Actin–Microtubule Crosstalk Imparts Stiffness to the Contractile Ring in Fission Yeast
Abstract
1. Introduction
2. Results
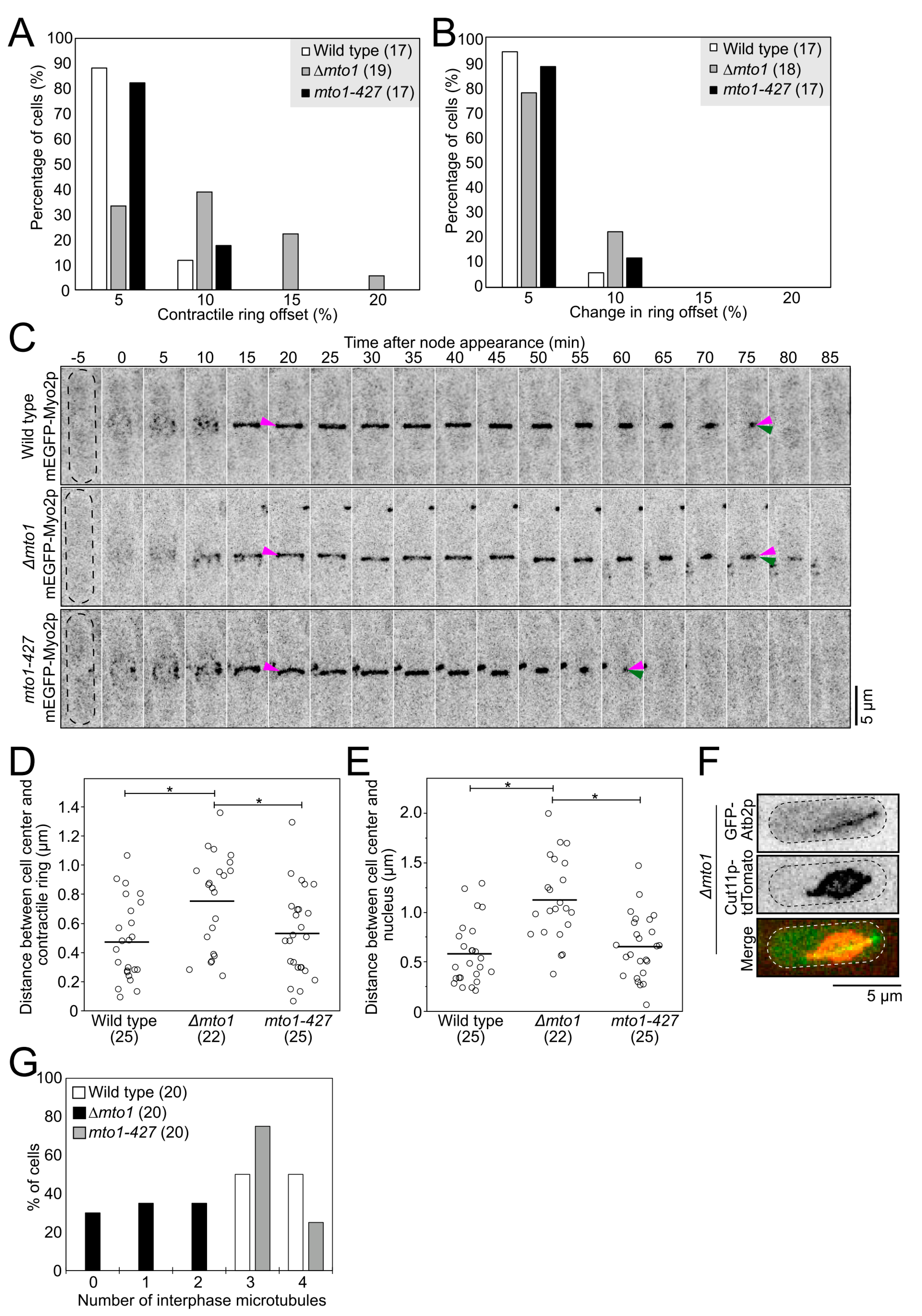
2.1. Myp2p Initiates PAA Microtubule Assembly without Directly Interacting with Mto1p
2.2. PAA Microtubules Form a Polygon of Ase1p Crosslinked Microtubules inside the Contractile Ring
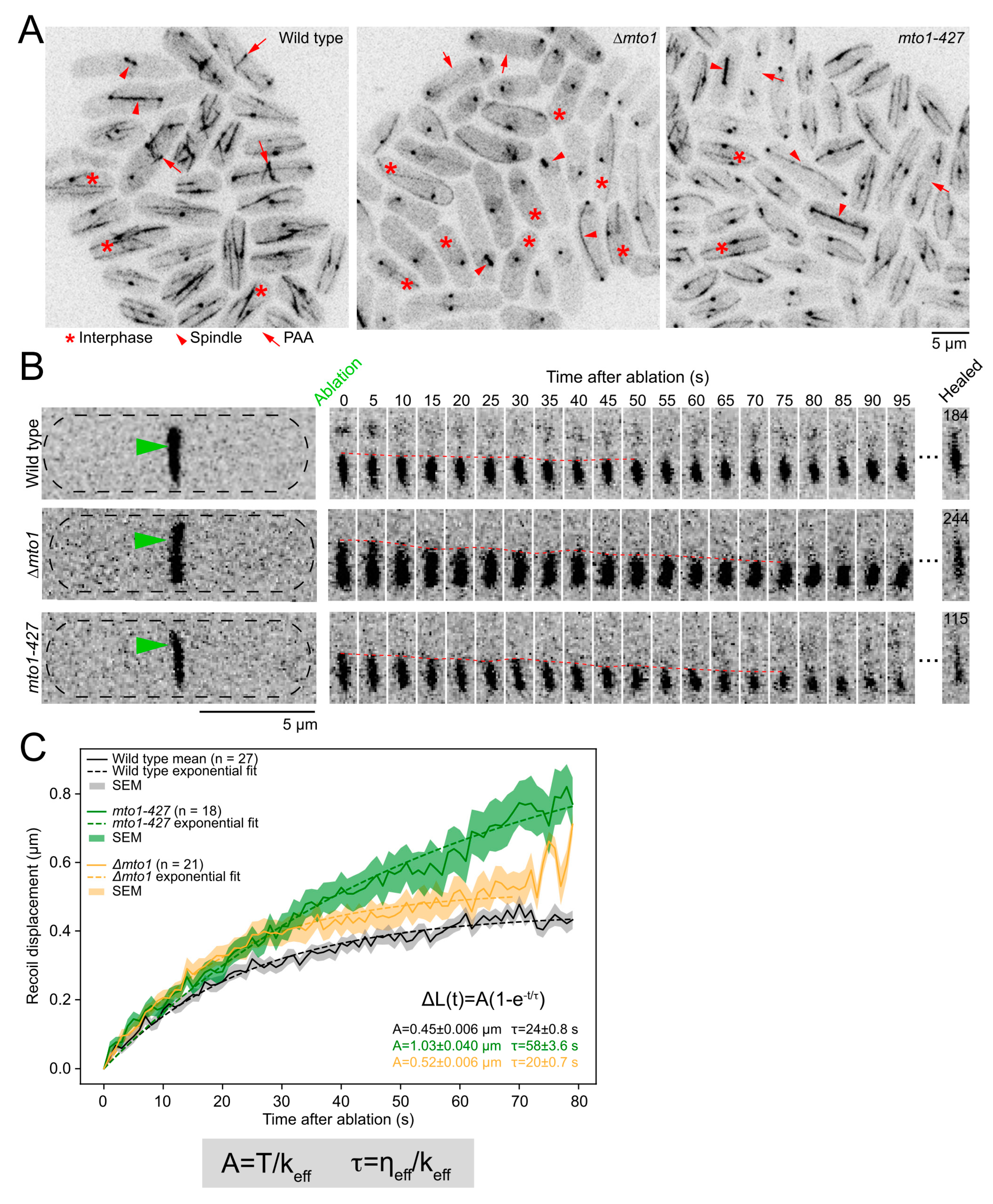
2.3. eMTOCs Impact the Mechanical Properties of the Contractile Ring
2.4. The Lack of PAA Microtubules Has No Impact on Cytokinesis
2.5. PAA Microtubules Alone Do Not Anchor the Contractile Ring
3. Discussion
4. Methods and Materials
4.1. Strains, Growing Conditions, and Genetic and Cellular Methods
4.2. Spinning-Disk Confocal Microscopy
4.3. Image Analyses
4.4. Ablation Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laan, L.; Pavin, N.; Husson, J.; Romet-Lemonne, G.; van Duijn, M.; López, M.P.; Vale, R.D.; Jülicher, F.; Reck-Peterson, S.L.; Dogterom, M. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 2012, 148, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, H.; Hyman, A.A. A cytokinesis furrow is positioned by two consecutive signals. Nature 2005, 436, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Kunda, P.; Baum, B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009, 19, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Dogterom, M.; Koenderink, G.H. Actin-microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 38–54. [Google Scholar] [CrossRef]
- Applewhite, D.A.; Grode, K.D.; Duncan, M.C.; Rogers, S.L. The actin-microtubule cross-linking activity of Drosophila Short stop is regulated by intramolecular inhibition. Mol. Biol. Cell 2013, 24, 2885–2893. [Google Scholar] [CrossRef]
- Preciado López, M.; Huber, F.; Grigoriev, I.; Steinmetz, M.O.; Akhmanova, A.; Koenderink, G.H.; Dogterom, M. Actin-microtubule coordination at growing microtubule ends. Nat. Commun. 2014, 5, 4778. [Google Scholar] [CrossRef]
- Sechi, S.; Piergentili, R.; Giansanti, M.G. Minor Kinases with Major Roles in Cytokinesis Regulation. Cells 2022, 11, 3639. [Google Scholar] [CrossRef]
