Loss-of-Function Variants in DRD1 in Infantile Parkinsonism-Dystonia
Abstract
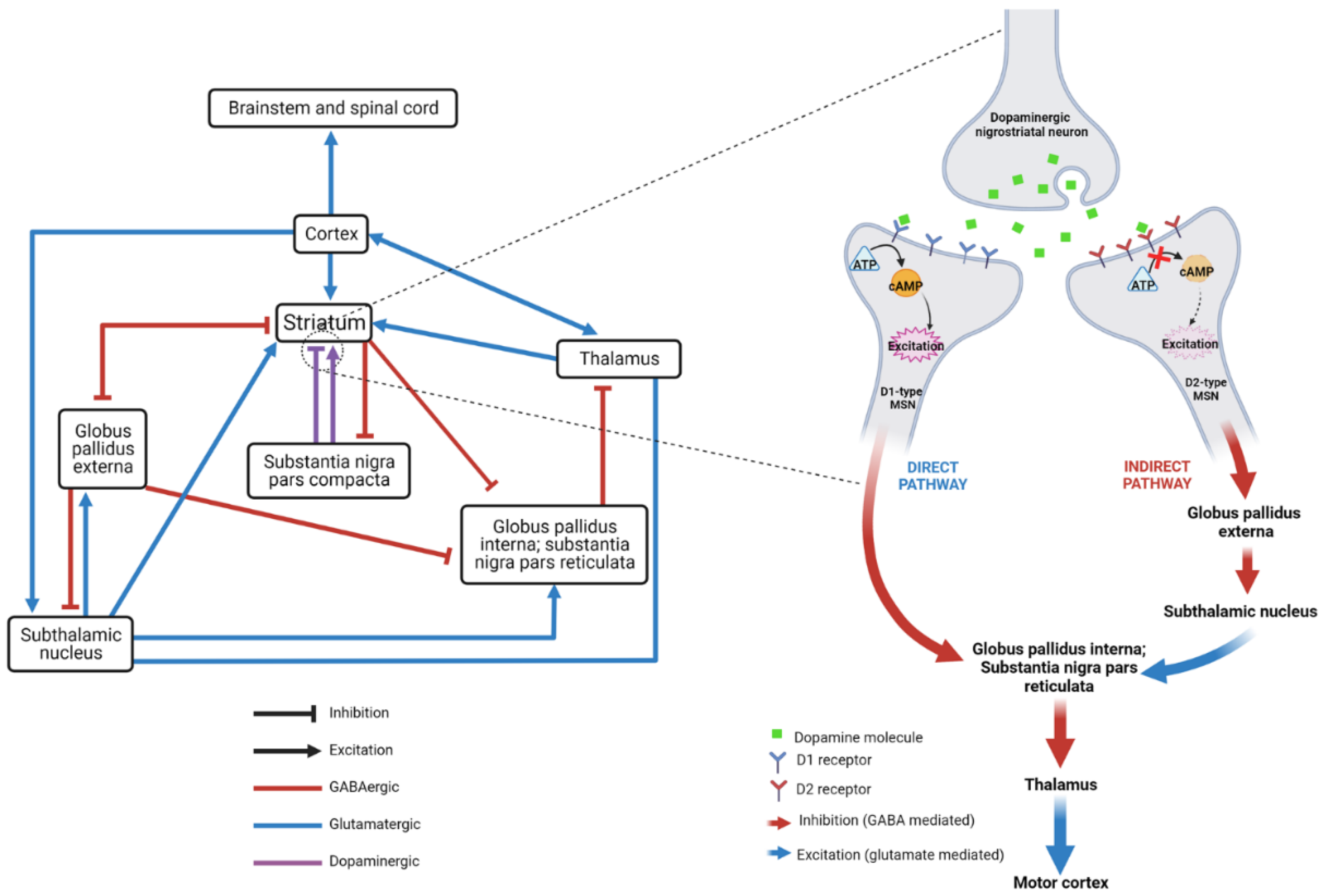
:1. Introduction
2. Materials and Methods
2.1. Ethics and Consent
2.2. Clinical Characterisation
2.3. Molecular Genetic Analysis
2.4. Site-Directed Mutagenesis, Cell Culture and Transfection
2.5. Western Blotting
2.6. D1 Surface Expression by Biotinylation
2.7. D1 Surface Expression by Immunofluorescence
2.8. cAMP Assay
2.9. Radiolabelled Ligand Binding Assay
2.10. Molecular Modelling
3. Results
3.1. Clinical Phenotyping Reveals a Complex Infantile Parkinsonism-Dystonia Phenotype
3.2. Molecular Genetic Analysis Identifies DRD1 as a Candidate Gene
3.3. DRD1-T37K Showed No Statistical Differences in Total Protein Expression and Cell Surface Localisation
3.4. DRD1-T37K Affected the cAMP Response on D1 Activation
3.5. Homology Modelling of DRD1-T37K Predicted Altered Ligand Binding, Confirmed In Vitro
3.6. DRD1-T37K Function Cannot Be Rescued by Dopamine Agonists
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Weitz, A.J.; Bernal-Casas, D.; Duffy, B.A.; Choy, M.K.; Kravitz, A.V.; Kreitzer, A.C.; Lee, J.H. Activation of Direct and Indirect Pathway Medium Spiny Neurons Drives Distinct Brain-wide Responses. Neuron 2016, 91, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.M.; Westlind-Danielsson, A. Dopamine receptors: Molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther. 1994, 64, 291–370. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.K. Molecular Profiling of Striatonigral and Striatopallidal Medium Spiny Neurons. In Past, Present, and Future, 1st ed.; International Review of Neurobiology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 89, pp. 1–35. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.L.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.; Papandreou, A.; Heales, S.J.; Kurian, M.A. Monoamine neurotransmitter disorders-Clinical advances and future perspectives. Nat. Rev. Neurol. 2015, 11, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.B.; Bezerra, M.A.; Rocha, M.E.; Barreto, G.E.; Kohlmeier, K.A. Higher zinc concentrations in hair of Parkinson’s disease are associated with psychotic complications and depression. J. Neural Transm. 2019, 126, 1291–1301. [Google Scholar] [CrossRef]
- Kaalund, S.S.; Newburn, E.N.; Ye, T.; Tao, R.; Li, C.; Deep-Soboslay, A.; Herman, M.M.; Hyde, T.M.; Weinberger, D.R.; Lipska, B.K.; et al. Contrasting changes in DRD1 and DRD2 splice variant expression in schizophrenia and affective disorders, and associations with SNPs in postmortem brain. Mol. Psychiatry 2014, 19, 1258–1266. [Google Scholar] [CrossRef]
- van der Weijden, M.; Rodriguez-Contreras, D.; Delnooz, C.; Robinson, B.; Condon, A.; Kielhold, M.; Stormezand, G.N.; Ma, K.Y.; Dufke, C.; Williams, J.T.; et al. A Gain-of-Function Variant in Dopamine D2 Receptor and Progressive Chorea and Dystonia Phenotype. Mov. Disord. 2021, 36, 729–739. [Google Scholar] [CrossRef]
