Evaluation of 2D and 3D Erythroid Differentiation Protocols Using Sickle Cell Disease and Healthy Donor Induced Pluripotent Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation and Characterization of iPSCs
2.2. Culture of iPSCs
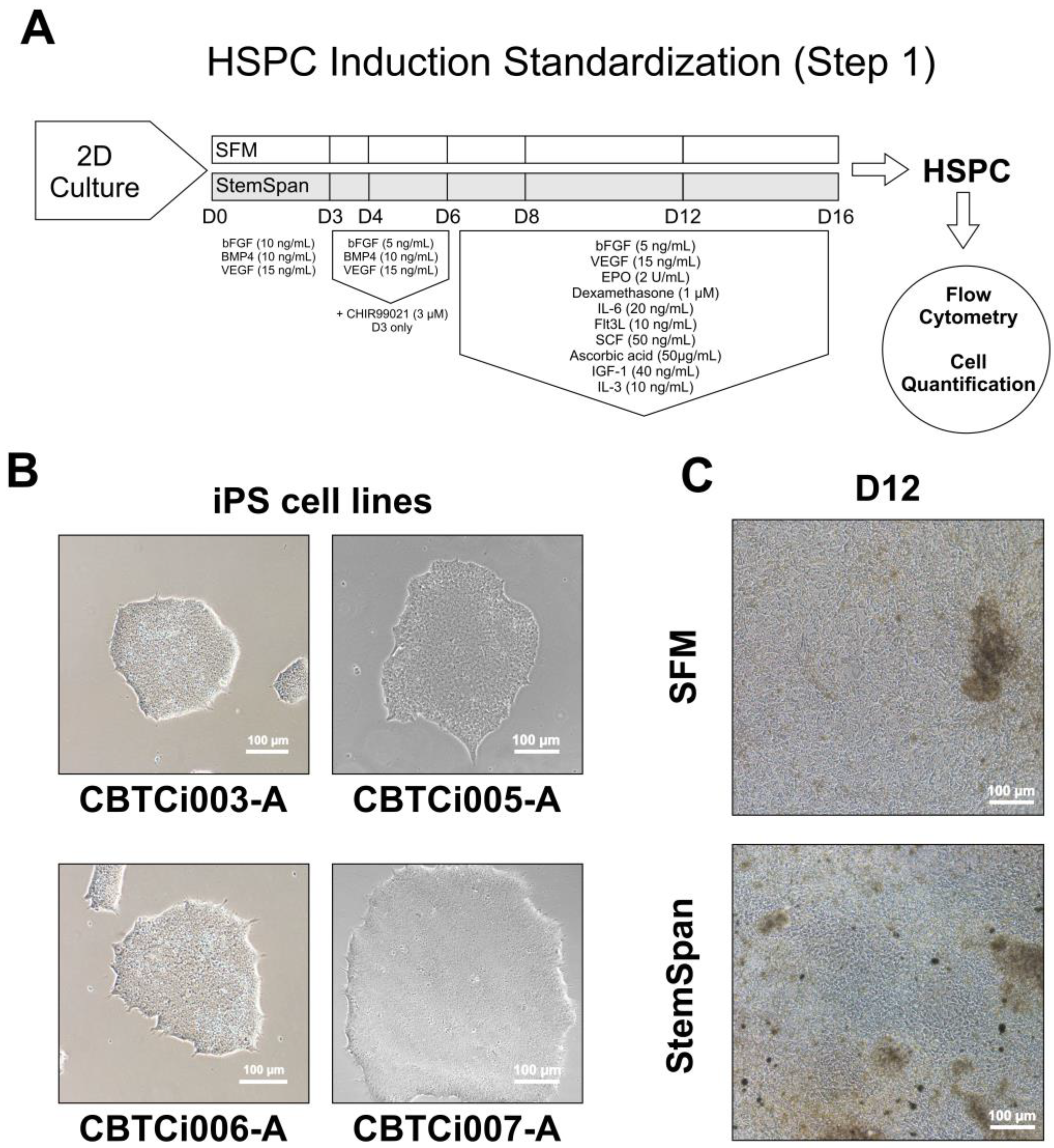
2.3. Differentiation Step 1—Hematopoietic Stem Progenitor Cell Induction
2.3.1. 2D Cultivation Method
2.3.2. 3D Cultivation Method—Embryoid Bodies (EBs)
2.4. Differentiation Step 2—HSCP towards Erythroid Progenitor Cells (EPCs)
2.5. Differentiation Step 3—Terminal Maturation of Erythroid Cells
2.6. Flow Cytometry
2.7. Colony Forming Unit (CFU) Assay
2.8. Morphological Evaluation
2.9. Analysis of Hemoglobin Expression by RT-qPCR
2.10. Investigation of βS-Globin Gene Cluster Haplotypes
2.11. Relevant Reagents and Statistical Analysis
3. Results
3.1. Induction of iPSCs towards HSPCs (Step 1)
3.2. Induction of HSPC towards EPCs (Step 2)
3.3. Maturation of Erythroid Cells (Step 3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Temperley, W.H.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013, 381, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Kato, J.G.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef] [Green Version]
- Rai, P.; Ataga, K.I. Drug Therapies for the Management of Sickle Cell Disease. F1000Research 2020, 9, 592. [Google Scholar] [CrossRef]
- Tozatto-Maio, K.; Girot, R.; Ly, I.D.; Silva, A.C.P.; Rocha, V.; Fernandes, F.; Diagne, I.; Benzerara, Y.; Dinardo, C.L.; Soler, J.P.; et al. Polymorphisms in Inflammatory Genes Modulate Clinical Complications in Patients with Sickle Cell Disease. Fron. Immunol. 2020, 11, 1664–3224. [Google Scholar] [CrossRef]
- Sebastiano, V.; Maeder, M.L.; Angstman, J.F.; Haddad, B.; Khayter, C.; Yeo, D.T.; Goodwin, M.J.; Hawkins, J.S.; Ramirez, C.L.; Batista, L.F.; et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells 2011, 29, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wang, Y.; Yan, W.; Smith, C.; Ye, Z.; Wang, J.; Gao, Y.; Mendelsohn, L.; Cheng, L. Production of Gene-Corrected Adult Beta Globin Protein in Human Erythrocytes Differentiated from Patient iPSCs after Genome Editing of the Sickle Point Mutation. Stem Cells 2015, 33, 1470–1479. [Google Scholar] [CrossRef] [Green Version]
- Park, I.H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef] [Green Version]
- Haro-Mora, J.J.; Uchida, N.; Demirci, S.; Wang, Q.; Zou, J.; Tisdale, J.F. Biallelic correction of sickle cell disease-derived induced pluripotent stem cells (iPSCs) confirmed at the protein level through serum-free iPS-sac/erythroid differentiation. Stem Cells Transl. Med. 2020, 9, 590–602. [Google Scholar] [CrossRef] [Green Version]
- Martins, G.L.S.; Paredes, B.D.; Azevedo, C.M.; Sampaio, G.L.A.; Nonaka, C.K.V.; Cavalcante, B.R.R.; Da Silva, K.N.; Pereira, C.S.E.; Soares, M.B.P.; Dos Santos, R.R.; et al. Generation of integration-free iPS cell lines from three sickle cell disease patients from the state of Bahia, Brazil. Stem Cell Res. 2018, 33, 10–14. [Google Scholar] [CrossRef]
