Uncovering Actions of Type 3 Deiodinase in the Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Procedures
2.2. Biochemical Parameters
2.3. Hepatic Histology and Immunofluorescence
2.4. Inflammatory Markers
2.5. Oxidative Stress Parameters
2.5.1. Carbonyl Content
2.5.2. Malondialdehyde Levels
2.5.3. Sulfhydryl Content
2.6. Antioxidant Defenses
2.6.1. Reduced Glutathione Concentrations
2.6.2. Glutathione Peroxide Activities
2.6.3. Glutathione Reductase Activities
2.6.4. Superoxide Dismutase Activities
2.7. Real-Time PCR
2.8. Mitochondrial Capacity
2.8.1. Mitochondrial Respiratory Parameters (Oxygen Consumption)
2.8.2. Complexes II, II-III, and IV
2.8.3. Activities of Glutamate Dehydrogenase (GDH), α-Ketoglutarate Dehydrogenase (α-KGDH) and Succinate Dehydrogenase (SDH)
2.9. Western Blot Analyses
2.10. D3 Activity
2.11. Statistical Analysis
3. Results
3.1. Sample Characteristics and Biochemical Parameters
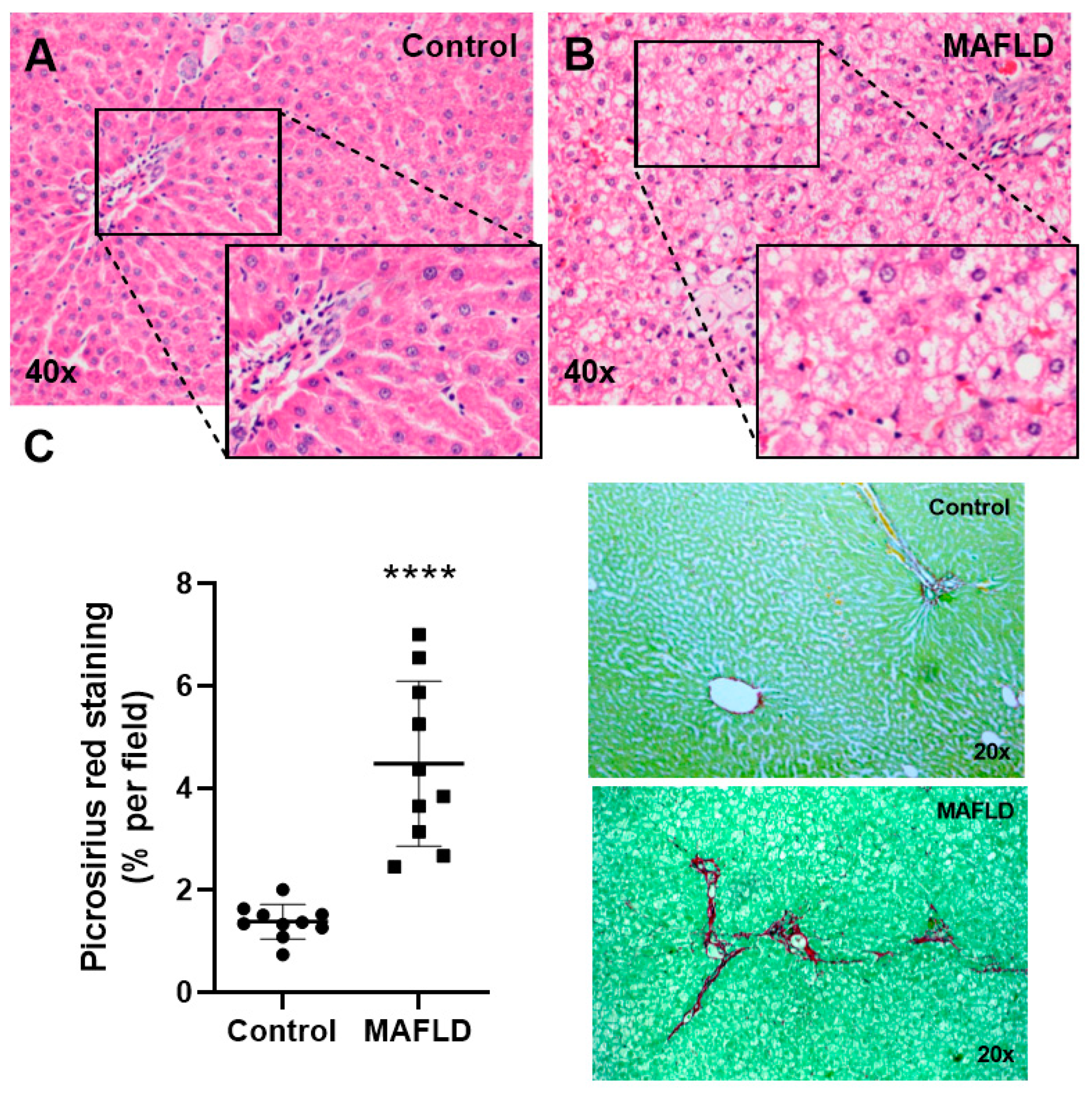
3.2. MAFLD Generates Histopathological Changes
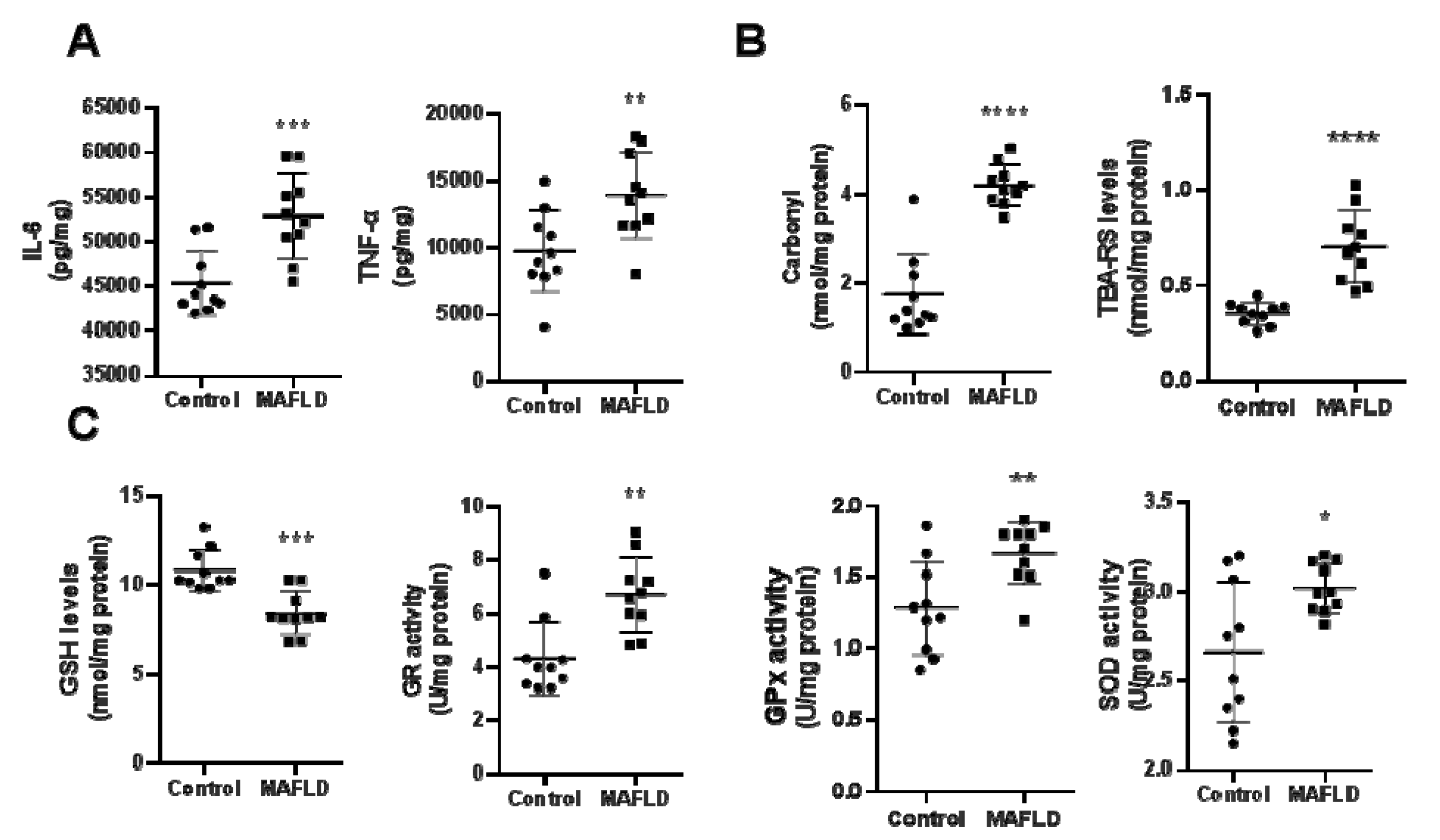
3.3. MAFLD Induces Inflammatory and REDOX State Parameters
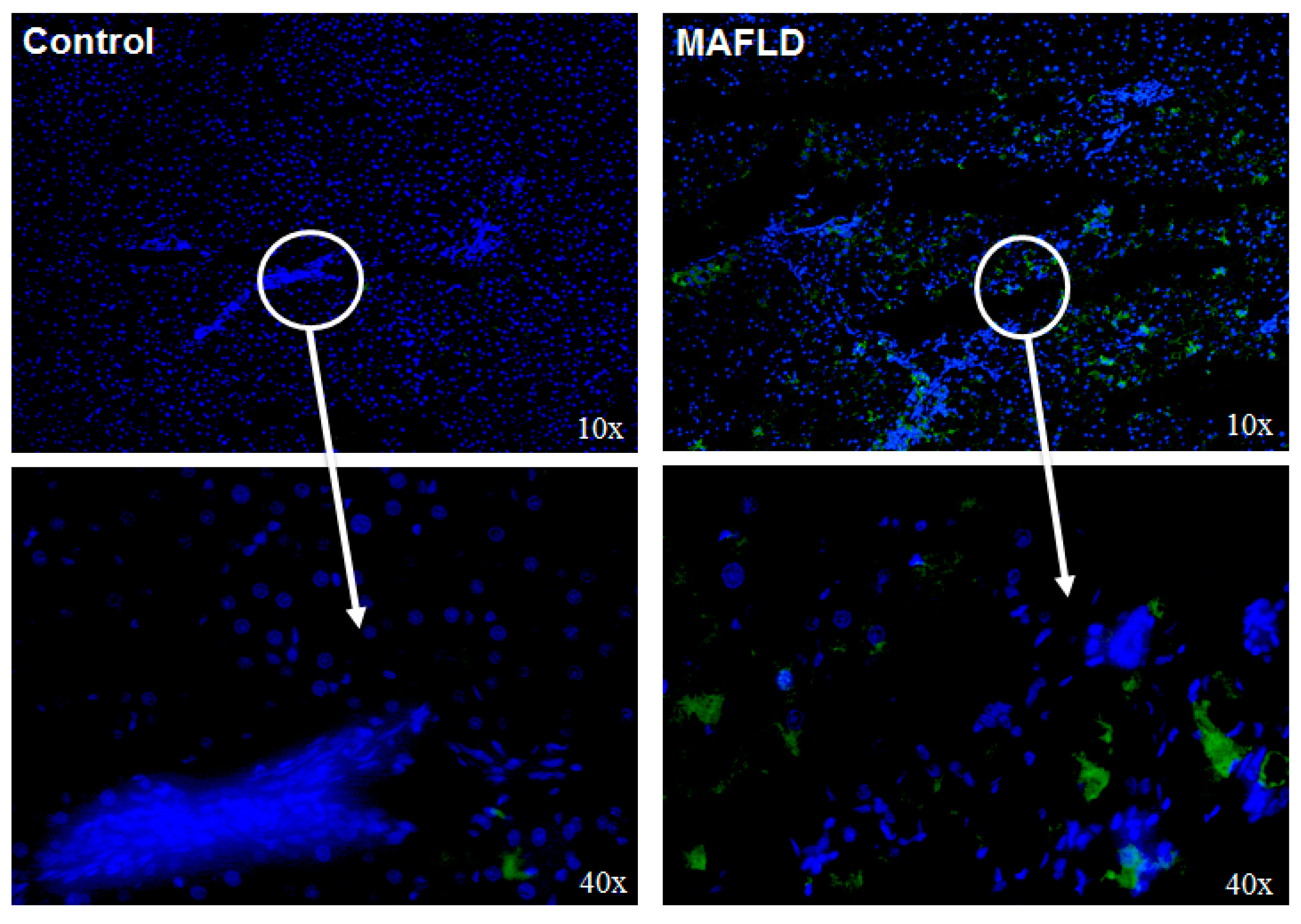
3.4. Altered Mechanisms of Thyroid Hormone Metabolism in MAFLD
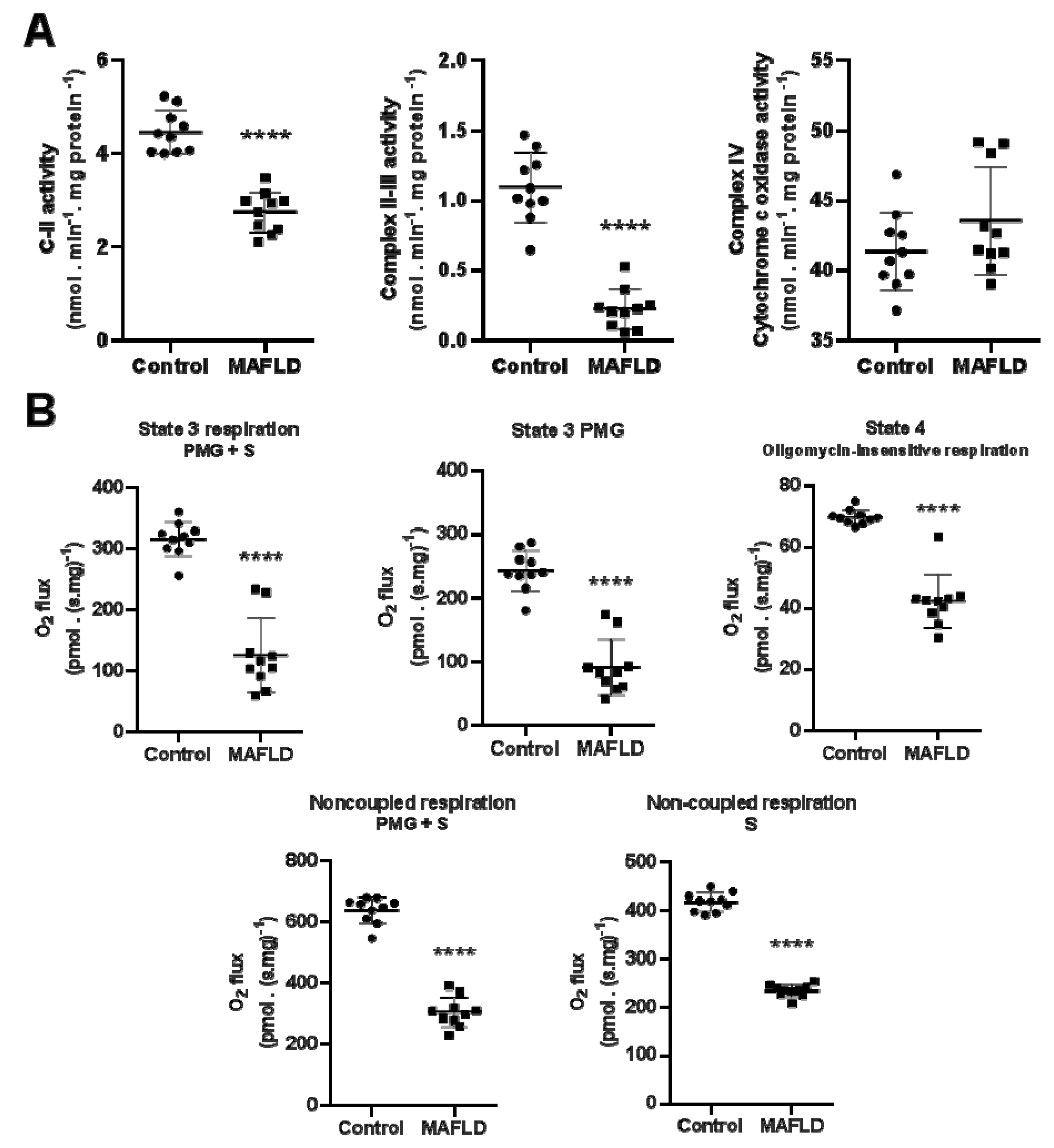
3.5. Mitochondrial Complexes Are Affected in MAFLD
3.6. MAFLD Induced Impairment of Mitochondrial Capacity
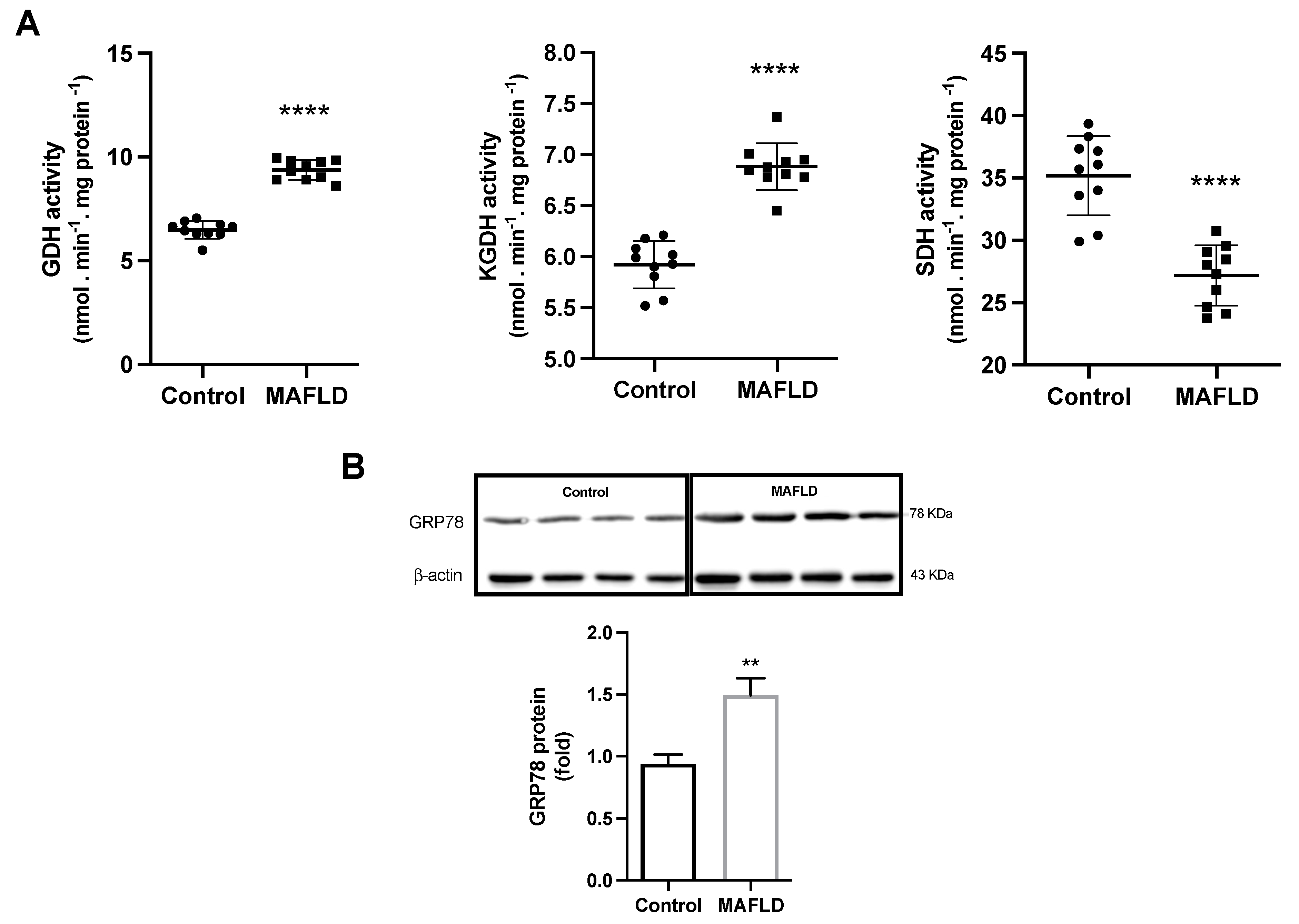
3.7. MAFLD Induces GDH, α-KGDH, SDH Enzymes and Endoplasmic Reticulum Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.E.; Koh, T.J.L.; Tang, A.S.P.; Quek, J.; Yong, J.N.; Tay, P.; Tan, D.J.H.; Lim, W.H.; Lin, S.Y.; Huang, D.; et al. Global Prevalence and Clinical Characteristics of Metabolic Associated Fatty Liver Disease. A Meta-Analysis and Systematic Review of 10,739,607 Individuals. J. Clin. Endocrinol. Metab. 2022, 107, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Hones, G.S.; Rakov, H.; Logan, J.; Liao, X.H.; Werbenko, E.; Pollard, A.S.; Praestholm, S.M.; Siersbaek, M.S.; Rijntjes, E.; Gassen, J.; et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E11323–E11332. [Google Scholar] [CrossRef]
