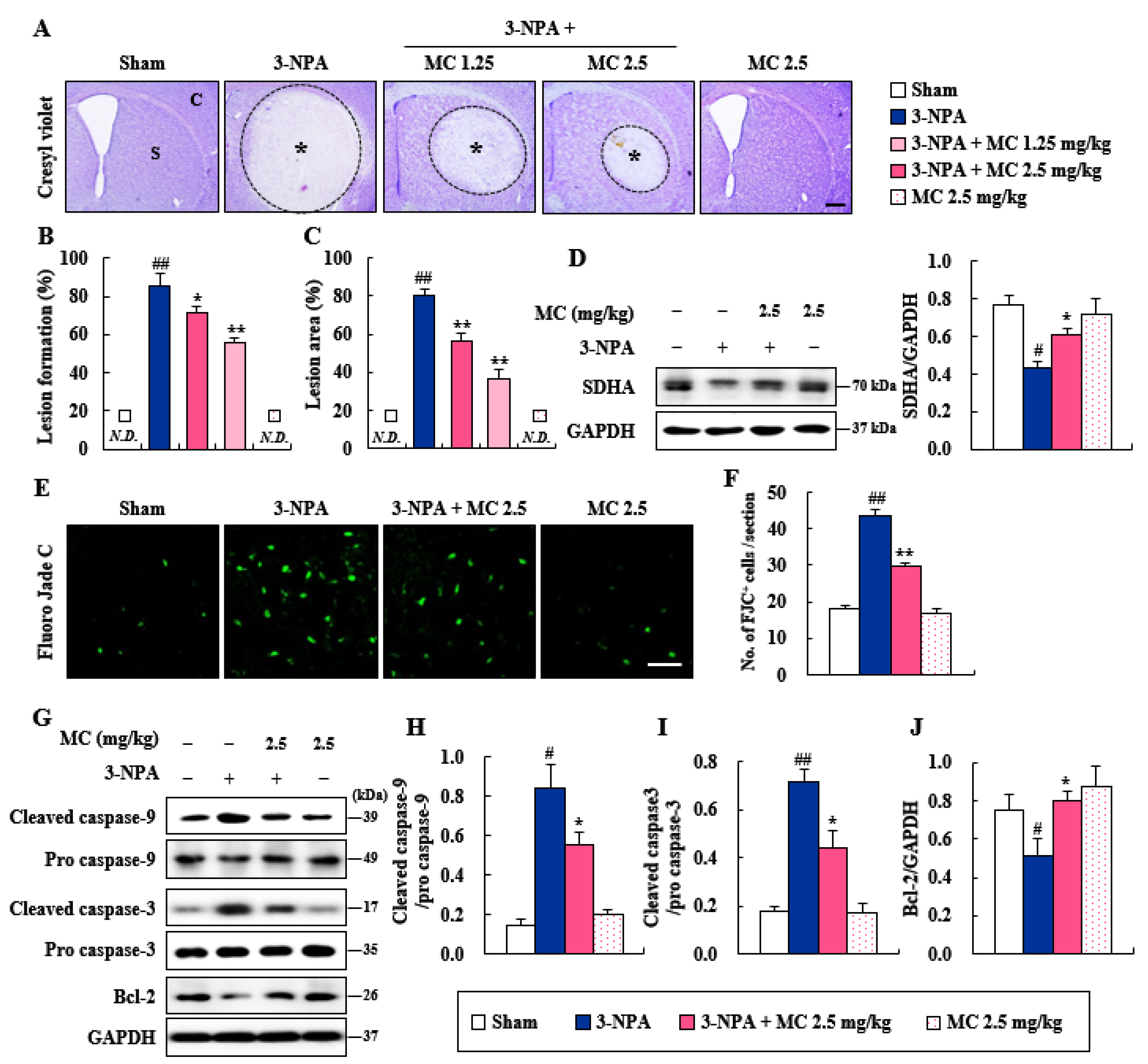
3.2. Effects of MC on Striatal Cell Death and Apoptosis Induced Following 3-NPA-Treatment
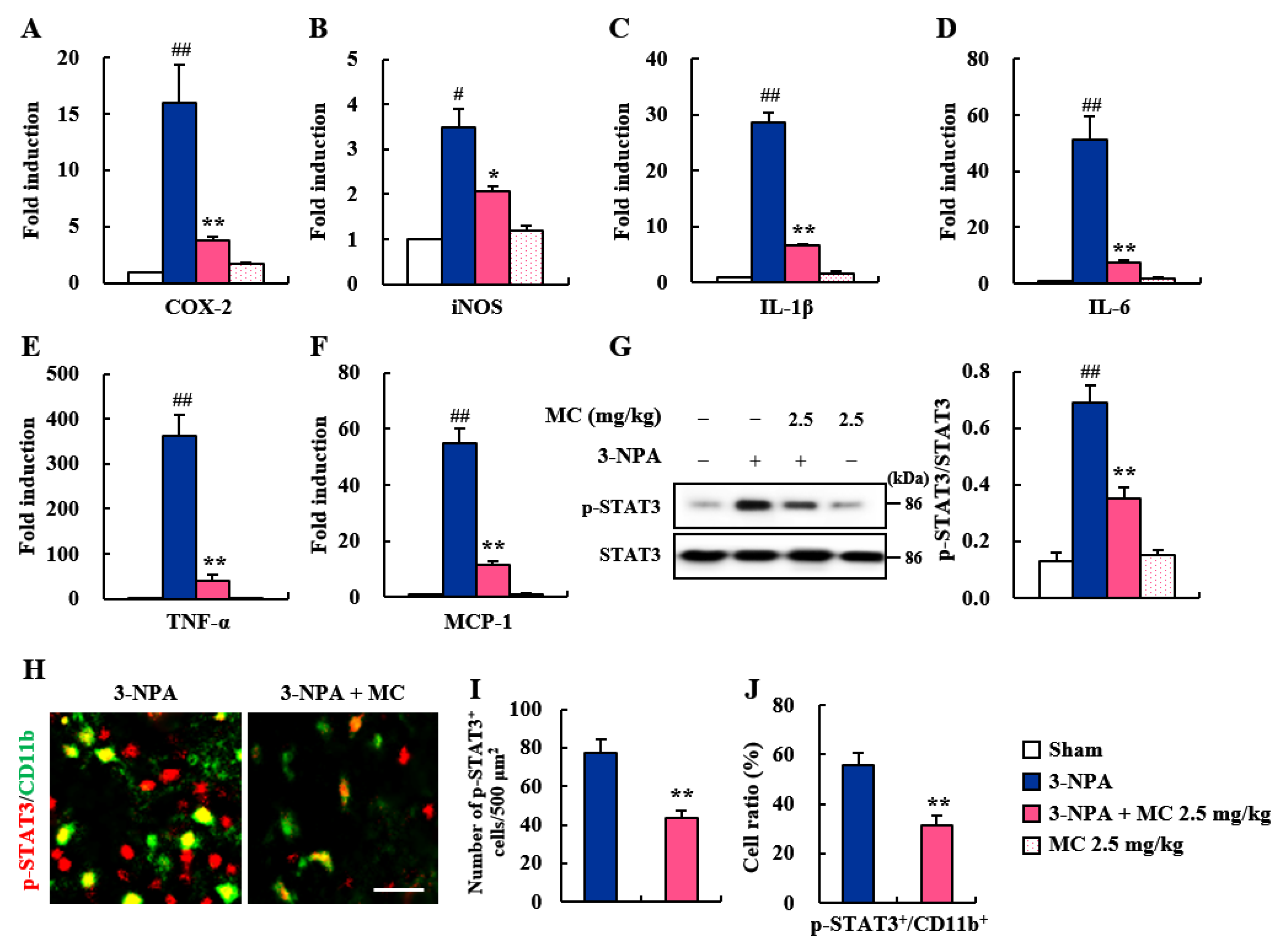
3.3. Effect of MC on Microglial Activation in the Striatum Following 3-NPA-Treatment
3.4. Effects of MC on Inflammatory Factors and STAT3 Pathways in the Striatum Following 3-NPA-Treatment
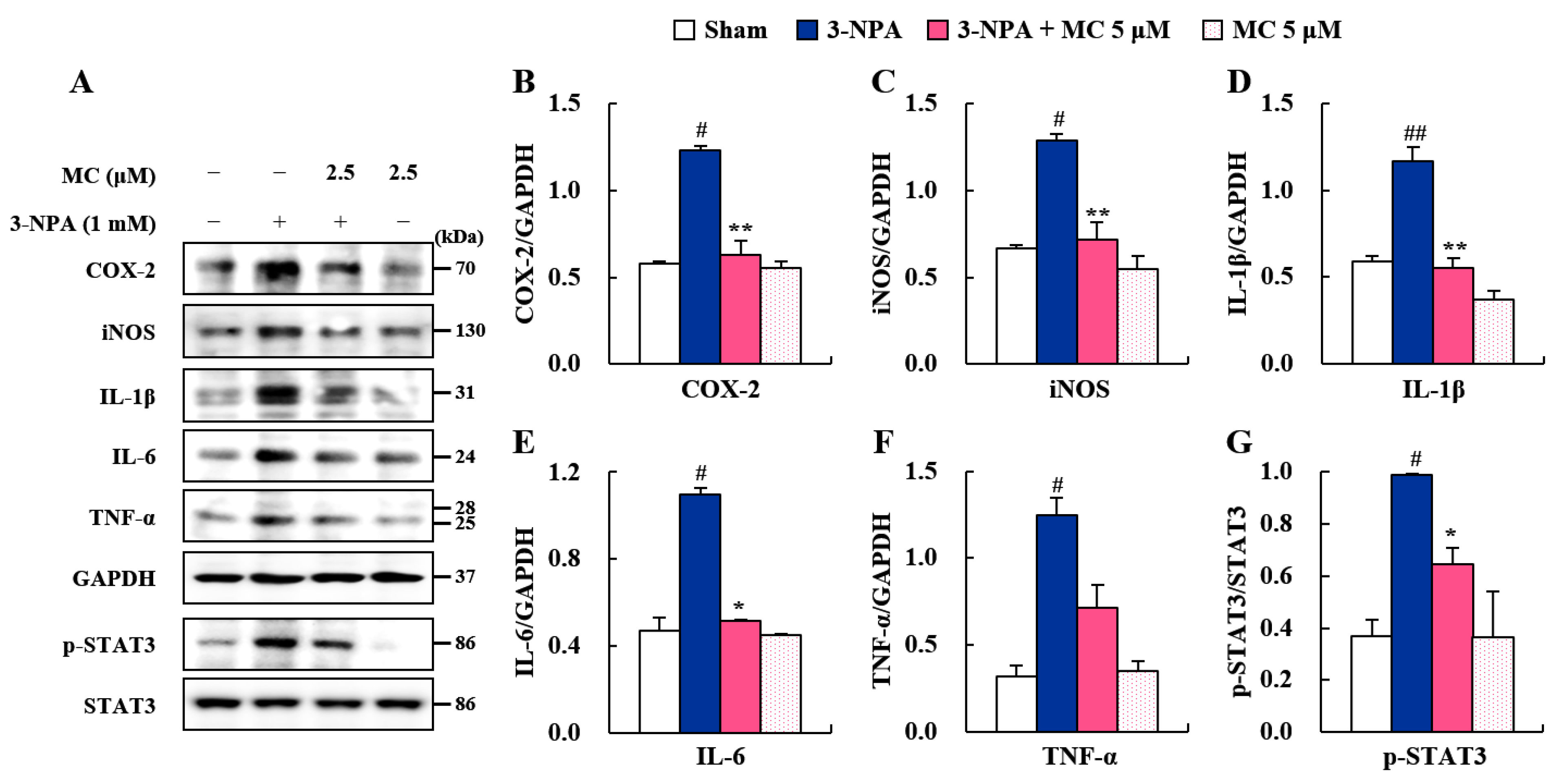
3.5. Effects of MC on Pro-Inflammatory Factors and STAT3 Pathways in 3-NPA-Induced BV2 Cells
3.6. Effect of MC on STHdh Cell Death via Microglial Downregulation by Inhibiting STAT3 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damiano, M.; Galvan, L.; Deglon, N.; Brouillet, E. Mitochondria in huntington’s disease. Biochim. Biophys. Acta. 2010, 1802, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Huntington Study Group. Tetrabenazine as antichorea therapy in huntington disease: A randomized controlled trial. Neurology 2006, 66, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, E.; Jacquard, C.; Bizat, N.; Blum, D. 3-nitropropionic acid: A mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in huntington’s disease. J. Neurochem. 2005, 95, 1521–1540. [Google Scholar] [CrossRef] [PubMed]
- Tunez, I.; Tasset, I.; La Cruz, V.P.-D.; Santamaria, A. 3-nitropropionic acid as a tool to study the mechanisms involved in huntington’s disease: Past, present and future. Molecules 2010, 15, 878–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.S.; Sun, G.; Cobessi, D.; Wang, A.C.; Shen, J.T.; Tung, E.Y.; Anderson, V.E.; Berry, E.A. 3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex ii, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006, 281, 5965–5972. [Google Scholar] [CrossRef] [Green Version]
- Trettel, F.; Rigamonti, D.; Hilditch-Maguire, P.; Wheeler, V.C.; Sharp, A.H.; Persichetti, F.; Cattaneo, E.; MacDonald, M.E. Dominant phenotypes produced by the hd mutation in sthdh(q111) striatal cells. Hum. Mol. Genet. 2000, 9, 2799–2809. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Jang, M.; Lee, M.J.; Choi, J.H.; Lee, S.J.; Kim, S.K.; Jang, D.S.; Cho, I.H. Schisandra chinensis stem ameliorates 3-nitropropionic acid-induced striatal toxicity via activation of the nrf2 pathway and inhibition of the mapks and nf-kappab pathways. Front. Pharm. 