Abstract
Background: Interleukin-1 blockade with anakinra leads to a transient increase in eosinophil blood count (eosinophils) in patients with acute myocardial infarction. We aimed to investigate the effect of anakinra on changes in eosinophils in patients with heart failure (HF) and their correlation with cardiorespiratory fitness (CRF). Methods: We measured eosinophils in 64 patients with HF (50% females), 55 (51–63) years of age, before and after treatment, and, in a subset of 41 patients, also after treatment cessation. We also evaluated CRF, measuring peak oxygen consumption (VO2) with a treadmill test. Results: Treatment with anakinra significantly and transiently increased eosinophils, from 0.2 [0.1–0.3] to 0.3 [0.1–0.4] × 103 cells/µL (p < 0.001) and from 0.3 [0.2–0.5] to 0.2 [0.1–0.3] × 103 cells/µL, with suspension (p < 0.001). Changes in eosinophils correlated with the changes in peak VO2 (Spearman’s Rho = +0.228, p = 0.020). Eosinophils were higher in patients with injection site reactions (ISR) (n = 8, 13%; 0.5 [0.4–0.6] vs. 0.2 [0.1–0.4] × 103 cells/µL, p = 0.023), who also showed a greater increase in peak VO2 (3.0 [0.9–4.3] vs. 0.3 [−0.6–1.8] mLO2·kg−1·min−1, p = 0.015). Conclusion: Patients with HF treated with anakinra experience a transient increase in eosinophils, which is associated with ISR and a greater improvement in peak VO2.
1. Introduction
Eosinophils are granulocytic white blood cells involved in health and disease. Normal levels in peripheral blood range between 0 and 0.5 × 109/L and increase in various diseases [,].
Classically, eosinophils have been considered as end-stage effector cells, mainly characterized by the release of granule-derived cytotoxic proteins and lipids, but they also have an immunoregulatory effect on other cells []. The role of eosinophils in cardiovascular diseases (CVDs) is not completely understood. While an increase in count, eosinophilia, is often considered pathologic [], a reduction in count, eosinopenia, during acute myocardial infarction (AMI) was associated with a higher incidence of heart failure (HF) and death [,].
Changes in eosinophil blood count have been reported in patients with rheumatoid arthritis receiving anakinra, a recombinant interleukin-1 (IL-1) receptor antagonist []. We recently described that in patients with ST-segment elevation myocardial infarction (STEMI), anakinra leads to a significant reduction in leukocyte count with a relative reduction in neutrophils and an increase in eosinophils []. In patients with STEMI, anakinra was also associated with a reduction in HF-related events [,,,].
Anakinra is also frequently associated with injection site reactions (ISR), characterized by erythema, inflammation, and pain. These reactions usually occur 1–2 weeks after the start of treatment, tend to be mild and transient, and eosinophils have been shown to play a role [,].
Whether anakinra is associated with changes in eosinophil count and whether these changes are associated with a different response in systemic inflammation (changes in C-reactive protein, CRP), cardiorespiratory fitness (CRF), cardiac systolic and diastolic function are unknown. Moreover, whether ISR represents an eosinophilic response to anakinra and is associated with a different response to treatment in patients with HF are also unknown. The aim of the study was to evaluate the role of the changes in eosinophil blood count in patients with HF treated with anakinra, and to determine whether changes in eosinophils correlate with clinical or functional parameters in patients with HF, and with the incidence of ISR.
2. Materials and Methods
2.1. Patient Population
We analyzed data from patients with HF who were treated with anakinra (Kineret®, Swedish Orphan Biovitrum, Waltham, MA, USA), and underwent blood sampling at baseline and during treatment. Transthoracic echocardiogram (TTE), cardiopulmonary exercise testing (CPX), and quality of life assessments were performed at each visit as well. In a portion of patients, the same assessment was repeated after discontinuation of the treatment.