- Peeters, R.P.; Visser, T.J. Metabolism of Thyroid Hormone. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Ogawa-Wong, A.; Carmody, C.; Le, K.; Marschner, R.A.; Larsen, P.R.; Zavacki, A.M.; Wajner, S.M. Modulation of Deiodinase Types 2 and 3 during Skeletal Muscle Regeneration. Metabolites 2022, 12, 612. [Google Scholar] [CrossRef]
- Lehnen, T.E.; Marschner, R.; Dias, F.; Maia, A.L.; Wajner, S.M. Oxidative remote induction of type 3 deiodinase impacts nonthyroidal illness syndrome. J. Endocrinol. 2020, 246, 237–246. [Google Scholar] [CrossRef]
- Raftopoulos, Y.; Gagne, D.J.; Papasavas, P.; Hayetian, F.; Maurer, J.; Bononi, P.; Caushaj, P.F. Improvement of hypothyroidism after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes. Surg. 2004, 14, 509–513. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol. Metab. 2014, 25, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; You, S.H.; Zhou, J.; Siddique, M.M.; Bay, B.H.; Zhu, X.; Privalsky, M.L.; Cheng, S.Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Zhou, J.; Tripathi, M.; Yau, W.W.; Boelen, A.; Singh, B.K.; Yen, P.M. Early induction of hepatic deiodinase type 1 inhibits hepatosteatosis during NAFLD progression. Mol. Metab. 2021, 53, 101266. [Google Scholar] [CrossRef] [PubMed]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The interplay between thyroid and liver: Implications for clinical practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef]
- Vallejo, C.G.; Seguido, A.M.; Testillano, P.S.; Risueno, M.C. Thyroid hormone regulates tubulin expression in mammalian liver. Effects of deleting thyroid hormone receptor-alpha or -beta. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E87–E94. [Google Scholar] [CrossRef] [PubMed]