2017, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of schisandra chinensis (turcz.) baill. (chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Zaklos-Szyda, M.; Blasiak, J.; Nowak, A.; Zhang, Z.; Zhang, B. Potential of schisandra chinensis (turcz.) baill. In human health and nutrition: A review of current knowledge and therapeutic perspectives. Nutrients 2019, 11, 333. [Google Scholar] [CrossRef] [Green Version]
- Panossian, A.; Wikman, G. Pharmacology of schisandra chinensis bail: An overview of russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yuan, C. Schisandra chinensis: A comprehensive review on its phytochemicals and biological activities. Arab. J. Chem. 2021, 14, 103310. [Google Scholar] [CrossRef]
- Kim, H.M.; Ryu, B.; Lee, J.S.; Choi, J.H.; Jang, D.S. Schisandrosides a-d, dibenzocyclooctadiene lignan glucosides from the roots of schisandra chinensis. Chem. Pharm. Bull. 2015, 63, 746–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.T.; Han, Q.B.; Zheng, Y.T.; Wang, R.R.; Yang, L.M.; Lu, Y.; Sang, S.Q.; Zheng, Q.T.; Zhao, Q.S.; Sun, H.D. Structure and anti-hiv activity of micrandilactones b and c, new nortriterpenoids possessing a unique skeleton from schisandra micrantha. Chem. Commun. 2005, 23, 2936–2938. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.M.; Kim, H.J.; Choi, J.H.; Jang, D.S. Kudsuphilactone b, a nortriterpenoid isolated from schisandra chinensis fruit, induces caspase-dependent apoptosis in human ovarian cancer a2780 cells. Arch. Pharm. Res. 2017, 40, 500–508. [Google Scholar] [CrossRef]
- Luo, D.; Xie, J.Z.; Zou, L.H.; Qiu, L.; Huang, D.P.; Xie, Y.F.; Xu, H.J.; Wu, X.D. Lanostane-type triterpenoids from ganoderma applanatum and their inhibitory activities on no production in lps-induced bv-2 cells. Phytochemistry 2020, 177, 112453. [Google Scholar] [CrossRef]
- Shi, Y.S.; Xia, H.M.; Wu, C.H.; Li, C.B.; Duan, C.C.; Che, C.; Zhang, X.J.; Li, H.T.; Zhang, Y.; Zhang, X.F. Novel nortriterpenoids with new skeletons and limonoids from the fruits of evodia rutaecarpa and their bioactivities. Fitoterapia 2020, 142, 104503. [Google Scholar] [CrossRef]