- Mencacci, N.E.; Steel, D.; Magrinelli, F.; Hsu, J.; Sarmiento, I.; Schifferli, M.; Muñoz, D.; Stefanis, L.; Lubbe, S.J.; Wood, N.W.; et al. Childhood-Onset Chorea Caused by a Recurrent De Novo DRD2 Variant. Mov. Disord. 2021, 36, 1472–1473. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Fang, L.; Xu, X. Using SOAPaligner for Short Reads Alignment. Curr. Protoc. Bioinform. 2013, 12, 1–17. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Cif, L.; Demailly, D.; Lin, J.P.; Barwick, K.E.; Sa, M.; Abela, L.; Malhotra, S.; Chong, W.K.; Steel, D.; Sanchis-Juan, A.; et al. KMT2B-related disorders: Expansion of the phenotypic spectrum and long-term efficacy of deep brain stimulation. Brain 2020, 143, 3242–3261. [Google Scholar] [CrossRef]
- Fitzgerald, T.W.; Gerety, S.S.; Jones, W.D.; van Kogelenberg, M.; King, D.A.; McRae, J.; Morley, K.I.; Parthiban, V.; Al-Turki, S.; Ambridge, K.; et al. Large-scale discovery of novel genetic causes of developmental disorders. Nature 2015, 519, 223–228. [Google Scholar]
- Gahl, W.A.; Markello, T.C.; Toro, C.; Fajardo, K.F.; Sincan, M.; Gill, F.; Carlson-Donohoe, H.; Gropman, A.; Pierson, T.M.; Golas, G.; et al. The NIH undiagnosed diseases program: Insights into rare diseases. Genet. Med. 2012, 14, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Smedley, D.; Smith, K.R.; Martin, A.; Thomas, E.A.; McDonagh, E.M.; Cipriani, V.; Ellingford, J.M.; Arno, G.; Tucci, A.; Vandrovcova, J.; et al. 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care—Preliminary Report. N. Engl. J. Med. 2021, 385, 1868–1880. [Google Scholar]
- Schobers, G.; Schieving, J.H.; Yntema, H.G.; Pennings, M.; Pfundt, R.; Derks, R.; Hofste, T.; de Wijs, I.; Wieskamp, N.; Heuvel, S.V.D.; et al. Reanalysis of exome negative patients with rare disease: A pragmatic workflow for diagnostic applications. Genome Med. 2022, 14, 1–10. [Google Scholar] [CrossRef]
- Zech, M.; Jech, R.; Boesch, S.; Škorvánek, M.; Weber, S.; Wagner, M.; Zhao, C.; Jochim, A.; Necpál, J.; Dincer, Y.; et al. Monogenic variants in dystonia: An exome-wide sequencing study. Lancet Neurol. 2020, 19, 908–918. [Google Scholar] [CrossRef]
- Kurian, M.A.; Zhen, J.; Cheng, S.-Y.; Li, Y.; Mordekar, S.R.; Jardine, P.; Morgan, N.V.; Meyer, E.; Tee, L.; Pasha, S.; et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J. Clin. Investig. 2009, 119, 1595–1603. [Google Scholar] [CrossRef]
- Kurian, M.A.; Li, Y.; Zhen, J.; Meyer, E.; Hai, N.; Christen, H.J.; Hoffmann, G.F.; Jardine, P.; von Moers, A.; Mordekar, S.R.; et al. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: An observational cohort and experimental study. Lancet Neurol. 2011, 10, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Carta, E.; Chung, S.-K.; James, V.M.; Robinson, A.; Gill, J.L.; Remy, N.; Vanbellinghen, J.-F.; Drew, C.J.G.; Cagdas, S.; Cameron, D.; et al. Mutations in the GlyT2 gene (SLC6A5) are a second major cause of startle disease. J. Biol. Chem. 2012, 287, 28975–28985. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Ortiz, M.E.; Seo, Y.; Posavi, M.; Cordon, M.C.; Clark, E.; Jain, N.; Charan, R.; Gallagher, M.D.; Unger, T.L.; Amari, N.; et al. GPNMB confers risk for Parkinson’s disease through interaction with α-synuclein. Science 2022, 377, eabk0637. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Weisburd, B.; Thomas, B.; Solomonson, M.; Ruderfer, D.M.; Kavanagh, D.; Hamamsy, T.; Lek, M.; Samocha, K.E.; Cummings, B.B.; et al. The ExAC browser: Displaying reference data information from over 60,000 exomes. Nucleic Acids Res. 2017, 45, D840–D845. [Google Scholar] [CrossRef] [Green Version]