The analysis included the pooled active treatment arms of four clinical trials. The pilot study of the safety and efficacy of Anakinra in Heart Failure (AIR-HF) [] was a phase 2, open-label, single-arm pilot trial that enrolled 7 patients with HF with reduced ejection fraction (HFrEF) and elevation of high-sensitivity CRP (>2 mg/L), baseline blood sampling, and CPX, and subsequently subjected them to treatment with anakinra 100 mg daily for 14 days, with repeat testing at the end of anakinra treatment. The pilot feasibility study of the safety and efficacy of Anakinra in Heart Failure With Preserved Ejection Fraction (D-HART) [] was a phase 2, pilot crossover trial that randomly assigned 12 patients with stable HF with preserved ejection fraction (HFpEF), and with significant symptoms (New York Heart Association [NYHA] class II or III) and baseline elevation of high-sensitivity CRP (≥2 mg/L) to receive either anakinra 100 mg daily for 14 days or placebo. Blood sampling and CPX were performed at the end of each 14-day treatment course, and 2 weeks after completing an experimental treatment course. The D-HART2 trial [] randomized 31 patients with HFpEF and elevated baseline high-sensitivity CRP (>2 mg/L) to either anakinra 100 mg daily (n = 21) or placebo (n = 10) for 12 weeks. Blood sampling and CPX were performed at 4, 12, and 24 weeks from enrollment. Finally, the Recently Decompensated Heart Failure Anakinra Response Trial (REDHART) trial [] enrolled patients with HF with HFrEF, and elevated CRP (>2 mg/L), within 14 days from discharge after a HF hospitalization. Patients were randomized to receive either anakinra 100 mg daily for 12 weeks (n = 20), anakinra 100 mg daily for 2 weeks (n = 20), or placebo (n = 20). Patients underwent blood sampling and CPX at 2, 4, 12, and 24 weeks. Patients with acute infections as well as severe asthma or chronic pulmonary obstructive diseases were excluded in the original trials. All studies were approved by the local Institutional Review Board and were conducted according to the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent.
2.2. Laboratory Data
A complete blood cell count (CBC) with differential count was obtained at baseline, during treatment with anakinra, and again after discontinuation of the treatment. White blood cell count, lymphocyte, neutrophil, eosinophil, and basophil counts were calculated using a hematology analyzer. We also calculated the leukocyte-to-eosinophil ratio (LER) and the neutrophil-to-eosinophil ratio (NER), as an expression of preferential eosinophilic maturation and increase. High-sensitivity CRP, chosen as a surrogate for IL-1 activity, and N-terminal pro B-type natriuretic peptide (NT-proBNP), a biomarker of myocardial strain, were also measured at each visit. Data from the last available laboratory test while on anakinra were collected as on-treatment, while data from last available laboratory test after treatment suspension were collected as off-treatment.
2.3. Cardiorespiratory Fitness, Cardiac Function, and Quality of Life
A supervised maximal aerobic CPX was administered using a conservative ramping treadmill protocol, as previously described [,,,]. The peak oxygen consumption (VO2), minute ventilation to carbon dioxide production (VE/VCO2) slope, exercise time, peak respiratory exchange ratio (RER), and the oxygen uptake efficiency slope (OUES) were considered.
Furthermore, all patients underwent TTE during the same outpatient visit as the laboratory data and prior to CPX, according to the study protocols []. The left-ventricular ejection fraction (LVEF) and pulsed-wave Doppler early mitral flow velocity to early mitral annulus tissue velocity (E/e′) ratio were considered as LV systolic and diastolic function indices, respectively.
Symptom burden and quality of life were assessed at each visit with the Duke Activity Status Index (DASI) [] and the Minnesota Living with Heart Failure (MLHFQ) questionnaire []. A lower DASI score reflects impaired perceived functional capacity, whereas a higher MLHFQ score reflects greater HF symptom burden.
2.4. Objective of the Analysis
The primary objective of this analysis was to evaluate changes in eosinophils in patients with HF treated with anakinra. Secondary objectives were to evaluate whether these changes are associated with the incidence of ISR and changes in CRP, CRF, and cardiac systolic and diastolic function.