- Landis, S.C.; Amara, S.G.; Asadullah, K.; Austin, C.P.; Blumenstein, R.; Bradley, E.W.; Crystal, R.G.; Darnell, R.B.; Ferrante, R.J.; Fillit, H.; et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012, 490, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.; Cho, I.H. Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the keap1-nrf2-are pathway and inhibiting the mapks and nf-kappab pathways. Mol. Neurobiol. 2016, 53, 2619–2635. [Google Scholar] [CrossRef]
- Jang, M.; Lee, M.J.; Cho, I.H. Ethyl pyruvate ameliorates 3-nitropropionic acid-induced striatal toxicity through anti-neuronal cell death and anti-inflammatory mechanisms. Brain Behav. Immun. 2014, 38, 151–165. [Google Scholar] [CrossRef]
- Jang, M.; Lee, M.J.; Kim, C.S.; Cho, I.H. Korean red ginseng extract attenuates 3-nitropropionic acid-induced huntington’s-like symptoms. Evid. Based Complement Altern. Med. 2013, 2013, 237207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Choi, J.H.; Chang, Y.; Lee, S.J.; Nah, S.Y.; Cho, I.H. Gintonin, a ginseng-derived ingredient, as a novel therapeutic strategy for huntington’s disease: Activation of the nrf2 pathway through lysophosphatidic acid receptors. Brain Behav. Immun. 2019, 80, 146–162. [Google Scholar] [CrossRef]
- Fernagut, P.O.; Diguet, E.; Stefanova, N.; Biran, M.; Wenning, G.K.; Canioni, P.; Bioulac, B.; Tison, F. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult c57bl/6 mice: Behavioural and histopathological characterisation. Neuroscience 2002, 114, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Elsevier Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Schmued, L.C.; Albertson, C.; Slikker, W., Jr. Fluoro-jade: A novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997, 751, 37–46. [Google Scholar] [CrossRef]