- NIH. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washingtion, DC, USA, 1996. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef]
- Longo, L.; Tonin Ferrari, J.; Rampelotto, P.H.; Hirata Dellavia, G.; Pasqualotto, A.; Oliveira, P.C.; Thadeu Schmidt Cerski, C.; Reverbel da Silveira, T.; Uribe-Cruz, C.; Alvares-da-Silva, M.R. Gut Dysbiosis and Increased Intestinal Permeability Drive microRNAs, NLRP-3 Inflammasome and Liver Fibrosis in a Nutritional Model of Non-Alcoholic Steatohepatitis in Adult Male Sprague Dawley Rats. Clin. Exp. Gastroenterol. 2020, 13, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Solari, J.I.G.; Filippi-Chiela, E.; Pilar, E.S.; Nunes, V.; Gonzalez, E.A.; Figueiro, F.; Andrade, C.F.; Klamt, F. Damage-associated molecular patterns (DAMPs) related to immunogenic cell death are differentially triggered by clinically relevant chemotherapeutics in lung adenocarcinoma cells. BMC Cancer 2020, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, A.; Viegas, C.M.; Tonin, A.M.; Busanello, E.N.; Grings, M.; Moura, A.P.; Leipnitz, G.; Wajner, M. Disturbance of redox homeostasis by ornithine and homocitrulline in rat cerebellum: A possible mechanism of cerebellar dysfunction in HHH syndrome. Life Sci. 2013, 93, 161–168. [Google Scholar] [CrossRef]
- Yagi, K. Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol. Biol. 1998, 108, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.W.; Armstrong, D. Reduced glutathione and glutathione disulfide. Methods Mol. Biol. 1998, 108, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A. Glutathione peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Marklund, S.L. Product of extracellular-superoxide dismutase catalysis. FEBS Lett. 1985, 184, 237–239. [Google Scholar] [CrossRef]
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle: New perspectives of mitochondrial physiology. Int. J. Biochem. Cell Biol. 2009, 41, 1837–1845. [Google Scholar] [CrossRef]