- Verma, V.; Mogilner, A.; Maresca, T.J. Classical and Emerging Regulatory Mechanisms of Cytokinesis in Animal Cells. Biology 2019, 8, 55. [Google Scholar] [CrossRef]
- Pardo, M.; Nurse, P. Equatorial retention of the contractile actin ring by microtubules during cytokinesis. Science 2003, 300, 1569–1574. [Google Scholar] [CrossRef]
- Samejima, I.; Miller, V.J.; Rincon, S.A.; Sawin, K.E. Fission yeast Mto1 regulates diversity of cytoplasmic microtubule organizing centers. Curr. Biol. 2010, 20, 1959–1965. [Google Scholar] [CrossRef]
- Hagan, I.M.; Hyams, J.S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 1988, 89, 343–357. [Google Scholar] [CrossRef]
- Hagan, I.; Yanagida, M. Evidence for cell cycle-specific, spindle pole body-mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 1997, 110, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Heitz, M.J.; Petersen, J.; Valovin, S.; Hagan, I.M. MTOC formation during mitotic exit in fission yeast. J. Cell Sci. 2001, 114, 4521–4532. [Google Scholar] [CrossRef] [PubMed]
- Mana-Capelli, S.; McLean, J.R.; Chen, C.T.; Gould, K.L.; McCollum, D. The kinesin-14 Klp2 is negatively regulated by the SIN for proper spindle elongation and telophase nuclear positioning. Mol. Biol. Cell 2012, 23, 4592–4600. [Google Scholar] [CrossRef]
- Bellingham-Johnstun, K.; Commer, B.; Levesque, B.; Tyree, Z.L.; Laplante, C. Imp2p forms actin-dependent clusters and imparts stiffness to the contractile ring. Mol. Biol. Cell 2022, 33, ar145. [Google Scholar] [CrossRef]
- Moshtohry, M.; Bellingham-Johnstun, K.; Elting, M.W.; Laplante, C. Laser ablation reveals the impact of Cdc15p on the stiffness of the contractile ring. Mol. Biol. Cell 2022, 33, br9. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kuhn, J.R.; Kovar, D.R.; Pollard, T.D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 2003, 5, 723–734. [Google Scholar] [CrossRef]
- Laplante, C.; Berro, J.; Karatekin, E.; Hernandez-Leyva, A.; Lee, R.; Pollard, T.D. Three myosins contribute uniquely to the assembly and constriction of the fission yeast cytokinetic contractile ring. Curr. Biol. 2015, 25, 1955–1965. [Google Scholar] [CrossRef]