- Paredes, B.D.; Martins, G.L.S.; Azevedo, C.M.; Sampaio, G.L.A.; Nonaka, C.K.V.; Da Silva, K.N.; Soares, M.B.P.; Dos Santos, R.R.; Souza, B.S.F. Generation of three control iPS cell lines for sickle cell disease studies by reprogramming erythroblasts from individuals without hemoglobinopathies. Stem Cell Res. 2019, 38, 101454. [Google Scholar] [CrossRef]
- Reis, L.J.C.; Picanço-Castro, V.; Paes, B.C.M.F.; Pereira, O.A.; Gyuricza, I.G.; De Araújo, F.T.; Morato-Marques, M.; Moreira, L.F.; Costa, E.B.O.; Dos Santos, T.P.M.; et al. Induced Pluripotent Stem Cell for the Study and Treatment of Sickle Cell Anemia. Stem Cells Int. 2017, 2017, 7492914. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, N.; Jaitly, S.; Goswami, S.G.; Jain, S.; Chakraborty, D.; Ramalingam, S. Generation and characterization of induced pluripotent stem cell line (IGIBi001-A) from a sickle cell anemia patient with homozygous β-globin mutation. Stem Cell Res. 2019, 39, 1873–5061. [Google Scholar] [CrossRef]
- Chou, B.K.; Mali, P.; Huang, X.; Ye, Z.; Dowey, S.N.; Resar, L.M.S.; Zou, C.; Zhang, Y.A.; Tong, J.; Chen, L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011, 21, 518–529. [Google Scholar] [CrossRef]
- Mali, P.; Chou, B.K.; Yen, J.; Ye, Z.; Zou, J.; Dowey, S.; Brodsky, R.A.; Ohm, J.E.; Yu, W.; Baylin, S.B.; et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 2010, 28, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Gianotti-Sommer, A.; Molina-Estevez, F.J.; Vanuytsel, K.; Skvir, N.; Leung, A.; Rozelle, S.S.; Shaikho, E.M.; Weir, I.; Jiang, Z.; et al. A Comprehensive, Ethnically Diverse Library of Sickle Cell Disease-Specific Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Paes, B.C.M.F.; Stabeli, L.C.J.R.; Costa, P.N.M.; Orellana, M.D.; Kashima, S.; Covas, T.D.; Picanço-Castro, V. Generation of hematopoietic stem/progenitor cells with sickle cell mutation from induced pluripotent stem cell in serum-free system. Hematol. Transfus. Cell Ther. 2021, 43, 156–164. [Google Scholar] [CrossRef]
- Martin, R.M.; Ikeda, K.; Cromer, M.K.; Uchida, N.; Nishimura, T.; Romano, R.; Tong, A.J.; Lemgart, V.T.; Camarena, J.; Pavel-Dinu, M.; et al. Highly Efficient and Marker-free Genome Editing of Human Pluripotent Stem Cells by CRISPR-Cas9 RNP and AAV6 Donor-Mediated Homologous Recombination. Cell Stem Cell 2019, 24, 821–828. [Google Scholar] [CrossRef]
- Cai, L.; Bai, H.; Mahairaki, V.; Gao, Y.; He, C.; Wen, Y.; Jin, Y.C.; Wang, Y.; Pan, R.L.; Qasba, A.; et al. A Universal Approach to Correct Various HBB Gene Mutations in Human Stem Cells for Gene Therapy of Beta-Thalassemia and Sickle Cell Disease. Stem Cells Transl. Med. 2018, 7, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Vanuytsel, K.; Matte, T.; Leung, A.; Naing, Z.H.; Morrison, T.; Chui, D.H.K.; Steinberg, M.H.; Murphy, G.J. Induced pluripotent stem cell-based mapping of β-globin expression throughout human erythropoietic development. Blood Adv. 2018, 2, 1998–2011. [Google Scholar] [CrossRef] [Green Version]