- Roginski, A.C.; Wajner, A.; Cecatto, C.; Wajner, S.M.; Castilho, R.F.; Wajner, M.; Amaral, A.U. Disturbance of bioenergetics and calcium homeostasis provoked by metabolites accumulating in propionic acidemia in heart mitochondria of developing rats. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165682. [Google Scholar] [CrossRef]
- Rustin, P.; Chretien, D.; Bourgeron, T.; Gerard, B.; Rotig, A.; Saudubray, J.M.; Munnich, A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 1994, 228, 35–51. [Google Scholar] [CrossRef]
- Fischer, J.C.; Ruitenbeek, W.; Berden, J.A.; Trijbels, J.M.; Veerkamp, J.H.; Stadhouders, A.M.; Sengers, R.C.; Janssen, A.J. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin. Chim. Acta 1985, 153, 23–36. [Google Scholar] [CrossRef]
- Melo, D.R.; Mirandola, S.R.; Assuncao, N.A.; Castilho, R.F. Methylmalonate impairs mitochondrial respiration supported by NADH-linked substrates: Involvement of mitochondrial glutamate metabolism. J. Neurosci. Res. 2012, 90, 1190–1199. [Google Scholar] [CrossRef]
- Tretter, L.; Adam-Vizi, V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 2000, 20, 8972–8979. [Google Scholar] [CrossRef]
- Fuziwara, C.S.; Kimura, E.T. High iodine blocks a Notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFbeta in thyroid follicular cells. Thyroid 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Huang, S.A.; Mulcahey, M.A.; Crescenzi, A.; Chung, M.; Kim, B.W.; Barnes, C.; Kuijt, W.; Turano, H.; Harney, J.; Larsen, P.R. Transforming growth factor-beta promotes inactivation of extracellular thyroid hormones via transcriptional stimulation of type 3 iodothyronine deiodinase. Mol. Endocrinol. 2005, 19, 3126–3136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sinkó, R.; Mohácsik, P.; Kővári, D.; Penksza, V.; Wittmann, G.; Mácsai, L.; Fonseca, T.L.; Bianco, A.C.; Fekete, C.; Gereben, B. Different Hypothalamic Mechanisms Control Decreased Circulating Thyroid Hormone Levels in Infection and Fasting-Induced Non-Thyroidal Illness Syndrome in Male Thyroid Hormone Action Indicator Mice. Thyroid 2022, 33, 109–118. [Google Scholar] [CrossRef]