- Foethke, D.; Makushok, T.; Brunner, D.; Nedelec, F. Force- and length-dependent catastrophe activities explain interphase microtubule organization in fission yeast. Mol. Syst. Biol. 2009, 5, 241. [Google Scholar] [CrossRef]
- Loiodice, I.; Staub, J.; Setty, T.G.; Nguyen, N.P.; Paoletti, A.; Tran, P.T. Ase1p organizes antiparallel microtubule arrays during interphase and mitosis in fission yeast. Mol. Biol. Cell 2005, 16, 1756–1768. [Google Scholar] [CrossRef]
- Yamashita, A.; Sato, M.; Fujita, A.; Yamamoto, M.; Toda, T. The roles of fission yeast ase1 in mitotic cell division, meiotic nuclear oscillation, and cytokinesis checkpoint signaling. Mol. Biol. Cell 2005, 16, 1378–1395. [Google Scholar] [CrossRef]
- Schuyler, S.C.; Liu, J.Y.; Pellman, D. The molecular function of Ase1p: Evidence for a MAP-dependent midzone-specific spindle matrix. Microtubule-associated proteins. J. Cell Biol. 2003, 160, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Janson, M.E.; Loughlin, R.; Loïodice, I.; Fu, C.; Brunner, D.; Nédélec, F.J.; Tran, P.T. Crosslinkers and motors organize dynamic microtubules to form stable bipolar arrays in fission yeast. Cell 2007, 128, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Kapitein, L.C.; Janson, M.E.; van den Wildenberg, S.M.; Hoogenraad, C.C.; Schmidt, C.F.; Peterman, E.J. Microtubule-driven multimerization recruits ase1p onto overlapping microtubules. Curr. Biol. 2008, 18, 1713–1717. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.; Chang, F. Effects of {gamma}-tubulin complex proteins on microtubule nucleation and catastrophe in fission yeast. Mol. Biol. Cell 2005, 16, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- McDargh, Z.; Wang, S.; Chin, H.F.; Thiyagarajan, S.; Karatekin, E.; Pollard, T.D.; O’Shaughnessy, B. Myosins generate contractile force and maintain organization in the cytokinetic contractile ring. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cortes, J.C.; Pujol, N.; Sato, M.; Pinar, M.; Ramos, M.; Moreno, B.; Osumi, M.; Ribas, J.C.; Perez, P. Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast. PLoS Genet. 2015, 11, e1005358. [Google Scholar] [CrossRef]
