The Anti-Inflammatory Peptide TnP Is a Candidate Molecule for Asthma Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. OVA-induced Mouse Model of Asthma and TnP Treatment
2.3. Bronchoalveolar Lavage Fluid (BAL) and Cellular Analysis
2.4. Determination of Airway Hyperresponsiveness (Ahr)
2.5. Titration of OVA-Specific Abs and Total IgE by ELISA
2.6. Quantification of Cytokines and Chemokines
2.7. Histology of the Lungs
2.8. Statistical Analysis
3. Results
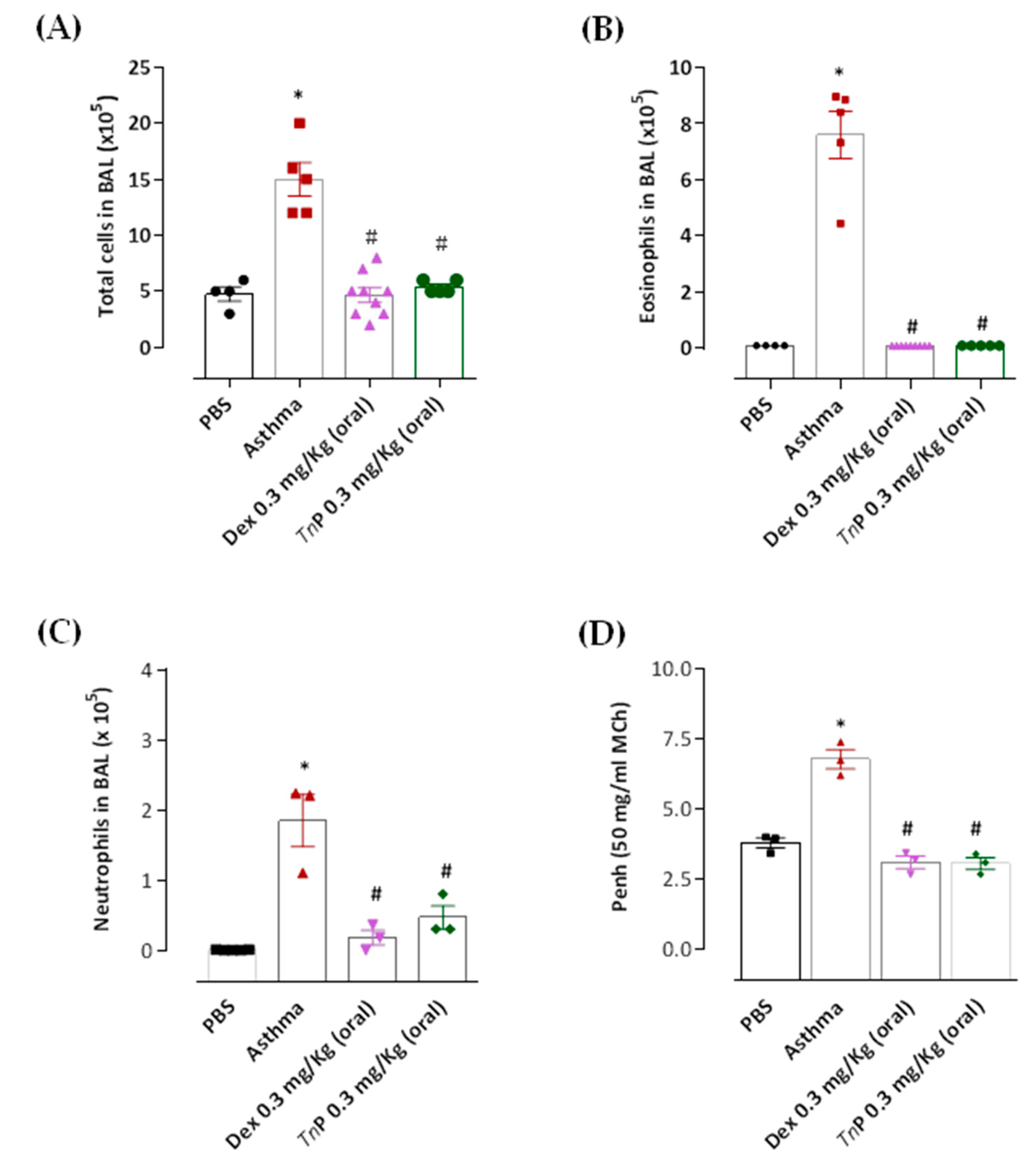
3.1. Effects of Oral Administration of TnP on Inflammatory Cell Recruitment in BAL and Ahr
3.2. TnP Treatment Attenuates Airway Remodeling Induced by the OVA Challenge
3.3. TnP Treatment Inhibits Specific Anaphylactic Antibody Production
3.4. TnP Suppresses OVA-Induced Th2-Associated Cytokine Production in BAL
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Schoettler, N.; Strek, M.E. Recent Advances in Severe Asthma: From Phenotypes to Personalized Medicine. Chest 2020, 157, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Gans, M.D.; Gavrilova, T. Understanding the immunology of asthma: Pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Kwah, J.H.; Peters, A.T. Asthma in adults: Principles of treatment. Allergy Asthma Proc. 2019, 40, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021 Executive Summary and Rationale for Key Changes. Am. J. Respir. Crit. Care Med. 2022, 205, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.; Hawiger, D. Current and Future Immunotherapies for Multiple Sclerosis. Sci. Med. 2021, 118, 334. [Google Scholar]
- Del Gatto, A.; Saviano, M.; Zaccaro, L. An Overview of Peptide-Based Molecules as Potential Drug Candidates for Multiple Sclerosis. Molecules 2021, 26, 5227. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Cabri, W.; Cantelmi, P.; Corbisiero, D.; Fantoni, T.; Ferrazzano, L.; Martelli, G.; Mattellone, A.; Tolomelli, A. Therapeutic Peptides Targeting PPI in Clinical Development: Overview, Mechanism of Action and Perspectives. Front. Mol. Biosci. 2021, 8, 697586. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Lima, C.; Pimenta, D.C.; Conceição, K.; Demasi, M.; Portaro, F.C.V. Peptídeos Cíclicos Antiinflamaórios e Antialérgicos WO2008009085A1: Monica Valdyrce Dos Anjos Lopes Ferreira; Cristália Produtos Químicos Farmacêuticos Ltd.a, Fundação de Amparo à Pesquisa Do Estado de São Paulo (FAPESP): São Paulo, Brazil, 2007. [Google Scholar]
- Komegae, E.N.; Souza, T.A.M.; Grund, L.Z.; Lima, C.; Lopes-Ferreira, M. Multiple Functional Therapeutic Effects of TnP: A Small Stable Synthetic Peptide Derived from Fish Venom in a Mouse Model of Multiple Sclerosis. PLoS ONE 2017, 12, e0171796. [Google Scholar] [CrossRef]
- Lima, C.; Eto, S.F.; Lopes-Ferreira, M. Shedding Light on the Drug–Target Prediction of the Anti-Inflammatory Peptide TnP with Bioinformatics Tools. Pharmaceuticals 2022, 15, 994. [Google Scholar] [CrossRef]