- Lehnen, T.E.; Santos, M.V.; Lima, A.; Maia, A.L.; Wajner, S. N-Acetylcysteine Prevents Low T3 Syndrome and Attenuates Cardiac Dysfunction in a Male Rat Model of Myocardial Infarction. Endocrinology 2017, 158, 1502–1510. [Google Scholar] [CrossRef][Green Version]
- Vidart, J.; Wajner, S.M.; Leite, R.S.; Manica, A.; Schaan, B.; Larsen, P.R.; Maia, A.L. N-acetylcysteine administration prevents nonthyroidal illness syndrome in patients with acute myocardial infarction: A randomized clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, 4537–4545. [Google Scholar] [CrossRef] [PubMed]
- Wajner, S.M.; Goemann, I.M.; Bueno, A.L.; Larsen, P.R.; Maia, A.L. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J. Clin. Investig. 2011, 121, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Dalan, R.; Cao, Y.; Bee, Y.M.; Chandran, K.; Cho, L.W.; Soh, S.B.; Teo, E.K.; Toh, S.-A.; Leow, M.K.S.; et al. Low-Dose Levothyroxine Reduces Intrahepatic Lipid Content in Patients With Type 2 Diabetes Mellitus and NAFLD. J. Clin. Endocrinol. Metab. 2018, 103, 2698–2706. [Google Scholar] [CrossRef]
- Ge, Y.; Yang, Y.; Jiang, Y.; Feng, C.; Li, B.; Sun, J.; Tang, X.; Shi, Y.; Le, G. Oxidized Pork Induces Hepatic Steatosis by Impairing Thyroid Hormone Function in Mice. Mol. Nutr. Food Res. 2022, 66, e2100602. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Canesi, L.; Salis, A.; Damonte, G.; Compalati, A.D.; Goglia, F.; Gallo, G.; Vergani, L. 3,5-diiodo-L-thyronine modifies the lipid droplet composition in a model of hepatosteatosis. Cell. Physiol. Biochem. 2014, 33, 344–356. [Google Scholar] [CrossRef]