- Lee, M.J.; Bing, S.J.; Choi, J.; Jang, M.; Lee, G.; Lee, H.; Chang, B.S.; Jee, Y.; Lee, S.J.; Cho, I.H. Ikkbeta-mediated inflammatory myeloid cell activation exacerbates experimental autoimmune encephalomyelitis by potentiating th1/th17 cell activation and compromising blood brain barrier. Mol. Neurodegener. 2016, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Chang, B.J.; Oh, S.; Nah, S.Y.; Cho, I.H. Korean red ginseng mitigates spinal demyelination in a model of acute multiple sclerosis by downregulating p38 mitogen-activated protein kinase and nuclear factor-kappab signaling pathways. J. Ginseng. Res. 2018, 42, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jang, M.; Choi, J.; Chang, B.S.; Kim, D.Y.; Kim, S.H.; Kwak, Y.S.; Oh, S.; Lee, J.H.; Chang, B.J.; et al. Korean red ginseng and ginsenoside-rb1/-rg1 alleviate experimental autoimmune encephalomyelitis by suppressing th1 and th17 cells and upregulating regulatory t cells. Mol. Neurobiol. 2016, 53, 1977–2002. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jang, M.; Choi, J.; Lee, G.; Min, H.J.; Chung, W.S.; Kim, J.I.; Jee, Y.; Chae, Y.; Kim, S.H.; et al. Bee venom acupuncture alleviates experimental autoimmune encephalomyelitis by upregulating regulatory t cells and suppressing th1 and th17 responses. Mol. Neurobiol. 2016, 53, 1419–1445. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, M.J.; Jang, M.; Kim, E.J.; Shim, I.; Kim, H.J.; Lee, S.; Lee, S.W.; Kim, Y.O.; Cho, I.H. An oriental medicine, hyungbangpaedok-san attenuates motor paralysis in an experimental model of multiple sclerosis by regulating the t cell response. PLoS ONE 2015, 10, e0138592. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, J.; Choi, S.Y.; Oh, S.B.; Park, K.; Kim, J.S.; Karin, M.; Lee, S.J. Cpg oligodeoxynucleotides induce expression of proinflammatory cytokines and chemokines in astrocytes: The role of c-jun n-terminal kinase in cpg odn-mediated nf-kappab activation. J. Neuroimmunol. 2004, 153, 50–63. [Google Scholar] [CrossRef]
- Cho, I.H.; Hong, J.; Suh, E.C.; Kim, J.H.; Lee, H.; Lee, J.E.; Lee, S.; Kim, C.H.; Kim, D.W.; Jo, E.K.; et al. Role of microglial ikkbeta in kainic acid-induced hippocampal neuronal cell death. Brain 2008, 131, 3019–3033. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ehara, A.; Ueda, S. Application of fluoro-jade c in acute and chronic neurodegeneration models: Utilities and staining differences. Acta Histochem. Cytochem. 