2.5. Statistical Analyses
Individual patient data were pooled in a single prespecified data set and analyzed. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Baseline characteristics were reported as number (percentage) or median [interquartile range]. The correlation between continuous variables was assessed using Spearman’s rank correlation coefficient. Categorical variables were compared using chi-square test or Fisher’s exact test, when indicated. The within-group paired differences were compared using the Wilcoxon signed-rank test. A 2-sided p-value of less than 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics 26 (IBM, Armonk, NY, USA).
3. Results
We identified 64 patients with HF who received anakinra treatment and had an available CBC with differential before and during anakinra treatment (on-treatment analysis). Among those, 41 (64%) also repeated the assessment after discontinuation of the treatment (off-treatment analysis). The median age was 55 (51–63) years, and 32 (50%) were biological females and 47 (73%) self-identified as Black/African-American. Baseline CRP was 6.2 (3.0–15.4) mg/L, LVEF was 49 (33–60)%, E/e’ was 14.2 (9.7–19.9), peak VO2 was 14.2 (11.6–16.9) mLO2·kg−1·min−1, and the VE/VCO2 slope was 31 (27–35). The duration of on-treatment with anakinra was 4 (2–12) weeks, whereas the time from discontinuation of anakinra to the latest off-treatment assessment was 12 (4–12) weeks. Baseline demographic and clinical characteristics are summarized in Table 1. There were no statistically significant differences in clinical characteristics between the patients with on-treatment analysis and those with off-treatment analysis (all p > 0.05).

Table 1.
Clinical characteristics of patients in the on-treatment and off-treatment analyses.
Treatment with anakinra was associated with a significant increase in eosinophils (from 0.2 [0.1–0.3] × 103 cells/µL to 0.3 [0.1–0.4] × 103 cells/µL, p < 0.001) and this change was reverted after the discontinuation of the treatment (from 0.3 [0.2–0.5] × 103 cells/µL to 0.2 [0.1–0.3] × 103 cells/µL, p < 0.001), as shown in Figure 1. A decrease in NER (from 21.0 [14.3–36.8] to 9.5 [6.4–20.9], p < 0.001) and LER (from 38.0 [21.7–56.0] to 19.0 [12.9–40.1], p = 0.001) during treatment was observed, and this was reversed after suspension (from 8.0 [5.3–13.0] to 18.5 [10.7–35.0] for NER, p < 0.001 and from 16.0 [11.5–27.7] to 31.0 [19.5–64.0] for LER, p < 0.001).
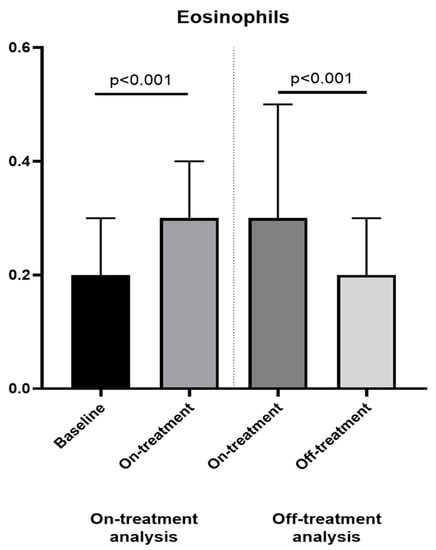
Figure 1.
Changes in eosinophils between baseline, on and off-treatment analyses. We found a significant change in eosinophils between baseline and on-treatment (on-treatment analysis, left panel) and between on and off-treatment (off-treatment analysis, right panel).
Comparing the patients treated for 2–4 weeks (N = 35) and patients treated for 12 weeks (N = 29), we found no significant changes in eosinophils in patients treated with anakinra for 2–4 weeks (from 0.2 [0.1–0.3] × 103 cells/µL to 0.2 [0.1–0.3] × 103 cells/µL, p = 0.18), and a statistically significant increase in eosinophils (from 0.2 [0.1–0.3] × 103 cells/µL to 0.4 [0.2–0.5] × 103 cells/µL, p < 0.001) in patients treated with anakinra for 12 weeks (absolute change in eosinophil was 0 [0–0.1] × 103 cells/µL and 0.2 [0–0.3] × 103 cells/µL, for patients treated for 2–4 and 12 weeks, respectively, p < 0.01) (Figure 2). The duration of treatment did not correlate with the changes in peak VO2. These data show a greater increase in eosinophils with longer duration of treatment.