- Uchida, N.; Haro-Mora, J.J.; Fujita, A.; Lee, D.Y.; Winkler, T.; Hsieh, M.M.; Tisdale, J.F. Efficient Generation of β-Globin-Expressing Erythroid Cells Using Stromal Cell-Derived Induced Pluripotent Stem Cells from Patients with Sickle Cell Disease. Stem Cells 2017, 35, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, M.H.; Kumar, S.; Murphy, G.J.; Vanuytsel, K. Sickle cell disease in the era of precision medicine: Looking to the future. Expert Rev. Precis. Med. Drug Dev. 2019, 4, 357–367. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Forouzesh, M.; Raoufi, S.; Ramazii, M.; Ghaedrahmati, F.; Farzaneh, M. Differentiation of human induced pluripotent stem cells into erythroid cells. Stem Cell Res. Ther. 2020, 11, 483. [Google Scholar] [CrossRef]
- Vodyanik, M.A.; Bork, J.A.; Thomson, J.A.; Slukvin, I.I. Human embryonic stem cell–derived CD34+ cells: Efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood 2005, 105, 317–626. [Google Scholar] [CrossRef] [Green Version]
- Salvagiotto, G.; Burton, S.; Daigh, C.A.; Rajesh, D.; Slukvin, I.I.; Seay, N.J. A defined, feeder-free, serum-free system to generate in vitro hematopoietic progenitors and differentiated blood cells from hESCs and hiPSCs. PLoS ONE 2011, 6, e17829. [Google Scholar] [CrossRef] [Green Version]
- Hirose, S.; Takayama, N.; Nakamura, S.; Nagasawa, K.; Ochi, K.; Hirata, S.; Yamazaki, S.; Yamaguchi, T.; Otsu, M.; Sano, S.; et al. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Rep. 2013, 1, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Ochi, K.; Takayama, N.; Hirose, S.; Nakahata, T.; Nakauchi, H.; Eto, K. Multicolor Staining of Globin Subtypes Reveals Impaired Globin Switching During Erythropoiesis in Human Pluripotent Stem Cells. Stem Cells Transl. Med. 2014, 3, 792–800. [Google Scholar] [CrossRef]
- Wang, J.; Hertz, L.; Ruppenthal, S.; El Nemer, W.; Connes, P.; Goede, J.S.; Bogdanova, A.; Birnbaumer, L.; Kaestner, L. Lysophosphatidic Acid-Activated Calcium Signaling Is Elevated in Red Cells from Sickle Cell Disease Patients. Cells 2021, 10, 456. [Google Scholar] [CrossRef]
- Adebiyi, M.G.; Manalo, J.M.; Xia, Y. Metabolomic and molecular insights into sickle cell disease and innovative therapies. Blood Adv. 2019, 3, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
- Hounkpe, B.W.; Fiusa, M.M.L.; Colella, M.P.; Da Costa, L.N.G.; Benatti, R.O.; Saad, S.T.O.; Costa, F.F.; Santos, M.N.O.d.; Paula, E.V.D. Role of innate immunity-triggered pathways in the pathogenesis of Sickle Cell Disease: A meta-analysis of gene expression studies. Sci. Rep. 2015, 5, 17822. [Google Scholar] [CrossRef] [Green Version]
- Aleluia, M.M.; Santiago, R.P.; da Guarda, C.C.; Fonseca, T.C.; Neves, F.I.; Quinto, R.S.; Figueiredo, C.V.; Yahouédéhou, S.C.; Oliveira, R.M.; Ferreira, J.R.; et al. Genetic modulation of fetal hemoglobin in hydroxyurea-treated sickle cell anemia. Am. J. Hematol. 2017, 92, E70–E72. [Google Scholar] [CrossRef] [Green Version]
- Dege, C.; Sturgeon, C.M. Directed Differentiation of Primitive and Definitive Hematopoietic Progenitors from Human Pluripotent Stem Cells. J. Vis. Exp. 2017, 129, 55196. [Google Scholar] [CrossRef]