- Iannucci, L.F.; Cioffi, F.; Senese, R.; Goglia, F.; Lanni, A.; Yen, P.M.; Sinha, R.A. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci. Rep. 2017, 7, 2023. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.L.; Cordeiro, A.; Oliveira, L.S.; de Paula, G.S.M.; Faustino, L.C.; Ortiga-Carvalho, T.M.; Oliveira, K.J.; Pazos-Moura, C.C. Thyroid hormone contributes to the hypolipidemic effect of polyunsaturated fatty acids from fish oil: In vivo evidence for cross talking mechanisms. J. Endocrinol. 2011, 211, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, I.; Gottschalk, B.; Jarc, A.; Bresilla, D.; Rost, R.; Obermayer-Pietsch, B.; Graier, W.F.; Madreiter-Sokolowski, C.T. T3-induced enhancement of mitochondrial Ca2+ uptake as a boost for mitochondrial metabolism. Free Radic. Biol. Med. 2022, 181, 197–208. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Di Girolamo, D.; Dentice, M. Metabolic Effects of the Intracellular Regulation of Thyroid Hormone: Old Players, New Concepts. Front. Endocrinol. 2018, 9, 474. [Google Scholar] [CrossRef]
- Caddeo, A.; Kowalik, M.A.; Serra, M.; Rapposelli, S.; Columbano, A.; Perra, A. TG68, a Novel Thyroid Hormone Receptor-beta Agonist for the Treatment of NAFLD. Int. J. Mol. Sci. 2021, 22, 13105. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.; Moussa, S.E.; McCarty, K.; Frias, J.P.; Taub, R.; Alkhouri, N. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH. Hepatol. Commun. 2021, 5, 573–588. [Google Scholar] [CrossRef]
- Romitti, M.; Ceolin, L.; Siqueira, D.R.; Ferreira, C.V.; Wajner, S.M.; Maia, A.L. Signaling pathways in follicular cell-derived thyroid carcinomas. Int. J. Oncol. 2013, 42, 19–28. [Google Scholar] [CrossRef]
- Zhou, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Camat, M.D.; James, A.; Wu, Y.; Ching, J.; Kovalik, J.-P.; et al. Thyroid Hormone Decreases Hepatic Steatosis, Inflammation, and Fibrosis in a Dietary Mouse Model of Nonalcoholic Steatohepatitis. Thyroid 2022, 32, 725–738. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Gottschalk, B.; Sokolowski, A.A.; Malli, R.; Graier, W.F. Dynamic Control of Mitochondrial Ca2+ Levels as a Survival Strategy of Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 614668. [Google Scholar] [CrossRef] [PubMed]