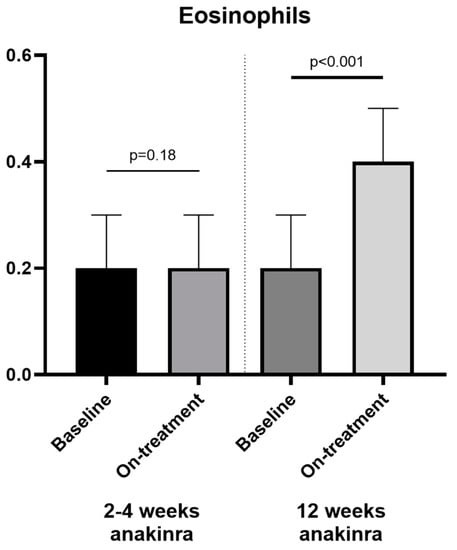
Figure 2.
Duration of anakinra treatment and changes in eosinophils. We found a significant change in eosinophils between baseline and on-treatment analysis in the patients who received anakinra for 12 weeks (right panel) and not in the 2–4 weeks treatment (left panel).
Of note, the changes in eosinophils over time were significantly positively correlated with the changes in peak VO2 (Spearman’s Rho = +0.228, p = 0.020), DASI score (Rho = +0.261, p = 0.015), and negatively with changes in CRP (Rho = −0.297, p = 0.002), NT-proBNP (Rho = −0.222, p = 0.041), and the E/e′ ratio (Rho = −0.288, p = 0.011). No significant correlations were found for changes in the other parameters (VE/VCO2 slope, Rho = −0.040, p = 0.690; exercise time, Rho = +0.185, p = 0.059; RER, Rho = +0.160, p = 0.11; OUES, Rho = +0.069, p = 0.849; LVEF, Rho = −0.029, p = 0.795; MLHFQ, Rho = −0.132, p = 0.227), as shown in Table 2.

Table 2.
Correlations between changes in eosinophils and peak VO2, DASI score, C-reactive protein, NT-proBNP, and E/e′ in patients with heart failure treated with anakinra.
Both changes in NER and LER correlated negatively with the changes in peak VO2 (Rho = −0.303, p = 0.002 and Rho = −0.344, p < 0.001, respectively) and DASI sore (Rho = −0.358, p = 0.001 and Rho = −0.360, p = 0.001, respectively), and positively with changes in CRP (Rho = +0.525, p < 0.001 and Rho = +0.504, p < 0.001, respectively). On the other hand, only LER correlated with MLHFQ score (Rho = +0.251, p = 0.026).
ISR were reported by eight (13%) patients (median age of 51 [45–59] years), of which four (50%) self-identified as females and four (50%) as Black/African-American. None of these ISR patients required drug discontinuation. There was no significant difference between patients with or without ISR at baseline; on the other hand, patients with ISR had higher on-treatment eosinophil counts (0.45 [0.38–0.60] × 103 cells/µL vs. 0.20 [0.10–0.40] × 103 cells/µL, p = 0.023), higher absolute peak VO2 (18.5 [14.5–25.2] mLO2·kg−1·min−1 vs. 14.9 [12.3–17.2] mLO2·kg−1·min−1, p = 0.044), larger change in peak VO2 (3.0 [0.9–4.3] mLO2·kg−1·min−1 vs. 0.3 [−0.6–1.8] mLO2·kg−1·min−1, p = 0.015), longer on-treatment exercise time (650 [490–830] seconds vs. 500 [370–583] seconds, p = 0.016), and lower on-treatment NT-proBNP levels (81 [13–120] pg/mL vs. 445 [95–1187] pg/mL, p = 0.003), as shown in Table 3 and Figure 3.

Table 3.
Clinical characteristics, cardiorespiratory fitness, and biomarkers in patients with and without injection site reactions.