- Kurosawa, H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng. 2007, 103, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Cerdan, C.; Hong, S.H.; Bhatia, M. Formation and Hematopoietic Differentiation of Human Embryoid Bodies by Suspension and Hanging Drop Cultures. Curr. Protoc. Stem Cell Biol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.S.; Davis, R.; Stanley, E.G.; Elefanty, A.G. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat. Protoc. 2008, 3, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, H.; Kobari, L.; Mazurier, C.; Tropel, P.; Giarratana, M.C.; Zanella-Cleon, I.; Kiger, L.; Wattenhofer-Donzé, M.; Puccio, H.; Hebert, N.; et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica 2010, 95, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Dorn, I.; Klich, K.; Arauzo-Bravo, M.J.; Radstaak, M.; Santourlidis, S.; Ghanjati, F.; Radke, T.F.; Psathaki, O.E.; Hargus, G.; Kramer, J.; et al. Erythroid differentiation of human induced pluripotent stem cells is independent of donor cell type of origin. Haematologica 2015, 100, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, M.; Bouhassira, E.E.; Nagel, R.L. Polymerase chain reaction amplification applied to the determination of β-like globin gene cluster haplotypes. Am. J. Hematol. 1989, 32, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protocols. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Feng, Q.; Lu, S.J.; Klimanskaya, I.; Gomes, I.; Kim, D.; Chung, Y.; Honig, G.R.; Kim, K.S.; Lanza, R. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 2010, 28, 704–712. [Google Scholar] [CrossRef]
- Smith, B.W.; Rozelle, S.S.; Leung, A.; Ubellacker, J.; Parks, A.; Nah, S.K.; French, D.; Gadue, P.; Monti, S.; Chui, D.H.; et al. The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood 2013, 18, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.; Varga, E.; Aarts, C.; Wust, T.; Kuijpers, T.; Von Lindern, M.; Van Den Akker, E. Efficient production of erythroid, megakaryocytic and myeloid cells, using single cell-derived iPSC colony differentiation. Stem Cell Res. 2018, 29, 232–244. [Google Scholar] [CrossRef]
- Vodyanik, M.A.; Thomson, J.A.; Slukvin, I.I. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 2006, 15, 2095–2105. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.; Gumenyuk, M.; Kang, H.; Vodyanik, M.; Yu, J.; Thomson, J.A.; Slukvin, I.I. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011, 20, 1639–1647. [Google Scholar] [CrossRef] [Green Version]
- Netsrithong, R.; Suwanpitak, S.; Boonkaew, B.; Trakarnsanga, K.; Chang, L.-J.; Tipgomut, C.; Vatanashevanopakorn, C.; Pattanapanyasat, K.; Wattanapanitch, M. Multilineage differentiation potential of hematoendothelial progenitors derived from human induced pluripotent stem cells. Stem Cell Res. Ther. 2020, 11, 481. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Mende, N.; Jolly, A.; Percin, G.I.; Günther, M.; Rostovskaya, M.; Krishnan, S.M.; Oostendorp, R.A.J.; Dahl, A.; Anastassiadis, K.; Höfer, T.; et al. Prospective isolation of nonhematopoietic cells of the niche and their differential molecular interactions with HSCs. Blood 2019, 134, 1214–1226. [Google Scholar] [CrossRef]
- Ulyanova, T.; Cherone, J.M.; Sova, P.; Papayannopoulou, T. α4-Integrin deficiency in human CD34+ cells engenders precocious erythroid differentiation but inhibits enucleation. Exp. Hematol. 2022, 108, 16–25. [Google Scholar] [CrossRef]