- Berardi, M.J.; Chou, J. Fatty acid flippase activity of UCP2 is essential for its proton transport in mitochondria. Cell Metab. 2014, 20, 541–552. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-kappaB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Wulf, A.; Harneit, A.; Kröger, M.; Kebenko, M.; Wetzel, M.G.; Weitzel, J.M. T3-mediated expression of PGC-1α via a far upstream located thyroid hormone response element. Mol. Cell. Endocrinol. 2008, 287, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, A.; Cellière, G.; Henkel, S.G.; Driesch, D.; Hoehme, S.; Hofmann, U.; Zellmer, S.; Godoy, P.; Sachinidis, A.; Blaszkewicz, M.; et al. Model-guided identification of a therapeutic strategy to reduce hyperammonemia in liver diseases. J. Hepatol. 2016, 64, 860–871. [Google Scholar] [CrossRef]
- Sinha, A.K.; Diricx, M.; Chan, L.P.; Liew, H.J.; Kumar, V.; Blust, R.; De Boeck, G. Expression pattern of potential biomarker genes related to growth, ion regulation and stress in response to ammonia exposure, food deprivation and exercise in common carp (Cyprinus carpio). Aquat. Toxicol. 2012, 122–123, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F.; Kircheis, G.; Hilger, N.; McPhail, M.J. Efficacy of l-Ornithine l-Aspartate for the Treatment of Hepatic Encephalopathy and Hyperammonemia in Cirrhosis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Exp. Hepatol. 2018, 8, 301–313. [Google Scholar] [CrossRef]
- Karaca, M.; Martin-Levilain, J.; Grimaldi, M.; Li, L.; Dizin, E.; Emre, Y.; Maechler, P. Liver Glutamate Dehydrogenase Controls Whole-Body Energy Partitioning Through Amino Acid-Derived Gluconeogenesis and Ammonia Homeostasis. Diabetes 2018, 67, 1949–1961. [Google Scholar] [CrossRef]

| Primers | Foward | Reverse |