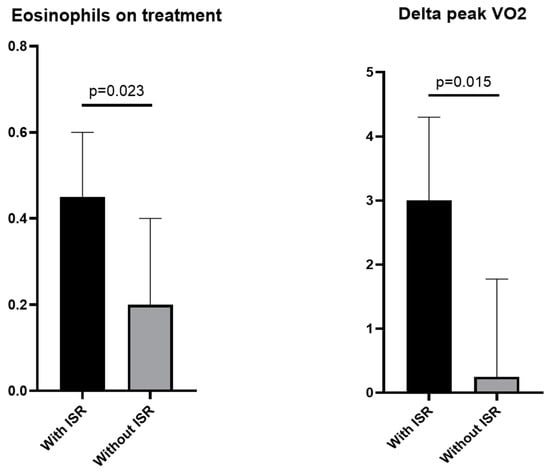
Figure 3.
Injection site reactions, eosinophils, and peak VO2 changes. We found a statistically significant difference in eosinophils (left panel) and change (delta) in peak VO2 (right panel) between patients who experienced and did not experience injection site reactions. Abbreviation: ISR, injection-site reactions.
4. Discussion
We herein report, for the first time, that IL-1 blockade with anakinra leads to a significant transient increase in eosinophils in patients with established HF, with greater changes with longer duration. These changes are reversed after discontinuation of treatment. Moreover, the changes in eosinophils were inversely related to markers of systemic inflammation (i.e., CRP) and directly related to greater cardiorespiratory fitness (peak VO2).
Eosinophilia after treatment with anakinra has been reported in as many as 9% of patients with rheumatoid arthritis [], although the exact mechanism and function is unknown. We recently reported an increase in eosinophils in patients with STEMI treated with anakinra [] in randomized clinical trials associated with a reduction in HF events. The effects of anakinra on eosinophils, and the implications of this in patients with HF treated with anakinra are, however, rather unexplored.
Eosinophilia is associated with severe cardiovascular manifestations such as endocarditis and thrombosis, endomyocardial fibrosis, and valvular disease []. The elevation of eosinophils, even if milder, has been associated with coronary artery disease prevalence []. On the other side, eosinophils have also recently been shown to have a cardioprotective role in preclinical models of AMI [], protecting cardiomyocytes from ischemia injury, reducing cardiomyocyte death, and regulating cardiac fibroblast activity and post-AMI inflammatory cell adhesion and infiltration. Eosinopenia, a reduction in eosinophils, has been reported as a surrogate of hyper-inflammation, linked to adverse cardiac remodeling and function following AMI [], and associated with worse clinical outcomes over long-term follow-up [,]. Vural A et al. [] showed that NER and LER are predictive for 6-month mortality and major adverse cardiovascular events in patients with acute decompensated HF with reduced EF. On this basis, the increased eosinophil counts observed during treatment with anakinra in STEMI patients [] could suggest a way by which the drug modulates the innate immune inflammatory response and prevents post-AMI HF events. We herein show a significant reduction in NER and LER during treatment with anakinra, which is reversed after suspension.
Injection-site reaction during treatment with anakinra is the most common side effect, reported in more than 70% of cases in some series, and this finding can be associated with an increase in circulating eosinophils and dermal infiltrate of eosinophils []. We showed for the first time that patients with ISR had increased eosinophil counts but also a greater response in CRF (greater improvements in peak VO2), supporting the concept that an enhanced eosinophilic response to anakinra is associated with clinical improvement. Accordingly, we also found a positive correlation between eosinophils, NER, LER, and the changes in peak VO2 and perceived functional capacity (DASI score). Whether the difference in improvement in CRF was dependent on the different baseline characteristics between patients with or without injection site reactions, or whether it was a biomarker of a greater response to the treatment cannot be determined with the current study and requires additional research. Moreover, whether the change in eosinophils is simply a biomarker or also a mediator of the anti-inflammatory mechanism of anakinra is not known. The inverse correlation between eosinophils and measures of impaired cardiac diastolic function (NT-proBNP, and E/e′ ratio) and the direct correlation with cardiorespiratory fitness (peak VO2) suggest a possible cardioprotective effect of eosinophils.