2009, 42, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Ikenari, T.; Kurata, H.; Satoh, T.; Hata, Y.; Mori, T. Evaluation of fluoro-jade c staining: Specificity and application to damaged immature neuronal cells in the normal and injured mouse brain. Neuroscience 2020, 425, 146–156. [Google Scholar] [CrossRef]
- Ferger, A.I.; Campanelli, L.; Reimer, V.; Muth, K.N.; Merdian, I.; Ludolph, A.C.; Witting, A. Effects of mitochondrial dysfunction on the immunological properties of microglia. J. Neuroinflammation 2010, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Lobsiger, C.S.; Cleveland, D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007, 10, 1355–1360. [Google Scholar] [CrossRef] [Green Version]
- Pavese, N.; Gerhard, A.; Tai, Y.F.; Ho, A.K.; Turkheimer, F.; Barker, R.A.; Brooks, D.J.; Piccini, P. Microglial activation correlates with severity in huntington disease: A clinical and pet study. Neurology 2006, 66, 1638–1643. [Google Scholar] [CrossRef]
- Jang, M.L.S.; Cho, I.H. Adeno-associated viral vector serotype dj-mediated overexpression of n171-82q-mutant huntingtin in the striatum of juvenile mice is a new model for huntington’s disease. Front. Cell. Neurosci. 2018, 12, 157. [Google Scholar] [CrossRef]
- Ben Haim, L.; Ceyzériat, K.; Carrillo-de Sauvage, M.A.; Aubry, F.; Auregan, G.; Guillermier, M.; Ruiz, M.; Petit, F.; Houitte, D.; Faivre, E.; et al. The jak/stat3 pathway is a common inducer of astrocyte reactivity in alzheimer’s and huntington’s diseases. J. Neurosci. 2015, 35, 2817–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.H.; Han, J.K.; Choi, M.; Kwon, Y.J.; Kim, S.J.; Yi, E.H.; Shin, J.C.; Cho, I.H.; Kim, B.H.; Jeong Kim, S.; et al. Dysfunction of microglial stat3 alleviates depressive behavior via neuron-microglia interactions. Neuropsychopharmacology 2017, 42, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Yang, S.; Huang, S.S.; Tang, B.S.; Guo, J.F. Microglial activation in the pathogenesis of huntington’s disease. Front. Aging Neurosci. 