- Eldor, J.I.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Rungarunlert, S.; Techakumphu, M.; Pirity, M.K.; Dinnyes, A. Embryoid body formation from embryonic and induced pluripotent stem cells: Benefits of bioreactors. World J. Stem Cells 2009, 1, 11–21. [Google Scholar] [CrossRef]
- Lengerke, C.; Grauer, M.; Niebuhr, N.I.; Riedt, T.; Kanz, L.; Park, I.-H.; Daley, G.Q. Hematopoietic Development from Human Induced Pluripotent Stem Cells. Ann. N. Y. Acad. Sci. 2009, 1176, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Guokai, C. Embryoid body formation from human pluripotent stem cells in chemically defined E8 media. In StemBook [Internet]; Harvard Stem Cell Institute: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Yoon, B.S.; Yoo, S.J.; Lee, J.E.; You, S.; Lee, H.T.; Yoon, H.S. Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation 2006, 74, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.D.; Gil, S.; Wilgo, M.; Pitt, A. Microporous membrane growth substrates for embryonic stem cell culture and differentiation. Methods Cell Biol. 2008, 86, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, G.; Wen, X.; Zhang, N. Formation of Well-defined Embryoid Bodies from Dissociated Human Induced Pluripotent Stem Cells using Microfabricated Cell-repellent Microwell Arrays. Sci. Rep. 2014, 4, 7402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy Buentello, D.; Koch, L.S.; Trujillo-de Santiago, G.; Alvarez, M.M.; Broersen, K. Use of standard U-bottom and V-bottom well plates to generate neuroepithelial embryoid bodies. PLoS ONE 2022, 17, e0262062. [Google Scholar] [CrossRef]
- Kessel, K.U.; Bluemke, A.; Schöler, H.R.; Zaehres, H.; Schlenke, P.; Dorn, I. Emergence of CD43-Expressing Hematopoietic Progenitors from Human Induced Pluripotent Stem Cells. Transfus. Med. Hemother. 2017, 44, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.; Ji, H.; Ehrlich, L. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y. Induced Tissue-Specific Stem Cells and Epigenetic Memory in Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018, 19, 930. [Google Scholar] [CrossRef] [Green Version]
- Vigilante, A.; Laddach, A.; Moens, N.; Meleckyte, R.; Leha, A.; Ghahramani, A.; Culley, O.J.; Kathuria, A.; Hurling, C.; Vickers, A.; et al. Identifying Extrinsic versus Intrinsic Drivers of Variation in Cell Behavior in Human iPSC Lines from Healthy Donors. Cell Rep. 2019, 26, 2078–2087.E3. [Google Scholar] [CrossRef] [Green Version]
- Merryweather-Clarke, A.T.; Tipping, A.J.; Lamikanra, A.A.; Fa, R.; Abu-Jamous, B.; Tsang, H.P.; Tsang, L.; Robson, K.J.H.; Nandi, N.K.; Roberts, D.J. Distinct gene expression program dynamics during erythropoiesis from human induced pluripotent stem cells compared with adult and cord blood progenitors. BMC Genom. 2016, 17, 817. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Lancelot, M.; Jajosky, R.; Deng, Q.; Deeb, K.; Saakadze, N.; Gao, Y.; Jaye, D.; Liu, S.; Stowell, S.R.; et al. Erythropoietic properties of human induced pluripotent stem cells-derived red blood cells in immunodeficient mice. Am. J. Hematol. 2022, 97, 194–202. [Google Scholar] [CrossRef]