5. Conclusions
Patients with HF treated with anakinra experience a transient increase in blood eosinophil counts. The eosinophil count may serve as a favorable prognostic biomarker for IL-1 blockade in HF, and patients with ISR may represent a subset of patients with an enhanced eosinophilic response that experience a greater benefit from the treatment. Furthermore, these data may also open the way to new mechanistic research about the roles of IL-1 and eosinophils in HF.
Author Contributions
Conceptualization, M.G., F.M., S.C., B.V.T. and A.A.; data curation, F.M., M.G.D.B., S.P., J.M. and A.-C.H.; formal analysis, M.G., F.M. and A.A.; investigation, M.G., F.M., M.G.D.B., J.M.C., A.H.T., S.P., J.M., A.V., A.-C.H., G.K.T., S.C., H.E.B., J.T., R.M., C.T., R.D.P., B.V.T. and A.A.; software, F.M.; supervision, B.V.T. and A.A.; writing—original draft, M.G.; writing—review and editing, F.M., M.G.D.B., J.M.C., A.H.T., A.V., G.K.T., S.C., H.E.B., J.T., R.M., C.T., R.D.P., B.V.T. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The present study provides a pooled analysis of the Pilot Study of the Safety and Efficacy of Anakinra in Heart Failure (AIR-HF) [] supported by award number UL1RR031990 from the National Center for Research Resources, and from an American Heart Association Scientist Development Grant. Pilot Feasibility Study of the Safety and Efficacy of Anakinra in Heart Failure With Preserved Ejection Fraction (D-HART) [] supported by an American Heart Association Scientist Development grant (10SDG303005) to Abbate, and by Clinical and Translational Science Award K12 training award (KL2RR031989) from the National Center for Research Resources to Van Tassell at Virginia Commonwealth University Center for Clinical and Translational Research; the D-HART2 trial [] supported by a grant from the National Heart, Lung, and Blood Institute (1R34HL118348) to Abbate and Van Tassell, a Clinical and Translational Science Award (UL1TR000058) from the National Center for Research Resources to the Virginia Commonwealth University Center for Clinical and Translational Research, and by Swedish Orphan Biovitrum (SOBI, Stockholm, Sweden) who provided the active drug (anakinra) and placebo free of charge; and the Recently Decompensated Heart Failure Anakinra Response Trial (REDHART) trial [] supported by a grant from the National Heart, Lung, and Blood Institute (1R34HL117026) to Abbate and Van Tassell, and by Swedish Orphan Biovitrum (SOBI, Stockholm, Sweden) who provided the active drug (anakinra) and placebo free of charge. No additional funding was provided for this analysis.
Institutional Review Board Statement
The present study provides a pooled analysis of four studies that were conducted according to the guidelines of the Declaration of Helsinki, and approved by the Virginia Commonwealth University Institutional Review Board (IRB): AIR-HF [] (IRB approval on 12 May 2011), DHART [] (HM14079, IRB approval on 18 October 2012), DHART-2 [] (HM20000118, IRB approval on 19 September 2014), and REDHART [] (HM15339, IRB approval on 8 August 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the four studies from which data were taken for the following pooled analysis.
Data Availability Statement
Data are available on request. Outcome is dependent upon review from the Institutional Review Board.
Conflicts of Interest
Abbate and Van Tassell have received grant support from and have served as consultants to Swedish Orphan Biovitrum (SOBI, Stockholm, Sweden) in the past. None of the other authors report any conflicts of interest regarding the content of this article.
References
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. 2020, 15, 179–209. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2019, 94, 1149–1167. [Google Scholar] [CrossRef] [PubMed]
- Akuthota, P.; Wang, H.B.; Spencer, L.A.; Weller, P.F. Immunoregulatory roles of eosinophils: A new look at a familiar cell. Clin. Exp. Allergy 2008, 38, 1254–1263. [Google Scholar] [CrossRef]
- Alkhalil, M.; Kearney, A.; Hegarty, M.; Stewart, C.; Devlin, P.; Owens, C.G.; Spence, M.S. Eosinopenia as an Adverse Marker of Clinical Outcomes in Patients Presenting with Acute Myocardial Infarction. Am. J. Med. 2019, 132, e827–e834. [Google Scholar] [CrossRef]
- Shah, A.D.; Denaxas, S.; Nicholas, O.; Hingorani, A.D.; Hemingway, H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: A CALIBER cohort study. Open Heart 2016, 3, e000477. [Google Scholar] [CrossRef]
- Swedish Orphan Biovitrum AB (publ). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf (accessed on 9 April 2023).