2017, 9, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, A.; Sandhir, R. Mitochondrial cofactors in experimental huntington’s disease: Behavioral, biochemical and histological evaluation. Behav. Brain Res. 2014, 261, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, A.; Ferrante, R.J.; Andreassen, O.A.; Dillmann, W.H.; Beal, M.F. Mice overexpressing 70-kda heat shock protein show increased resistance to malonate and 3-nitropropionic acid. Exp. Neurol. 2002, 176, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, E. The 3-np model of striatal neurodegeneration. Curr. Protoc. Neurosci. 2014, 67, 1–9. [Google Scholar] [CrossRef]
- Giulian, D.; Vaca, K. Inflammatory glia mediate delayed neuronal damage after ischemia in the central nervous system. Stroke 1993, 24, I84–I90. [Google Scholar]
- Yun, J.H.; Lee, D.H.; Jeong, H.S.; Kim, H.S.; Ye, S.K.; Cho, C.H. Stat3 activation in microglia exacerbates hippocampal neuronal apoptosis in diabetic brains. J. Cell Physiol. 2021, 236, 7058–7070. [Google Scholar] [CrossRef]
- Tai, Y.F.; Pavese, N.; Gerhard, A.; Tabrizi, S.J.; Barker, R.A.; Brooks, D.J.; Piccini, P. Imaging microglial activation in huntington’s disease. Brain Res. Bull. 2007, 72, 148–151. [Google Scholar] [CrossRef]
- Kabba, J.A.; Xu, Y.; Christian, H.; Ruan, W.; Chenai, K.; Xiang, Y.; Zhang, L.; Saavedra, J.M.; Pang, T. Microglia: Housekeeper of the central nervous system. Cell Mol. Neurobiol. 2017, 38, 53–71. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Holst, J.; Coffre, M.; Mielke, L.; de Pauw, A.; Lhocine, N.; Smith, A.M.; Rutschman, R.; Kaushal, D.; Shen, Y.; et al. General nature of the stat3-activated anti-inflammatory response. J. Immunol. 2006, 177, 7880–7888. [Google Scholar] [CrossRef] [Green Version]
- Clausen, B.H.; Lambertsen, K.L.; Babcock, A.A.; Holm, T.H.; Dagnaes-Hansen, F.; Finsen, B. Dagnaes-Hansen and B. Finsen. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflammation 2008, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Miyamoto, T.; Yoshida, H.; Asakawa, M.; Kawasumi, M.; Kobayashi, T.; Morioka, H.; Chiba, K.; Toyama, Y.; Yoshimura, A. Il-1beta and tnfalpha-initiated il-6-stat3 pathway is critical in mediating inflammatory cytokines and rankl expression in inflammatory arthritis. Int. Immunol. 