- Arasada, R.; Pollard, T.D. Contractile ring stability in S. pombe depends on F-BAR protein Cdc15p and Bgs1p transport from the Golgi complex. Cell Rep. 2014, 8, 1533–1544. [Google Scholar] [CrossRef]
- Tran, P.T.; Doye, V.; Chang, F.; Inoue, S. Microtubule-dependent nuclear positioning and nuclear-dependent septum positioning in the fission yeast Schizosaccharomyces [correction of Saccharomyces] pombe. Biol. Bull. 2000, 199, 205–206. [Google Scholar] [CrossRef]
- Tran, P.T.; Marsh, L.; Doye, V.; Inoue, S.; Chang, F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 2001, 153, 397–411. [Google Scholar] [CrossRef]
- Daga, R.R.; Yonetani, A.; Chang, F. Asymmetric microtubule pushing forces in nuclear centering. Curr. Biol. 2006, 16, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Bellingham-Johnstun, K.; Anders, E.C.; Ravi, J.; Bruinsma, C.; Laplante, C. Molecular organization of cytokinesis node predicts the constriction rate of the contractile ring. J. Cell Biol. 2021, 220, e202008032. [Google Scholar] [CrossRef] [PubMed]
- Laplante, C.; Huang, F.; Tebbs, I.R.; Bewersdorf, J.; Pollard, T.D. Molecular organization of cytokinesis nodes and contractile rings by super-resolution fluorescence microscopy of live fission yeast. Proc. Natl. Acad. Sci. USA 2016, 113, E5876–E5885. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, M.R.; Laplante, C.; Chin, H.F.; Guirao, B.; Karatekin, E.; Pollard, T.D.; O’Shaughnessy, B. Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev. Cell 2014, 29, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Gittes, F.; Mickey, B.; Nettleton, J.; Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 1993, 120, 923–934. [Google Scholar] [CrossRef]
- Janson, M.E.; Dogterom, M. A bending mode analysis for growing microtubules: Evidence for a velocity-dependent rigidity. Biophys. J. 2004, 87, 2723–2736. [Google Scholar] [CrossRef]
- Roque, H.; Ward, J.J.; Murrells, L.; Brunner, D.; Antony, C. The fission yeast XMAP215 homolog Dis1p is involved in microtubule bundle organization. PLoS ONE 2010, 5, e14201. [Google Scholar] [CrossRef]