- Wegler, M.; Jia, X.; Alders, M.; Bouman, A.; Chen, J.; Duan, X.; Lauzon, J.L.; Mathijssen, I.B.; Sticht, H.; Syrbe, S.; et al. De novo variants in the PABP domain of PABPC1 lead to developmental delay. Genet. Med. 2022, 24, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- do Costa, M.C.; Paulson, H.L. Toward understanding Machado-Joseph disease. Prog. Neurobiol. 2012, 97, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Oo, J.A.; Irmer, B.; Günther, S.; Warwick, T.; Pálfi, K.; Ponce, J.I.; Teichmann, T.; Pflüger-Müller, B.; Gilsbach, R.; Brandes, R.P.; et al. ZNF354C is a transcriptional repressor that inhibits endothelial angiogenic sprouting. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.N.; Velic, A.; Soliz, J.; Shi, Y.; Li, K.; Wang, S.; Weaver, J.L.; Sen, J.; Abbott, S.B.G.; Lazarenko, R.M.; et al. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 2015, 348, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Bockaert, J.; Pin, J.P. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevich, V.V.; Gurevich, E.V. Plethora of functions packed into 45 kDa arrestins: Biological implications and possible therapeutic strategies. Cell. Mol. Life Sci. 2019, 76, 4413–4421. [Google Scholar] [CrossRef]
- Xu, M.; Moratalla, R.; Gold, L.H.; Hiroi, N.; Koob, G.F.; Graybiel, A.M.; Tonegawa, S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 1994, 79, 729–742. [Google Scholar] [CrossRef]
- Tran, A.H.; Tamura, R.; Uwano, T.; Kobayashi, T.; Katsuki, M.; Ono, T. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc. Natl. Acad. Sci. USA 2005, 102, 2117–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Sato, A.; Kitsukawa, T.; Momiyama, T.; Yamamori, T.; Sasaoka, T. Distinct motor impairments of dopamine D1 and D2 receptor knockout mice revealed by three types of motor behavior. Front. Integr. Neurosci. 2014, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Durieux, P.F.; Schiffmann, S.N.; D’Exaerde, A.D.K. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 2012, 31, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Lee, H.G.; Han, K.A. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci. 2007, 27, 7640–7647. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.; Hidalgo, S.; Campusano, J.M. Dop1R1, a type 1 dopaminergic receptor expressed in Mushroom Bodies, modulates Drosophila larval locomotion. PLoS ONE 2020, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kottler, B.; Faville, R.; Bridi, J.C.; Hirth, F. Inverse Control of Turning Behavior by Dopamine D1 Receptor Signaling in Columnar and Ring Neurons of the Central Complex in Drosophila. Curr. Biol. 2019, 29, 567–577. [Google Scholar] [CrossRef]