- Lima, C.; Maleski, A.L.A.; Bernardo, J.T.G.; Zelli, V.C.; Komegae, E.N.; Lopes-Ferreira, M. TnP Peptide Suppresses Experimental Autoimmune Encephalomyelitis (EAE) in a Preclinical Mouse Model. Front. Immunol. 2022, 13, 857692. [Google Scholar] [CrossRef] [PubMed]
- Falcao, M.A.P.; Banderó Walker, C.I.; Rodrigo Disner, G.; Batista-Filho, J.; Silva Soares, A.B.; Balan-Lima, L.; Lima, C.; Lopes-Ferreira, M. Knockdown of MiR-26a in Zebrafish Leads to Impairment of the Anti-Inflammatory Function of TnP in the Control of Neutrophilia. Fish Shellfish Immunol. 2021, 114, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Disner, G.R.; Falcão, M.A.P.; Lima, C.; Lopes-Ferreira, M. In Silico Target Prediction of Overexpressed MicroRNAs from Lps-Challenged Zebrafish (Danio Rerio) Treated with the Novel Anti-Inflammatory Peptide TnP. Int. J. Mol. Sci. 2021, 22, 7117. [Google Scholar] [CrossRef]
- Batista-Filho, J.; Falcão, M.A.P.; Maleski, A.L.A.; Soares, A.B.S.; Balan-Lima, L.; Disner, G.R.; Lima, C.; Lopes-Ferreira, M. Early Preclinical Screening Using Zebrafish (Danio Rerio) Reveals the Safety of the Candidate Anti-Inflammatory Therapeutic Agent TnP. Toxicol. Rep. 2021, 8, 13–22. [Google Scholar] [CrossRef]
- Van Hulst, G.; Bureau, F.; Desmet, C.J. Eosinophils as Drivers of Severe Eosinophilic Asthma: Endotypes or Plasticity? Int. J. Mol. Sci. 2021, 22, 150. [Google Scholar] [CrossRef]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma HHS Public Access. Trends Immunol. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Nabe, T. Steroid-Resistant Asthma and Neutrophils. Biol. Pharm. Bull. 2020, 43, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Halwani, R.; Al-Muhsen, S.; Al-Jahdali, H.; Hamid, Q. Role of Transforming Growth Factor-β in Airway Remodeling in Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Radermecker, C.; Louis, R.; Bureau, F.; Marichal, T. Role of Neutrophils in Allergic Asthma. Curr. Opin. Immunol. 2018, 54, 28–34. [Google Scholar] [CrossRef]
- Shefrin, A.E.; Goldman, R.D. Use of dexamethasone and prednisone in acute asthma exacerbations in pediatric patients. Can. Fam. Physician 2009, 55, 704–706. [Google Scholar] [PubMed]
- Banoth, B.; Verma, A.; Bhalla, K.; Khanna, A.; Holla, S.; Yadav, S. Comparative effectiveness of oral dexamethasone vs. oral prednisolone for acute exacerbation of asthma: A randomized control trial. J. Family Med. Prim. Care 2022, 11, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.; Makrinioti, H.; Phillips, B. Can Low-Dose Dexamethasone Be Used Instead of Prednisolone in Acute Asthma Attacks? Arch. Dis. Child 2021, 106, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and Non-Immunologic Mechanisms Leading to Airway Remodeling in Asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and Mast Cells in Allergic Disease. Nat. Med. 2014, 18, 693–704. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; Castro, M.; et al. Plasma IL6 Levels, Metabolic Dysfunction, and Asthma Severity: A Cross-Sectional Analysis of Two Cohorts. Lancet Respir. Med. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Thompson, A.; Ciccarelli, O. Towards treating progressive multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 589–590. [Google Scholar] [CrossRef]
- Garner, O.; Ramey, J.S.; Hanania, N.A. Management of Life-Threatening Asthma. Chest 2022, 162, 747–756. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V. Structure, Function, and Therapeutic Potential of Marine Bioactive Peptides. Mar. Drugs 2019, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Margelidon-Cozzolino, V.; Tsicopoulos, A.; Chenivesse, C.; de Nadai, P. Role of Th17 Cytokines in Airway Remodeling in Asthma and Therapy Perspectives. Front. Allergy 2022, 3. [Google Scholar] [CrossRef] [PubMed]
- Brannan, J.D.; Lougheed, M.D. Airway Hyperresponsiveness in Asthma: Mechanisms, Clinical Significance, and Treatment. Front. Physiol. 2012, 3, 460. [Google Scholar] [CrossRef]
- Banno, A.; Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. Bidirectional interaction of airway epithelial remodeling and inflammation in asthma. Clin. Sci. 2020, 134, 1063–1079. [Google Scholar] [CrossRef]
- Kardas, G.; Kuna, P.; Panek, M. Biological Therapies of Severe Asthma and Their Possible Effects on Airway Remodeling. Front. Immunol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Halayko, A.J.; Gosens, R.; Panettieri, R.A., Jr.; Camoretti-Mercado, B.; Penn, R.B. ATS Assembly on Respiratory Structure and Function. An Official American Thoracic Society Research Statement: Current Challenges Facing Research and Therapeutic Advances in Airway Remodeling. Am. J. Respir. Crit. Care Med. 2017, 195, e4–e19. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, C.; Falcão, M.A.P.; Pinto, F.J.; Bernardo, J.T.G.; Lopes-Ferreira, M. The Anti-Inflammatory Peptide TnP Is a Candidate Molecule for Asthma Treatment. Cells 2023, 12, 924. https://doi.org/10.3390/cells12060924
Lima C, Falcão MAP, Pinto FJ, Bernardo JTG, Lopes-Ferreira M. The Anti-Inflammatory Peptide TnP Is a Candidate Molecule for Asthma Treatment. Cells. 2023; 12(6):924. https://doi.org/10.3390/cells12060924
Chicago/Turabian StyleLima, Carla, Maria Alice Pimentel Falcão, Felipe Justiniano Pinto, Jefferson Thiago Gonçalves Bernardo, and Monica Lopes-Ferreira. 2023. "The Anti-Inflammatory Peptide TnP Is a Candidate Molecule for Asthma Treatment" Cells 12, no. 6: 924. https://doi.org/10.3390/cells12060924
APA StyleLima, C., Falcão, M. A. P., Pinto, F. J., Bernardo, J. T. G., & Lopes-Ferreira, M. (2023). The Anti-Inflammatory Peptide TnP Is a Candidate Molecule for Asthma Treatment. Cells, 12(6), 924. https://doi.org/10.3390/cells12060924