- Braun, M.; Drummond, D.R.; Cross, R.A.; McAinsh, A.D. The kinesin-14 Klp2 organizes microtubules into parallel bundles by an ATP-dependent sorting mechanism. Nat. Cell Biol. 2009, 11, 724–730. [Google Scholar] [CrossRef]
- Dundon, S.E.R.; Pollard, T.D. Microtubule nucleation promoters Mto1 and Mto2 regulate cytokinesis in fission yeast. Mol. Biol. Cell 2020, 31, 1846–1856. [Google Scholar] [CrossRef]
- Proctor, S.A.; Minc, N.; Boudaoud, A.; Chang, F. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr. Biol. 2012, 22, 1601–1608. [Google Scholar] [CrossRef]
- Bahler, J.; Wu, J.Q.; Longtine, M.S.; Shah, N.G.; McKenzie, A., 3rd; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Keeney, J.B.; Boeke, J.D. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics 1994, 136, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Miura, K. Bleach correction ImageJ. plugin for compensating the photobleaching of time-lapse sequences. F1000Research 2020, 9, 1494. [Google Scholar] [CrossRef]
- Ward, J.J.; Roque, H.; Antony, C.; Nédélec, F. Mechanical design principles of a mitotic spindle. eLife 2014, 3, e03398. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellingham-Johnstun, K.; Tyree, Z.L.; Martinez-Baird, J.; Thorn, A.; Laplante, C. Actin–Microtubule Crosstalk Imparts Stiffness to the Contractile Ring in Fission Yeast. Cells 2023, 12, 917. https://doi.org/10.3390/cells12060917
Bellingham-Johnstun K, Tyree ZL, Martinez-Baird J, Thorn A, Laplante C. Actin–Microtubule Crosstalk Imparts Stiffness to the Contractile Ring in Fission Yeast. Cells. 2023; 12(6):917. https://doi.org/10.3390/cells12060917
Chicago/Turabian StyleBellingham-Johnstun, Kimberly, Zoe L. Tyree, Jessica Martinez-Baird, Annelise Thorn, and Caroline Laplante. 2023. "Actin–Microtubule Crosstalk Imparts Stiffness to the Contractile Ring in Fission Yeast" Cells 12, no. 6: 917. https://doi.org/10.3390/cells12060917
APA StyleBellingham-Johnstun, K., Tyree, Z. L., Martinez-Baird, J., Thorn, A., & Laplante, C. (2023). Actin–Microtubule Crosstalk Imparts Stiffness to the Contractile Ring in Fission Yeast. Cells, 12(6), 917. https://doi.org/10.3390/cells12060917