|---|---|---|
| D1 | 5′-ATTTGACCAGTTCAAGAGACTCGTAG-3′ | 5′-GGCGTGAGCTTCTTCAATGTA-3′ |
| D3 | 5′-TTCCAGAGCCAGCACATCCT-3′ | 5′-ACGTCGCGCTGGTACTTAGTG-3′ |
| Ucp2 | 5′-TCAACTGTACTGAGCTGGTGACCTA-3′ | 5′-GGAGGTCGTCTGTCATGAGGTT-3′ |
| Ciclofilin A | 5′-GTCAACCCCACCGTGTTCTTC-3′ | 5′-ACTTGCCACCAGTGCCATTATG-3′ |
| Variables | Control | MAFLD | p Value |
|---|---|---|---|
| Weight (g) | 535.5 (±45.14) | 644.2 (±42.15) | <0.01 |
| Glucose (mg/dL) | 278.2 (±56.76) | 353.4 (±64.30) | <0.05 |
| Triglycerides (mg/dL) | 79.33 (±14.71) | 105.6 (±23.71) | <0.05 |
| LDL cholesterol (mg/dL) | 16.65 (±2.74) | 23.41 (±6.63) | <0.05 |
| HDL cholesterol (mg/dL) | 53.23 (±9.77) | 31.27 (±6.63) | <0.01 |
| Total cholesterol (mg/dL) | 78.74 (±11.72) | 101.8 (±27.54) | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marschner, R.A.; Roginski, A.C.; Ribeiro, R.T.; Longo, L.; Álvares-da-Silva, M.R.; Wajner, S.M. Uncovering Actions of Type 3 Deiodinase in the Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Cells 2023, 12, 1022. https://doi.org/10.3390/cells12071022
Marschner RA, Roginski AC, Ribeiro RT, Longo L, Álvares-da-Silva MR, Wajner SM. Uncovering Actions of Type 3 Deiodinase in the Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Cells. 2023; 12(7):1022. https://doi.org/10.3390/cells12071022
Chicago/Turabian StyleMarschner, Rafael Aguiar, Ana Cristina Roginski, Rafael Teixeira Ribeiro, Larisse Longo, Mário Reis Álvares-da-Silva, and Simone Magagnin Wajner. 2023. "Uncovering Actions of Type 3 Deiodinase in the Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)" Cells 12, no. 7: 1022. https://doi.org/10.3390/cells12071022
APA StyleMarschner, R. A., Roginski, A. C., Ribeiro, R. T., Longo, L., Álvares-da-Silva, M. R., & Wajner, S. M. (2023). Uncovering Actions of Type 3 Deiodinase in the Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Cells, 12(7), 1022. https://doi.org/10.3390/cells12071022