2011, 23, 701–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabot, S.; Williams, G.; Yong, V.W. Microglial production of tnf-alpha is induced by activated t lymphocytes. Involvement of vla-4 and inhibition by interferonbeta-1b. J. Clin. Investig. 1997, 100, 604–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riazi, K.; Galic, M.A.; Kuzmiski, J.B.; Ho, W.; Sharkey, K.A.; Pittman, Q.J. Microglial activation and tnfalpha production mediate altered cns excitability following peripheral inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 17151–17156. [Google Scholar] [CrossRef] [Green Version]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, R.; Yuan, T.; Yang, Z.; Zhang, Q.; Wang, W.W.; Lin, L.B.; Zhu, M.Q.; Gao, J.M. Ulmoidol, an unusual nortriterpenoid from eucommia ulmoides oliv. Leaves prevents neuroinflammation by targeting the pu.1 transcriptional signaling pathway. Bioorg. Chem. 2021, 116, 105345. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, M.; Choi, J.H.; Jang, D.S.; Cho, I.-H. Micrandilactone C, a Nortriterpenoid Isolated from Roots of Schisandra chinensis, Ameliorates Huntington’s Disease by Inhibiting Microglial STAT3 Pathways. Cells 2023, 12, 786. https://doi.org/10.3390/cells12050786
Jang M, Choi JH, Jang DS, Cho I-H. Micrandilactone C, a Nortriterpenoid Isolated from Roots of Schisandra chinensis, Ameliorates Huntington’s Disease by Inhibiting Microglial STAT3 Pathways. Cells. 2023; 12(5):786. https://doi.org/10.3390/cells12050786
Chicago/Turabian StyleJang, Minhee, Jong Hee Choi, Dae Sik Jang, and Ik-Hyun Cho. 2023. "Micrandilactone C, a Nortriterpenoid Isolated from Roots of Schisandra chinensis, Ameliorates Huntington’s Disease by Inhibiting Microglial STAT3 Pathways" Cells 12, no. 5: 786. https://doi.org/10.3390/cells12050786
APA StyleJang, M., Choi, J. H., Jang, D. S., & Cho, I.-H. (2023). Micrandilactone C, a Nortriterpenoid Isolated from Roots of Schisandra chinensis, Ameliorates Huntington’s Disease by Inhibiting Microglial STAT3 Pathways. Cells, 12(5), 786. https://doi.org/10.3390/cells12050786