- Luderman, K.D.; Jain, P.; Benjamin Free, R.; Conroy, J.L.; Aubé, J.; Sibley, D.R.; Frankowski, K.J. Development of pyrimidone D1 dopamine receptor positive allosteric modulators. Bioorganic Med. Chem. Lett. 2021, 31, 127696. [Google Scholar] [CrossRef]
- Lewis, M.A.; Hunihan, L.; Watson, J.; Gentles, R.G.; Hu, S.; Huang, Y.; Bronson, J.; Macor, J.E.; Beno, B.R.; Ferrante, M.; et al. Discovery of D1 dopamine receptor positive allosteric modulators: Characterization of pharmacology and identification of residues that regulate species selectivity. J. Pharmacol. Exp. Ther. 2015, 354, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, T.S.; Gupta, N.; San Sebastian, W.; Imamura-Ching, J.; Viehoever, A.; Grijalvo-Perez, A.; Fay, A.J.; Seth, N.; Lundy, S.M.; Seo, Y.; et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, G.; Rickhag, M.; Leo, D.; Lycas, M.D.; Ridderstrøm, P.H.; Weikop, P.; Lilja, J.H.; Rifes, P.; Herborg, F.; Woldbye, D.; et al. Disruption of the PDZ domain–binding motif of the dopamine transporter uniquely alters nanoscale distribution, dopamine homeostasis, and reward motivation. J. Biol. Chem. 2021, 297, 101361. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Barral, S.; Barrigon, C.D.L.F.; Lignani, G.; Erdem, F.A.; Wallings, R.; Privolizzi, R.; Rossignoli, G.; Alrashidi, H.; Heasman, S.; et al. Gene therapy restores dopamine transporter expression and ameliorates pathology in iPSC and mouse models of infantile parkinsonism. Sci. Transl. Med. 2021, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hyland, K.; Surtees, R.A.H.; Heales, S.J.R.; Bowron, A.; Howells, D.W.; Smith, I. Cerebrospinal fluid concentrations of pterins and metabolites of serotonin and dopamine in a pediatric reference population. Pediatr Res. 1993, 34, 10–14. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reid, K.M.; Steel, D.; Nair, S.; Bhate, S.; Biassoni, L.; Sudhakar, S.; Heys, M.; Burke, E.; Kamsteeg, E.-J.; Genomics England Research Consortium; et al. Loss-of-Function Variants in DRD1 in Infantile Parkinsonism-Dystonia. Cells 2023, 12, 1046. https://doi.org/10.3390/cells12071046
Reid KM, Steel D, Nair S, Bhate S, Biassoni L, Sudhakar S, Heys M, Burke E, Kamsteeg E-J, Genomics England Research Consortium, et al. Loss-of-Function Variants in DRD1 in Infantile Parkinsonism-Dystonia. Cells. 2023; 12(7):1046. https://doi.org/10.3390/cells12071046
Chicago/Turabian StyleReid, Kimberley M., Dora Steel, Sanjana Nair, Sanjay Bhate, Lorenzo Biassoni, Sniya Sudhakar, Michelle Heys, Elizabeth Burke, Erik-Jan Kamsteeg, Genomics England Research Consortium, and et al. 2023. "Loss-of-Function Variants in DRD1 in Infantile Parkinsonism-Dystonia" Cells 12, no. 7: 1046. https://doi.org/10.3390/cells12071046
APA StyleReid, K. M., Steel, D., Nair, S., Bhate, S., Biassoni, L., Sudhakar, S., Heys, M., Burke, E., Kamsteeg, E.-J., Genomics England Research Consortium, Hameed, B., Zech, M., Mencacci, N. E., Barwick, K., Topf, M., & Kurian, M. A. (2023). Loss-of-Function Variants in DRD1 in Infantile Parkinsonism-Dystonia. Cells, 12(7), 1046. https://doi.org/10.3390/cells12071046