- Del Buono, M.G.; Damonte, J.I.; Trankle, C.R.; Kadariya, D.; Carbone, S.; Thomas, G.; Turlington, J.; Markley, R.; Canada, J.M.; Biondi-Zoccai, G.G.; et al. Effect of interleukin-1 blockade with anakinra on leukocyte count in patients with ST-segment elevation acute myocardial infarction. Sci. Rep. 2022, 12, 1254. [Google Scholar] [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef]
- Abbate, A.; Wohlford, G.F.; Del Buono, M.G.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Kadariya, D.; Trankle, C.R.; Biondi-Zoccai, G.; Lipinski, M.J.; et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: Results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharm. 2022, 8, 503–510. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Damonte, J.I.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Trankle, C.R.; Kang, L.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Effect of IL-1 Blockade with Anakinra on Heart Failure Outcomes in Patients with Anterior Versus Nonanterior ST Elevation Myocardial Infarction. J. Cardiovasc. Pharm. 2022, 79, 774–780. [Google Scholar] [CrossRef]
- Kaiser, C.; Knight, A.; Nordstrom, D.; Pettersson, T.; Fransson, J.; Florin-Robertsson, E.; Pilstrom, B. Injection-site reactions upon Kineret (anakinra) administration: Experiences and explanations. Rheumatol. Int. 2012, 32, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Vila, A.T.; Puig, L.; Fernandez-Figueras, M.T.; Laiz, A.M.; Vidal, D.; Alomar, A. Adverse cutaneous reactions to anakinra in patients with rheumatoid arthritis: Clinicopathological study of five patients. Br. J. Dermatol. 2005, 153, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Arena, R.A.; Toldo, S.; Mezzaroma, E.; Azam, T.; Seropian, I.M.; Shah, K.; Canada, J.; Voelkel, N.F.; Dinarello, C.A.; et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS ONE 2012, 7, e33438. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 Blockade in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Canada, J.; Carbone, S.; Trankle, C.; Buckley, L.; Oddi Erdle, C.; Abouzaki, N.A.; Dixon, D.; Kadariya, D.; Christopher, S.; et al. Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 2017, 10, e004373. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Boineau, R.E.; Higginbotham, M.B.; Lee, K.L.; Mark, D.B.; Califf, R.M.; Cobb, F.R.; Pryor, D.B. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am. J. Cardiol. 1989, 64, 651–654. [Google Scholar] [CrossRef]
- Rector, T.S.; Kubo, S.H.; Cohn, J.N. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am. J. Cardiol. 1993, 71, 1106–1107. [Google Scholar] [CrossRef]
- Pongdee, T.; Manemann, S.M.; Decker, P.A.; Larson, N.B.; Moon, S.; Killian, J.M.; Liu, H.; Kita, H.; Bielinski, S.J. Rethinking blood eosinophil counts: Epidemiology, associated chronic diseases, and increased risks of cardiovascular disease. J. Allergy Clin. Immunol. Glob. 2022, 1, 233–240. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Liu, T.; Deng, Z.; Fang, W.; Zhang, X.; Li, J.; Huang, Q.; Liu, C.; Wang, Y.; et al. Eosinophils improve cardiac function after myocardial infarction. Nat. Commun. 2020, 11, 6396. [Google Scholar] [CrossRef]
- Toor, I.S.; Rückerl, D.; Thomson, A.; Tang, K.; Newby, D.E.; Rossi, A.G.; Allen, J.E.; Grey, G.A. Eosinophils have an essential role in cardiac repair following myocardial infarction. Heart 2017, 103, A152. [Google Scholar] [CrossRef]
- Vural, A.; Aydin, E. The Predictive Value of Eosinophil Indices for Major Cardiovascular Events in Patients with Acute Decompensated HFrEF. Medicina 2022, 58, 1455. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).