- Bernecker, C.; Ackermann, M.; Lachmann, N.; Rohrhofer, L.; Zaehres, H.; Araúzo-Bravo, M.J.; Van Den Akker, E.; Schlenke, P.; Dorn, I. Enhanced Ex Vivo Generation of Erythroid Cells from Human Induced Pluripotent Stem Cells in a Simplified Cell Culture System with Low Cytokine Support. Stem Cells Dev. 2019, 28, 1540–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakarnsanga, K.; Wilson, M.C.; Lau, W.; Singleton, B.K.; Parsons, S.F.; Sakuntanaga, P.; Kurita, R.; Nakamura, Y.; Anstee, D.J.; Frayne, J. Induction of adult levels of β-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL. Haematologica 2014, 99, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Kobari, L.; Yates, F.; Oudrhiri, N.; Francina, A.; Kiger, L.; Mazurier, C.; Rouzbeth, S.; El-Nemer, W.; Hebert, N.; Giarratana, M.C.; et al. Human pluripotent stem cells can reach complete terminal maturation: In vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica 2012, 97, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, Y.; Lu, X.; Li, W.; Zhang, Y.; Mao, B.; Pan, X.; Li, X.; Zhou, Y.; An, Q.; et al. Inhibition of aryl hydrocarbon receptor signaling promotes the terminal differentiation of human erythroblasts. J. Mol. Cell Biol. 2022, 14, mjac001. [Google Scholar] [CrossRef]





| Gene | Primer | Amplicon (pb) | Digestion Product | Annealing Temperature | Restriction Enzime |
|---|---|---|---|---|---|
| 5′γG | 3 and 4 | 650 | 450 + 200 | 57 °C | XmnI * |
| γG/γA | 5 and 6 | 780 | 440 + 340 | 60 °C | HindIII |
| γG/γA | 6 and 7 | 760 | 360 + 400 | 62 °C | HindIII |
| Ψβ | 8 and 9 | 700 | 360 + 340 | 60 °C | HincII |
| 3′ψβ | 10 and 11 | 590 | 470 + 120 | 57 °C | HincII |
| iPSC Line | Phenotype | βS Haplotypes |
|---|---|---|
| CBTCi003-A | Healthy (HbAA) | Not evaluated |
| CBTCi005-A | Sickle cell anemia (HbSS) | Benin/CAR |
| CBTCi006-A | Sickle cell anemia (HbSS) | Benin/Benin |
| CBTCi007-A | Sickle cell anemia (HbSS) | Benin/CAR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, G.L.S.; Nonaka, C.K.V.; Rossi, E.A.; de Lima, A.V.R.; Adanho, C.S.A.; Oliveira, M.S.; Yahouedehou, S.C.M.A.; de Souza, C.L.e.M.; Gonçalves, M.d.S.; Paredes, B.D.; et al. Evaluation of 2D and 3D Erythroid Differentiation Protocols Using Sickle Cell Disease and Healthy Donor Induced Pluripotent Stem Cells. Cells 2023, 12, 1121. https://doi.org/10.3390/cells12081121
Martins GLS, Nonaka CKV, Rossi EA, de Lima AVR, Adanho CSA, Oliveira MS, Yahouedehou SCMA, de Souza CLeM, Gonçalves MdS, Paredes BD, et al. Evaluation of 2D and 3D Erythroid Differentiation Protocols Using Sickle Cell Disease and Healthy Donor Induced Pluripotent Stem Cells. Cells. 2023; 12(8):1121. https://doi.org/10.3390/cells12081121
Chicago/Turabian StyleMartins, Gabriele Louise Soares, Carolina Kymie Vasques Nonaka, Erik Aranha Rossi, Adne Vitória Rocha de Lima, Corynne Stephanie Ahouefa Adanho, Moisés Santana Oliveira, Setondji Cocou Modeste Alexandre Yahouedehou, Clarissa Lima e Moura de Souza, Marilda de Souza Gonçalves, Bruno Diaz Paredes, and et al. 2023. "Evaluation of 2D and 3D Erythroid Differentiation Protocols Using Sickle Cell Disease and Healthy Donor Induced Pluripotent Stem Cells" Cells 12, no. 8: 1121. https://doi.org/10.3390/cells12081121






